CELL WALL SYNTHESIS CELL WALL SYNTHESIS INHIBITORS INHIBITORS A N.VIJAY KUMAR A N.VIJAY KUMAR June 8, 2022 June 8, 2022

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

CELL WALL SYNTHESIS CELL WALL SYNTHESIS INHIBITORSINHIBITORS
A N.VIJAY KUMARA N.VIJAY KUMARApril 11, 2023April 11, 2023

Site and Mechanism of action Site and Mechanism of action of Antibioticsof Antibiotics

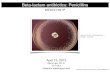
PenicilliumPenicillium
The name Penicillium comes from Penicillus = brush, and this is based on the brush-like appearance of the fruiting structures

IntroductionIntroduction The penicillins constitute one of the most important groups The penicillins constitute one of the most important groups
of antibiotics. of antibiotics.
Although numerous other antimicrobial agents have been Although numerous other antimicrobial agents have been
produced since the first penicillin became available, these produced since the first penicillin became available, these
still are widely used, major antibiotics, and new derivatives still are widely used, major antibiotics, and new derivatives
of the basic penicillin nucleus still are being produced.of the basic penicillin nucleus still are being produced.
Many of these have unique advantages, such that members Many of these have unique advantages, such that members
of this group of antibiotics are presently the drugs of choice of this group of antibiotics are presently the drugs of choice
for a large number of infectious diseases.for a large number of infectious diseases.

1928 - Alexander Fleming 1928 - Alexander Fleming
Bread mold (Bread mold (Penicillium notatumPenicillium notatum) growing on petri ) growing on petri
dishdish
1939 - Florey, Chain, and Associates1939 - Florey, Chain, and Associates
Began work on isolating and synthesizing large Began work on isolating and synthesizing large
amounts of Penicillin.amounts of Penicillin.
1941 – introduced in antibacterial therapy1941 – introduced in antibacterial therapy
HistoryHistory

the fused beta-lactam structure (shown in the blue and the fused beta-lactam structure (shown in the blue and red rings, red rings, a free carboxyl acid group (shown in red bottom right), a free carboxyl acid group (shown in red bottom right), one or more substituted amino acid side chains (shown one or more substituted amino acid side chains (shown in black). in black). The lactam structure can also be viewed as the The lactam structure can also be viewed as the covalent bonding of pieces of two amino acids - covalent bonding of pieces of two amino acids - cysteine (blue) and valine (red). cysteine (blue) and valine (red).
StructureStructure

thiazolidine ring (A) connected to a b-lactam ring (B), to which is attached a side chain (R).

The penicillin nucleus itself is the chief structural
requirement for biological activity
Metabolic transformation or chemical alteration of
this portion of the molecule causes loss of all
significant antibacterial activity

Clinically useful families of beta-Clinically useful families of beta-
lactam compounds include the lactam compounds include the
PenicillinsPenicillins
CephalosporinsCephalosporins
MonobactamsMonobactams
CarbapenemsCarbapenems

Mechanisms of ActionMechanisms of Action
All penicillin derivatives produce their All penicillin derivatives produce their
bactericidal effects by inhibition of bacterial cell bactericidal effects by inhibition of bacterial cell
wall synthesis. wall synthesis.
Specifically, the cross linking of peptides on the Specifically, the cross linking of peptides on the
polysaccharide chain is prevented. polysaccharide chain is prevented.
If cell walls are improperly made cell walls allow If cell walls are improperly made cell walls allow
water to flow into the cell causing it to burst. water to flow into the cell causing it to burst.

Penicillin Penicillin
Bind (PBP) on the cell wall of susceptible bacteriaBind (PBP) on the cell wall of susceptible bacteria
Inhibits transpeptidationInhibits transpeptidation
Prevents peptidoglycan synthesis Prevents peptidoglycan synthesis
Cell wall deficient forms spheroplasts & Cell wall deficient forms spheroplasts & filamentous forms filamentous forms
Autolysis Autolysis
Cell death (bactericidal action) Cell death (bactericidal action)

MOA: Cell Wall productionMOA: Cell Wall production
The cell walls of bacteria are essential for their normal The cell walls of bacteria are essential for their normal
growth and development. growth and development.
Peptidoglycan is a heteropolymeric component of the cell Peptidoglycan is a heteropolymeric component of the cell
wall that provides rigid mechanical stability by virtue of its wall that provides rigid mechanical stability by virtue of its
highly cross-linked latticework structure highly cross-linked latticework structure
The peptidoglycan is composed of glycan chains, which are The peptidoglycan is composed of glycan chains, which are
linear strands of two alternating amino sugars (N-linear strands of two alternating amino sugars (N-
acetylglucosamine and N-acetylmuramic acid) that are acetylglucosamine and N-acetylmuramic acid) that are
cross-linked by peptide chains. (NAG-NAM). cross-linked by peptide chains. (NAG-NAM).

