Journal of Dermatology and Clinical Research Cite this article: Poon F, Heyes C, Scardamaglia L, Martyres R, Chatfield S, et al. (2017) Severe Dystrophic Calcinosis Cutis: The Need for Early Intervention and Optimal Treatment of Juvenile Dermatomyositis. J Dermatolog Clin Res 5(2): 1095. Central *Corresponding author Dr Flora Poon, Dermatology Registrar, The Royal Melbourne Hospital, Grattan Street, Parkville, VIC, Australia 3050, Email: flora Submitted: 01 February 2017 Accepted: 11 March 2017 Published: 22 March 2017 Copyright © 2017 Poon et al. OPEN ACCESS Keywords • Juvenile dermatomyositis • Dystrophic calcinosis cutis Case Report Severe Dystrophic Calcinosis Cutis: The Need for Early Intervention and Optimal Treatment of Juvenile Dermatomyositis Poon F 1 *, Heyes C 1 , Scardamaglia L 1 , Martyres R 2 , Chatfield S 3 , and Varigos G 1 1 Department of Dermatology, The Royal Melbourne Hospital, Australia 2 Department of General Medicine, The Royal Melbourne Hospital, Australia 3 Department of Rheumatology, The Royal Melbourne Hospital, Australia Abstract Dystrophic calcinosis cutis (DCC) is a severe, debilitating sequelae of juvenile dermato- myositis (DM) that can have significant morbidity. Treatment of extensive DCC can be very challenging with inconsistent results and limited evidence to guide best therapy. We present a case of a particularly severe form of DCC presenting in an adult, which highlights the necessity of early optimisation of juvenile DM treatment to help prevent this occurring. ABBREVIATIONS JDM: Juvenile Dermatomyositis; DCC: Dystrophic Calcinosis Cutis INTRODUCTION Dystrophic calcinosis cutis (DCC) is a severe debilitating entity secondary to juvenile dermatomyositis (DM) that can have significant morbidity later in life. DCC is characterised by the abnormal deposition of calcium salts within skin, subcutaneous tissue, muscles, and tendons in the setting of normal serum calcium and phosphate levels. Juvenile DM and DCC progressing into adulthood is an uncommon occurrence. We present a rare case of a patient with juvenille DM who presents with DCC after a prolonged period of being lost to follow-up. CASE PRESENTATION A 49-year-old Caucasian woman was referred to a tertiary centre for assessment of painful large nodules located over her bilateral posterior thighs, elbows, flexor arms and posterior torso that had been gradually worsening over 10-years with initial nodules noticed in her twenties. This was associated with joint contractures that limited her physical mobility resulting in deconditioning and psychological stress including anxiety and depression. The patient had been diagnosed with juvenile DM aged 12 whilst living in regional Australia. She had been treated with oral prednisolone initiated by her general practitioner and subsequently was lost to follow-up without any regular rheumatology care. In her teenage years, she began to develop calcific deposits that caused contractures of her bilateral achilles tendons which required tendon lengthening. Her comorbidities include hypertension and hyperandrogenism secondary to ovarian enlargement for which she underwent a bilateral salpingo-oophrectomy. Clinical examination revealed multiple irregular protuberant painful masses in the bilateral posterior thighs and torso (Figure 1) that cause the patient significant discomfort and limited her range of movement including the ability to sit down for prolonged periods. She displayed generalised muscle atrophy of the upper and lower limbs however had 4+/5 power symmetrical bilaterally. Violaceous papules (Gottron’s papules) were identified over dorsal hands overlying the metacarpophalyngeal and proximal interphalyngeal joints and bilateral elbows. She had a fixed flexion deformity (less than 10 degrees) of her right Figure 1 Protuberant and indurated masses in the bilateral posterior thighs and torso.

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Journal of Dermatology and Clinical Research
Cite this article: Poon F, Heyes C, Scardamaglia L, Martyres R, Chatfield S, et al. (2017) Severe Dystrophic Calcinosis Cutis: The Need for Early Intervention and Optimal Treatment of Juvenile Dermatomyositis. J Dermatolog Clin Res 5(2): 1095.
