The Pauling Principle Linus Pauling Pauling discovered that in many cases the type of bonding — whether ionic or covalent (formed by a sharing of electrons between bonded atoms) — could be determined from a substance’s electronegativity value. He established an electronegativity scale of the elements for use in bonds of an intermediate character (having both ionic and covalent bonding); the smaller the difference in electronegativity between two atoms, the more the bond between them approaches a purely covalent bond. http://lpi.oregonstate.edu/ about/linus-pauling-biography e-mail: [email protected] BSc (Hons) Chemistry web: plymouth.ac.uk/courses/undergraduate/bsc-chemistry Chemistry with Foundation year: plymouth.ac.uk/courses/undergraduate/bsc-chemistry-with-foundation-year 0.7 0.9 The Electronegativity Game Types of bond in a compound can be determined using The Pauling Principle, see right, which uses the electronegativity scale, shown below. The difference between two atoms’ electronegativity values, that make up the bond, tells you the TYPE of bond that is present. Electronegativity is the ability of an atom to draw electron density towards itself in a bond. Difference in Electronegativity 0 1.0 0.5 1.5 2.0 + C—C Pure Covalent Weakly Polar Covalent Polar Covalent C—H C—N C—O O—H C—F Na + Cl – + - + - Strongly Polar Covalent Mainly Ionic Character + - - + C C Cl – Na + + - C F C O H 2.1 Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.7 Fr 0.7 Be 1.5 Mg 1.2 Ca 1.0 Sr 1.0 Ba 0.9 Ra 0.9 B 2.0 Si 1.8 As 2.0 Te 2.1 Ge 1.8 Sb 1.9 Po 2.0 O 3.5 N 3.0 C 2.5 S 2.5 P 2.1 Se 2.4 F 4.0 Cl 3.0 Br 2.8 I 2.5 At 2.2 Al 1.5 Ga 1.6 In 1.7 Tl 1.8 Sc 1.3 Ti 1.5 V 1.6 Cr 1.6 Mn 1.5 Fe 1.8 Co 1.8 Ni 1.8 Cu 1.9 Zn 1.6 Y 1.2 Zr 1.4 Nb 1.6 Mo 1.8 Tc 1.9 Ru 2.2 Rh 2.2 Pd 2.2 Ag 1.9 Cd 1.7 La - 1.0 - 1.2 Hf 1.3 Ta 1.5 W 1.7 Re 1.9 Os 2.2 Ir 2.2 Pt 2.2 Au 2.4 Hg 1.9 Sn 1.8 Pb 1.9 Bi 1.9

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

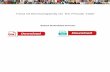
The Pauling Principle Linus Pauling
Pauling discovered that in many cases the type of bonding — whether ionic or covalent (formed by a sharing of electrons between bonded atoms) — could be determined from a substance’s electronegativity value. He established an electronegativity scale of the elements for use in bonds of an intermediate character (having both ionic and covalent bonding); the smaller the difference in electronegativity between two atoms, the more the bond between them approaches a purely covalent bond.
http://lpi.oregonstate.edu/about/linus-pauling-biography
e-mail: [email protected] (Hons) Chemistry web: plymouth.ac.uk/courses/undergraduate/bsc-chemistryChemistry with Foundation year: plymouth.ac.uk/courses/undergraduate/bsc-chemistry-with-foundation-year
0.7 0.9
The Electronegativity GameTypes of bond in a compound can be determined using The Pauling Principle, see right, which uses the electronegativity scale, shown below. The difference between two atoms’ electronegativity values, that make up the bond, tells you the TYPE of bond that is present. Electronegativity is the ability of an atom to draw electron density towards itself in a bond.
Difference in Electronegativity0 1.00.5 1.5 2.0 +
C—C
Pure Covalent Weakly Polar Covalent
Polar Covalent
C—H C—N C—O O—H C—F Na+ Cl–+ - + -
Strongly Polar Covalent Mainly Ionic Character
+ -- +
C C Cl–Na++ -
C FC O
H2.1
Li1.0
Na0.9
K0.8
Rb0.8
Cs0.7
Fr0.7
Be1.5
Mg1.2
Ca1.0
Sr1.0
Ba0.9
Ra0.9
B2.0
Si1.8
As2.0
Te2.1
Ge1.8
Sb1.9
Po2.0
O3.5
N3.0
C2.5
S2.5
P2.1
Se2.4
F4.0
Cl3.0
Br2.8
I2.5
At2.2
Al1.5
Ga1.6
In1.7
Tl1.8
Sc1.3
Ti1.5
V1.6
Cr1.6
Mn1.5
Fe1.8
Co1.8
Ni1.8
Cu1.9
Zn1.6
Y1.2
Zr1.4
Nb1.6
Mo1.8
Tc1.9
Ru2.2
Rh2.2
Pd2.2
Ag1.9
Cd1.7
La-
1.0 - 1.2Hf1.3
Ta1.5
W1.7
Re1.9
Os2.2
Ir2.2
Pt2.2
Au2.4
Hg1.9
Sn1.8
Pb1.9
Bi1.9
Related Documents