Binding to PBPs results in:Binding to PBPs results in:
Inhibition of transpeptidase: Inhibition of transpeptidase: transpeptidase transpeptidase
catalyzes the cross-linking of the pentaglycine bridge catalyzes the cross-linking of the pentaglycine bridge
with the fourth residue (D-Ala) of the pentapeptide. The with the fourth residue (D-Ala) of the pentapeptide. The
fifth residue (also D-Ala) is released during this reaction. fifth residue (also D-Ala) is released during this reaction.
Spheroblasts are formed.Spheroblasts are formed.
Structural irregularities: Structural irregularities: binding to PBPs may result binding to PBPs may result
in abnormal elongation, abnormal shape, cell wall in abnormal elongation, abnormal shape, cell wall
defects.defects.
Mechanisms of Action Mechanisms of Action Cont…Cont…


Comparison of the structure and composition of gram-positive and gram-negative cell walls.

CLASSIFICATION CLASSIFICATION NARROW SPECTRUM PENICILLINSNARROW SPECTRUM PENICILLINS
ββ-lactamase sensitive(NATURAL PENICILLINS)-lactamase sensitive(NATURAL PENICILLINS)
Acid resistantAcid resistant
- Penicillin V (oral)- Penicillin V (oral)
Acid labileAcid labile- Penicillin-G (benzyl - Penicillin-G (benzyl
penicillin)(I.M,IV)penicillin)(I.M,IV)- Procaine penicillin-- Procaine penicillin-
G(I.M,depot inj)G(I.M,depot inj)- Benzathine penicillin-G(I.M, - Benzathine penicillin-G(I.M,
depot inj)depot inj)

ββ-lactamase resistant(ANTISTEPHYLOCOCCAL -lactamase resistant(ANTISTEPHYLOCOCCAL
PENICILLINS)PENICILLINS)
Acid resistantAcid resistant
- Cloxacillin - Cloxacillin
- Dicloxacillin - Dicloxacillin
- flucloxacillin- flucloxacillin
Acid labileAcid labile
- Methicillin (I.M,I.V)- Methicillin (I.M,I.V) - Nafcillin (I.M,I.V) - Nafcillin (I.M,I.V)

EXTENDED SPECTRUM PENICILLINSEXTENDED SPECTRUM PENICILLINS
Acid resistantAcid resistant
• AminopenicillinsAminopenicillins: Ampicillin, Amoxicillin, Bacampicillin, : Ampicillin, Amoxicillin, Bacampicillin,
Talampicillin Talampicillin
Acid labileAcid labile ((ANTIPSEUDOMONAL PENICILLINS)ANTIPSEUDOMONAL PENICILLINS)
• CarboxypenicillinsCarboxypenicillins: Carbenicillin, Ticarcillin: Carbenicillin, Ticarcillin
• UreidopenicillinsUreidopenicillins: Piperacillin, Mezlocillin, Azlocillin: Piperacillin, Mezlocillin, Azlocillin
BETA LACTAMASE INHIBITORSBETA LACTAMASE INHIBITORS
• Sulbactam, Tazobactam, Clavulanic acidSulbactam, Tazobactam, Clavulanic acid

Natural penicillinsNatural penicillins
Listed as antistaphylococcalListed as antistaphylococcal
Obtained from fermentations of the mold Obtained from fermentations of the mold
Penicillium chrysogenumPenicillium chrysogenum
Penicillin G (benzylpenicillin) and Penicillin Penicillin G (benzylpenicillin) and Penicillin
VV

Antimicrobial spectrum: Antimicrobial spectrum: Penicillin GPenicillin G

PharmacokineticsPharmacokineticsOral administration of Penicillin G:Oral administration of Penicillin G:
Acid labileAcid labile
About one-third of an orally administered dose of PenG is About one-third of an orally administered dose of PenG is
absorbed from the intestinal tract under favorable conditions. absorbed from the intestinal tract under favorable conditions.
Gastric juice at pH 2 rapidly destroys the antibiotic.Gastric juice at pH 2 rapidly destroys the antibiotic.
Parenteral Administration of Penicillin G:Parenteral Administration of Penicillin G:
From I.M site absorption is rapid and completeFrom I.M site absorption is rapid and complete
Peak plasma levels attained in 30minPeak plasma levels attained in 30min