Central
*Corresponding authorDr Flora Poon, Dermatology Registrar, The Royal Melbourne Hospital, Grattan Street, Parkville, VIC, Australia 3050, Email: flora
Submitted: 01 February 2017
Accepted: 11 March 2017
Published: 22 March 2017
Copyright© 2017 Poon et al.
OPEN ACCESS
Keywords• Juvenile dermatomyositis• Dystrophic calcinosis cutis
Case Report
Severe Dystrophic Calcinosis Cutis: The Need for Early Intervention and Optimal Treatment of Juvenile DermatomyositisPoon F1*, Heyes C1, Scardamaglia L1, Martyres R2, Chatfield S3, and Varigos G1
1Department of Dermatology, The Royal Melbourne Hospital, Australia2Department of General Medicine, The Royal Melbourne Hospital, Australia3Department of Rheumatology, The Royal Melbourne Hospital, Australia
Abstract
Dystrophic calcinosis cutis (DCC) is a severe, debilitating sequelae of juvenile dermato-myositis (DM) that can have significant morbidity. Treatment of extensive DCC can be very challenging with inconsistent results and limited evidence to guide best therapy. We present a case of a particularly severe form of DCC presenting in an adult, which highlights the necessity of early optimisation of juvenile DM treatment to help prevent this occurring.
ABBREVIATIONS JDM: Juvenile Dermatomyositis; DCC: Dystrophic Calcinosis
Cutis
INTRODUCTIONDystrophic calcinosis cutis (DCC) is a severe debilitating
entity secondary to juvenile dermatomyositis (DM) that can have significant morbidity later in life. DCC is characterised by the abnormal deposition of calcium salts within skin, subcutaneous tissue, muscles, and tendons in the setting of normal serum calcium and phosphate levels. Juvenile DM and DCC progressing into adulthood is an uncommon occurrence. We present a rare case of a patient with juvenille DM who presents with DCC after a prolonged period of being lost to follow-up.
CASE PRESENTATIONA 49-year-old Caucasian woman was referred to a tertiary
centre for assessment of painful large nodules located over her bilateral posterior thighs, elbows, flexor arms and posterior torso that had been gradually worsening over 10-years with initial nodules noticed in her twenties. This was associated with joint contractures that limited her physical mobility resulting in deconditioning and psychological stress including anxiety and depression. The patient had been diagnosed with juvenile DM aged 12 whilst living in regional Australia. She had been treated with oral prednisolone initiated by her general practitioner and subsequently was lost to follow-up without any regular rheumatology care. In her teenage years, she began to develop
calcific deposits that caused contractures of her bilateral achilles tendons which required tendon lengthening. Her comorbidities include hypertension and hyperandrogenism secondary to ovarian enlargement for which she underwent a bilateral salpingo-oophrectomy.
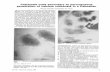
Clinical examination revealed multiple irregular protuberant painful masses in the bilateral posterior thighs and torso (Figure 1) that cause the patient significant discomfort and limited her range of movement including the ability to sit down for prolonged periods. She displayed generalised muscle atrophy of the upper and lower limbs however had 4+/5 power symmetrical bilaterally. Violaceous papules (Gottron’s papules) were identified over dorsal hands overlying the metacarpophalyngeal and proximal interphalyngeal joints and bilateral elbows. She had a fixed flexion deformity (less than 10 degrees) of her right
Figure 1 Protuberant and indurated masses in the bilateral posterior thighs and torso.

Poon et al. (2017)Email:
J Dermatolog Clin Res 5(2): 1095 (2017) 2/5
Central
elbow. The patient also displayed concomitant sicca symptoms consisting of dry mouth and dry eyes.
Serology tests revealed a positive Antinuclear Antibody (ANA) with a Homogenous Titre of 640. Extractable Nuclear Antigen (ENA) testing were negative, specifically negative Anti-Ro and Anti-La antigens. Creatine kinase (CK) was 100 [Reference Interval (RI): 30-170 IU/L], CRP 17 [RI: <5 mg/L], ESR 19 [RI: 2-12 mm in 1 hr] and a mild leucocytosis was present (WCC 11.3 x 109 /L) [RI: 140-400 x 109 /L]. Her corrected calcium represented the upper range of normal at 2.57 mmol/L [RI: 2.1 – 2.6 mmol/L] with a normal serum phosphate level of 0.96 mmol/L [RI: 0.75-1.5 mmol/L]. Parathyroid hormone (PTH) testing revealed a normal level of 6.7 pmo/L [RI: 1.7-7.5 pmol/L].
A bone mineral density (BMD) scan revealed a femoral Z score of - 0.3 and a lumbar spine T = + 1.1 which is in normal range. Computed tomography of her thighs revealed predominantly intramuscular calcification with large intramuscular peripherally calcified soft tissue masses in the posterior thigh compartment bilaterally (Figure 2). The largest lesion in the hamstrings measuring 85 x 75 x 290mm on the left and 43 x 46 x 250mm on the right.
The patient was diagnosed with extensive dystrophic calcinosis cutis (DCC) and concomitant inflammatory joint and cutaneous symptoms secondary to her inadequately treated juvenile DM that has likely progressed into adulthood.
Management required a multidisciplinary approach that was co-ordinated by dermatology with input from rheumatology, plastics and reconstructive surgery, pain medicine and endocrinology. Diltiazem extended-release 360mg daily, prednisolone 7.5mg daily and probenecid 500mg twice-daily were commenced initially. The patient declined plastics
and reconstructive surgical intervention given the extensive undertaking required to excise the intramuscular calcification. Pain was managed with pregabalin (75mg twice-daily) under the care of a pain specialist and she continues to have regular follow-up with her psychiatrist for management of depression and anxiety. Furthermore, use of photo-protective measures and non-pharmacologic interventions involving physical therapy to assist with stretches, prevention of worsening contractures and building muscle strength were encouraged.
On review 3 months later, the patient’s inflammatory joint and cutaneous rash had resolved. Despite this, she did not notice any meaningful improvement in her pain or reduction in the dystrophic calcification. Bisphosphonates were considered however given the limited evidence demonstrating its efficacy and the patient’s normal BMD, its use was deemed currently premature.
The patient’s DCC continues to persist causing significant debilitating effects that have a negative impact on her quality of life. DCC is a well-known sequel of juvenile dermatomyositis and can be a very challenging disease entity to treat especially when extensive. This case highlights extensive complicating DCC manifesting into adulthood after a chronic period of inadequately treated JDM. It raises the valid point questioning whether earlier adequate treatment of her JDM, would have prevented such severe DCC that has now manifested into her adult life.
DISCUSSION Calcinosis cutis can be sub-classified into four categories:
dystrophic (due to localised tissue damage), metastatic (due to abnormal calcium or phosphate metabolism), iatrogenic (eg. parenteral calcium or phosphate administration) or idiopathic. Calcinosis cutis affects up to 40% of patients with juvenile DM [1]. Juvenile DM and DCC progressing into adulthood are uncommon occurrences, which at times can also have unpredictable clinical patterns.
The presence of myositis specific auto-antibodies can provide strong evidence for the diagnosis of DM. Myositis specific antibodies may also aid the possibility of predicting clinical patterns. The presence of anti-p155 myositis associated antibody (MAA) can also predict worse cutaneous involvement and p140 can predict calcinosis, severity of disease course and persistent disease activity [2,3]. Although these antibodies were not measured in our patient’s case, perhaps there may have been a role in assisting with predicting clinical disease course earlier. Despite this, muscle enzymes are not always elevated at the diagnosis of juvenile DM and are poorly responsive to changing disease activity. This is also complicated also by the incompletely understood pathogenesis of DCC.
One postulation involves tissue necrosis caused by tissue injury that may result in alkaline phosphatase release by damaged lysosomes [4]. This subsequently may cause inhibition of calcium phosphate crystallisation and elevated intracellular calcium levels and deposition [5]. Calcium homeostasis may also be disturbed by ectopic sources and this would be important to exclude as part of the work up. Lobo et al., (2008) have suggested the manifestation of DCC may be a localised process rather an imbalance of calcium homeostasis [6]. This could be possible
Figure 2 Coronal Computed Tomography view illustrating intramuscular calcification with large peripherally calcified soft tissue masses in the posterior thigh compartments bilaterally.