PenG is distributed widely throughout the body, but the PenG is distributed widely throughout the body, but the
concentrations in various fluids and tissues differ widely. concentrations in various fluids and tissues differ widely.
Approx. 60% of the PenG in plasma is reversibly bound to albumin. Approx. 60% of the PenG in plasma is reversibly bound to albumin.
Significant amounts appear in liver, bile, kidney, semen, joint fluid, Significant amounts appear in liver, bile, kidney, semen, joint fluid,
lymph, and intestinelymph, and intestine
CSF:CSF: Penicillin does not readily enter the CSF when the meninges Penicillin does not readily enter the CSF when the meninges
are normal. However, when the meninges are acutely inflamed, are normal. However, when the meninges are acutely inflamed,
penicillin penetrates into the CSF more easily.penicillin penetrates into the CSF more easily.
Little metabolized because rapid excretionLittle metabolized because rapid excretion
Pharmacokinetics Pharmacokinetics Cont…Cont…

The half-time for elimination is about 30 minutes in The half-time for elimination is about 30 minutes in
normal adults (upto 10 hours in renal failure) . normal adults (upto 10 hours in renal failure) .
Approx. 10% of the drug is eliminated by glomerular Approx. 10% of the drug is eliminated by glomerular
filtration and filtration and 90% by tubular secretion90% by tubular secretion..
While While probenecidprobenecid markedly markedly ↓↓ss the tubular secretion ss the tubular secretion
of the penicillins, this is not the only factor responsible of the penicillins, this is not the only factor responsible
for the elevated plasma concentrations of the antibiotic for the elevated plasma concentrations of the antibiotic
that follow its administration. that follow its administration.
Pharmacokinetics Pharmacokinetics Cont…Cont…

Preparations and dosePreparations and dose
Benzylpenicillin (sodium and Benzylpenicillin (sodium and potassium salts)potassium salts)
Repository preparations:Repository preparations: Insoluble salts, only I.M injection never I.V injInsoluble salts, only I.M injection never I.V inj
Procaine penicillinProcaine penicillin Benzathine penicillinBenzathine penicillin

Unitage of Penicillin The IU of penicillin is the specific penicillin activity The IU of penicillin is the specific penicillin activity
contained in 0.6 mg of the crystalline sodium salt of contained in 0.6 mg of the crystalline sodium salt of
penicillin G.penicillin G.
1mg of pure penicillin G sodium thus equals 1667 units; 1mg of pure penicillin G sodium thus equals 1667 units;
1mg of pure penicillin G potassium represents 1595 units. 1mg of pure penicillin G potassium represents 1595 units.
The dosage and the antibacterial potency of the The dosage and the antibacterial potency of the
semisynthetic penicillins are expressed in terms of semisynthetic penicillins are expressed in terms of
weight.weight.

Therapeutic Uses Pneumococcal InfectionsPneumococcal Infections
Pneumococcal MeningitisPneumococcal Meningitis Pneumococcal PneumoniaPneumococcal Pneumonia
Streptococcal InfectionsStreptococcal Infections Streptococcal Pharyngitis (including Scarlet Fever)Streptococcal Pharyngitis (including Scarlet Fever) Streptococcal Pneumonia, Arthritis, Meningitis, and EndocarditisStreptococcal Pneumonia, Arthritis, Meningitis, and Endocarditis
Staphylococcal InfectionsStaphylococcal Infections Meningococcal InfectionsMeningococcal Infections Gonococcal InfectionsGonococcal Infections Syphilis, Actinomycosis, Diphtheria, AnthraxSyphilis, Actinomycosis, Diphtheria, Anthrax Clostridial InfectionsClostridial Infections Fusospirochetal InfectionsFusospirochetal Infections Rat-Bite FeverRat-Bite Fever Listeria InfectionsListeria Infections Lyme DiseaseLyme Disease ErysipeloidErysipeloid Surgical Procedures in Patients with Valvular Heart DiseaseSurgical Procedures in Patients with Valvular Heart Disease Tetanus and gas gangreneTetanus and gas gangrene Prophylactic uses:Prophylactic uses:
Rheumatic fever, bacterial endocarditis and agranulocytosis ptsRheumatic fever, bacterial endocarditis and agranulocytosis pts

Mechanisms of Bacterial Resistance to Penicillins
Resistance to penicillins and other beta Resistance to penicillins and other beta lactams is due to one of four general lactams is due to one of four general mechanisms:mechanisms: Inactivation of the antibiotic by beta Inactivation of the antibiotic by beta
lactamaselactamase Modification of target PBPsModification of target PBPs Impaired penetration of drug to target Impaired penetration of drug to target
PBPsPBPs The presence of an efflux pump.The presence of an efflux pump.