Poon et al. (2017)Email:
J Dermatolog Clin Res 5(2): 1095 (2017) 3/5
Central
given our patient had normal phosphate levels and a corrected calcium level within the upper range of normal.
Another theory relates to possible activation of macrophages which are typically found, along with pro-inflammatory cytokines, in calcium rich fluid [7]. This may explain the use of intravenous immunoglobulin (IVIG) where effector macrophages can be suppressed [7] that may contribute to its anti-inflammatory properties. Despite this, the mechanism by which IVIG eradicates DCC remains uncertain and conflicting efficacy data exists.
Complications of DCC include infection, ulceration, pain, disfigurement and restricted range of movement in limbs. Overall, it can also have a significant negative impact on quality of life, as our patient’s experience demonstrates.
Treatment of DCC is currently based on limited evidence with inconsistent therapeutic success. Evidence based therapeutic options for DCC are summarised in Table (1). Our priorities in managing this patient included controlling any residual DM activity to minimise any progression of calcification, targeted
Table 1: Summary of management options for dystrophiccal cinosis cutis and levels of evidence .
TREATMENT LEVEL OF EVIDENCE
Oral TherapiesColchicine• MOA: Inhibits inflammation by concentrating
in leukocytes and interfering with lysosomal degranulation
• Dose: 1mg daily
Level IIIcRetrospective study evaluating 78 patients with calcinosis cutis and autoimmune
connective tissue diseaseMixed results• 1/8 patients given less than 1.2g/day achieved complete resolution• 2/18 patients achieved partial response(Balin et al, 2012) [9]
Minocycline• MOA: Antibiotic with anti-inflammatory
properties• Dose: 200mg daily
Level IIIcRetrospective study evaluating 78 patients with calcinosis cutis and autoimmune
connective tissue diseaseMixed results• 1/6 patients partial response at less than 200mg/day• 2/16 patients failed to respond• 3/6 patients had unknown response to treatment(Balin et al, 2012) [9]
Diltiazem• MOA: Inhibits intracellular calcium influx
within macrophages• Dose: 2-4mg/kg/day
Level IIIcRetrospective study evaluating 78 patients with calcinosis cutis and autoimmune
connective tissue diseaseMixed results• 9/17 patients showed partial response at less than 180mg daily• 3/17 patients had insufficient documentation of outcome(Balin et al, 2012) [9]
Probenecid• MOA: Increases renal phosphate clearance&
anti-inflammatory actions• Dose: 500mg twice-daily
Level IVCase report• 19-year-old male showed reduced pain and improvement in calcification at 3-5 months.(Eddy et al, 1997) [10]
Warfarin• MOA: Vitamin K has a role in calcium binding
within bones and also calcium deposition within soft tissues
• Dose: 1mg daily
Level IVCase report• Reduction in the amount of subcutaneous calcium and mobility improvement in a 27-year-old
male with DM and calcinosisunversalis.(Berger et al, 1998) [11]
Intravenous TherapiesBisphosphonates• MOA: Inhibits macrophage pro-inflammatory
cytokine production (IL 1, IL 6 and TNF alpha)• Dose: variabledepending on type
bisphosphonate
Level IVCase series3 patients with juvenile DM received pamidronate at 1mg/kg/day for 3 consecutive days, repeated
every month. Complete resolution of calcinosis in 1 case and satisfactory response in others.(Marco et al, 2010) [12]
treatment of the DCC and alleviating the sequelae of pain, functional limitations and any possible associated psychological distress.
In consultation with Rheumatology, it was felt our patient’s normal CK level and the absence of cutaneous or myopathic symptoms of DM did not warrant aggressive immune suppressive therapy. Following case reports [8] and a retrospective study [9] demonstrating the successful use of diltiazem for calcinosis cutis in patients with autoimmune connective tissue disease, this was commenced. Probenecid 500mg twice-daily was added based on the anti-inflammatory properties, although evidence on its use is controversial [10].
Bisphosphonates have value in its ability to reduce calcium turnover resulting in reduced calcium deposition within soft tissue with success reported in children [7]. In consultation with Endocrinology, bisphosphonates were considered not appropriate at this point in time given the patient’s normal BMD, although may be considered in the near future.

Poon et al. (2017)Email:
J Dermatolog Clin Res 5(2): 1095 (2017) 4/5
Central
The literature describes a wide array of different treatment options for DCC, however data on efficacy is limited and often conflicting therefore making consensus on the most appropriate treatment regimen difficult. Furthermore, the required duration of therapy to address DCC is also uncertain, though a case report demonstrating marked improvement in calcinosis cutis after 2.5-years of diltiazem use has been reported [8]. Surgical resection is also usually considered last line due to the chance of surgical trauma possibly inducing further calcification, not to mention the complexity of excising calcification that crosses multiple tissue planes and likely poor healing post-operatively.
This case highlights the severe effects of DCC that has progressed into adulthood following inadequately treated JDM. It supports the notion that early and aggressive therapy may prevent or stabilise organ damage and disease sequaele such as calcinosis [18], a condition that is associated with significant morbidity due to pain and infection. Our patient is currently able to ambulate independently however have significant difficulties with activities of daily living. The patient remains on diltiazem and probenecid. Regular photo-protective measures are employed to minimise the chance of myositis and disease activation. She continues to have regular follow-up and monitoring with the multidisciplinary team.
Methylprednisolone + Methotrexate• MOA:Methotrexate: Antiproliferative and
immunosuppressive effectsMethylprednisolone: intermediate acting anti-
inflammatory agent• Dose:Methotrexate: 7-15mg weekly (titrated)Methylprednisolone: 30mg/kg/day (max dose
1g)
Level IVCase seriesMixed results• 6/12 paediatric patients with juvenile DM treated within 6 weeks of diagnosis of juvenile DM,
did not develop calcinosis cutis• 2/6 paediatric patients with juvenile DM treated over 5 months of diagnosis developed
calcinosis cutis.(Al-Mayouf et al, 2000) [13]
Immunoglobulin (IVIG)• MOA: Multiple postulated theories including
suppression of activated macrophages and anti-inflammatory properties
• Dose: 2g/kg in divided doses once per month x 3 months
Level IVCase reportSuccessful treatment:• 56-year-old female with CREST syndrome and associated DCC affecting fingers received IVIG
2g/day over 4 days once a month. After 3 cycles, patient was symptom free.(Schanz et al, 2008) [14]Failed treatment:• 2 patients with DM experiencing extensive and progressive DCC despite IVIG treatment at 2g/
kg/month in divided doses for nearly 5 years.(Kalajian et al, 2009) [15]
BiologicsInfliximab• MOA: Anti TNF- α• Indicated for patients with juvenile DM
refractory to previously proposed treatments• Dose: 3mg/kg
Level IVCase series• 5 patients with refractory juvenile DM with calcinosis showed regression in calcinosisat
follow-up intervals of 8 and 30 months.(Riley et al, 2008) [16]
Surgical Intervention
Surgical Excision/ Debulking• Primary goal: Adjunctive treatment of
symptomatic areas to help relieve pain and improve functional limitations
Level IVRetrospective analysis of 9 patients with scleroderma and digital calcinosis treated with
high speed digital burr debulking
Mixed results• Patients with discrete areas of calcinosis cutis including those with 1-2 affected digits did
much better than patients with diffuse disease and multiple affected digits.(Lapner and Goetz, 2014) [17]
MOA: Mechanism of Action
The pathogenesis of DCC is poorly understood and a more thorough understanding of the underlying disease mechanism is critical in finding new novel treatments. Higher order evidence guiding treatment regimens for DCC is limited and currently there is uncertainty about which management option(s) is best. Multimodal therapies should be considered. Early initiation of optimal treatment for juvenile DM is likely necessary to help prevent the sequelae of extensive DCC. It is imperative patients with adult and juvenile DM are followed up on a regular basis by Rheumatology and Dermatology to be able to identify and treat inflammatory symptoms of DM and possible DCC early.
REFERENCES1. Koler R, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;
64: 1565-1572.
2. Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore). 2013; 92: 223-224.
3. Tansley SL, McHugh. Myositis specific and associated autoantibodies in the diagnosis and management of juvenile and adult idiopathic inflammatory myopathies. Curr Rheum Rep. 2014; 16: 464.
4. Eich D, Scharffetter-Kochanek K, Weihrauch J, Krieg T, Hunzelmann N. Calcinosis of the cutis and subcutis: an unusual nonimmunologic adverse reaction to subcutaneous injections of low molecular weight

Poon et al. (2017)Email:
J Dermatolog Clin Res 5(2): 1095 (2017) 5/5
Central
Poon F, Heyes C, Scardamaglia L, Martyres R, Chatfield S, et al. (2017) Severe Dystrophic Calcinosis Cutis: The Need for Early Intervention and Optimal Treat-ment of Juvenile Dermatomyositis. J Dermatolog Clin Res 5(2): 1095.
Cite this article
calicium containing heparins. J Am Academy Dermatol. 2004; 50: 210-214.
5. Bhatia S, Silverberg NB, Don PC, Weinberg JM. Extensive calcinosis cutis in association with systemic lupus erythematosus. Acta DermVenereol. 2001; 81: 446-447.
6. Lobo IM, Machado S, Teixeira, Selores M. Calcinosis cutis: A rare feature of adult dermatomyositis. Dermatology Online Journal. 2008; 14: 10.
7. Mukamel M, Horev G, Mimouni M. New insight into calcinosis of juvenile dermatomyositis: A study of composition and treatment. J Peadiatrics. 2001; 138: 763-766.
8. Vinen CS, Patel S, Bruckner FE. Regression of calcinosis associated with adult dermatomyositis following diltiazem therapy. Rheumatology. 2000; 39: 333-334.
9. Balin SJ, Wetter DA, Anderson LK, Davis MD. Calcinosis cutis occurring in association with autoimmune connective tissue disease: The Mayo experience with 78 patients, 1996-2000. Arch Dermatol. 2012; 148: 455-462.
10. Eddy MC, Leelawattana R, McAllister WH, Whyte MP. Clacinosisunverisalis complicating juvenile dermatomyositis: resolution during probenecid therapy. J Clin endocrinol Metabol. 1997; 82: 3536-3542.
11. Berger RG, Featherstone GL, Raasch RH, McCartney WH, Hadler NM.
Treatment of calcinosisuniversalis with low dose warfarin. Am J Med. 1998; 83: 72-76.
12. Marco P, Calvo P, Lopez MB. Effectiveness of the treatment with intravenous pamidronate in calcinosis in juvenile dermatomyositis. Clin Exp Rheumatol. 2010; 28: 135-140.
13. Al-Mayouf S, Al-Mazyed A, Bahabri S. Efficacy of early treatment of severe juvenile dermatomyositis with intravenous methylprednisolone and methotrexate. Clin Rheumatol. 2000; 19; 138-141.
14. Schanz S, Ulmer A, Fierlbeck G. Response of dystrophic calcification to intravenous immunoglobulin. Arch Dermatol. 2008; 144: 585.
15. Kaliajan AH, Perryman JH and Callen JP. Intravenous immunoglobulin therapy for dystrophic calcinosiscutis: Unreliable in our hands. JAMA Dermatol. 2009; 145: 334.
16. Riley P, McCann LJ, Maillard SM, Woo P, Murray KJ, Pilkington CA. Effectiveness of infliximab in the treatment of refractory juvenile dermatomyositis with calcinosis. Rheumatology. 2008; 47: 877-880.
17. Lapner MA, Goetz TJ. High speed burr debulking of digital calcinosis cutis in scleroderma patients. Am J hand surg. 2014; 39: 503.
18. Enders FB, Bader-Meunier B, Baildam E, Tamas Constantin, Pavla Dolezalova, Brian M Feldman, et al. Consensus based reocmmendations for management of juvenile dermatomyositis. Ann Rheum Dis. 2016; 76: 329-340.
Related Documents



![Case Report Metastatic Calcinosis Cutis: A Case in a Child ...downloads.hindawi.com/journals/crihem/2015/384821.pdf · phoblastic and myeloid acute leukemia []. Metastatic cal-cinosis](https://static.cupdf.com/doc/110x72/5f903fad69bb713af81a8e96/case-report-metastatic-calcinosis-cutis-a-case-in-a-child-phoblastic-and-myeloid.jpg)