Other resistance Other resistance mechanismsmechanisms
A reductionA reduction in the permeability of the in the permeability of the outer membrane.outer membrane.
Thus there is a decreased ability of Thus there is a decreased ability of the drug to penetrate to the target the drug to penetrate to the target site.site.
The occurrence of modified penicillin The occurrence of modified penicillin binding sites. This mechanism is binding sites. This mechanism is responsible in methicillin resistance responsible in methicillin resistance in in Pneumococci.Pneumococci.

Adverse effectsAdverse effects
Hypersensitivity ReactionsHypersensitivity Reactions
The basis of which is the fact that degradation The basis of which is the fact that degradation
products of penicillin combine with host protein and products of penicillin combine with host protein and
become antigenic.become antigenic.

In approximate order of decreasing frequency, In approximate order of decreasing frequency,
manifestations of allergy to penicillins include manifestations of allergy to penicillins include
maculopapular rash, urticarial rash, fever, maculopapular rash, urticarial rash, fever,
bronchospasm, vasculitis, serum sickness, bronchospasm, vasculitis, serum sickness,
exfoliative dermatitis, Stevens-Johnson syndrome, exfoliative dermatitis, Stevens-Johnson syndrome,
and anaphylaxis and anaphylaxis
The incidence of such reaction is 5-8% of the Pts The incidence of such reaction is 5-8% of the Pts
receiving Penicillins receiving Penicillins
Adverse effects Adverse effects Cont…Cont…

Very high doses of penicillin G can Very high doses of penicillin G can
cause seizures in kidney failure.cause seizures in kidney failure.
Pain at I.M injection sitePain at I.M injection site
Nausea on oral ingestionNausea on oral ingestion
Thromboplebitis of injected veinThromboplebitis of injected vein
Adverse effects Adverse effects Cont…Cont…

Penicillin VPenicillin V
Orally activeOrally active
Used for the treatment of bacteremia and Used for the treatment of bacteremia and
oral infectionsoral infections
Higher minimum bactericidal concentrationHigher minimum bactericidal concentration

Semisynthetic penicillinsSemisynthetic penicillins The major draw backs of benzylpenicillin are:The major draw backs of benzylpenicillin are:
Inactivation by gastric acidInactivation by gastric acid
Short duration of actionShort duration of action
Poor penetration into the CSFPoor penetration into the CSF
Narrow spectrum of activityNarrow spectrum of activity
Susceptibility to PenicillinaseSusceptibility to Penicillinase
Development of resistanceDevelopment of resistance
Possibility of anaphylaxisPossibility of anaphylaxis

Penicillinase-resistant Penicillinase-resistant penicillinspenicillins
(antistaphylococcal penicillins)(antistaphylococcal penicillins)
These congeners have side chains that protect the These congeners have side chains that protect the
beta lactam ring from attack by staphylococcal beta lactam ring from attack by staphylococcal
penicillinasepenicillinase
Indicated in infections caused by penicillinase Indicated in infections caused by penicillinase
producing staphylococci (drugs of choice, except in producing staphylococci (drugs of choice, except in
MRSA)MRSA)
Methicillin, CloxacillinMethicillin, Cloxacillin
Oxacillin, Nafcillin, DicloxacillinOxacillin, Nafcillin, Dicloxacillin

Methicillin:Methicillin:
Acid labile Acid labile
Not used clinically, except to identify Not used clinically, except to identify
resistant strainsresistant strains
MRSA is susceptible to Vancomycin/linezolid MRSA is susceptible to Vancomycin/linezolid
and rarely Ciprofloxacinand rarely Ciprofloxacin
Penicillinase-resistant Penicillinase-resistant penicillinspenicillins
(antistaphylococcal penicillins)(antistaphylococcal penicillins)Cont…Cont…

Cloxacillin:Cloxacillin:
Acid resistantAcid resistant
More active than methicillinMore active than methicillin
Less active against PnG sensitive organisms: should not be used Less active against PnG sensitive organisms: should not be used
as its substituteas its substitute
Incompletely but dependably absorbed (oral route)Incompletely but dependably absorbed (oral route)
>90% protein bound, eliminated primarily by kidney, also partly by >90% protein bound, eliminated primarily by kidney, also partly by
liverliver
Plasma half life is about 1hrPlasma half life is about 1hr
Penicillinase-resistant Penicillinase-resistant penicillinspenicillins
(antistaphylococcal penicillins)(antistaphylococcal penicillins)Cont…Cont…

Extended spectrum Extended spectrum penicillinspenicillins
Active against a variety of gram-negative bacilli as wellActive against a variety of gram-negative bacilli as well
Can be grouped according to their spectrum of activityCan be grouped according to their spectrum of activity
1. 1. Aminopenicillins:Aminopenicillins:
Ampicillins:Ampicillins:
Active against all organisms sensitive to PnG; in Active against all organisms sensitive to PnG; in
addition, many gram-negative bacilliaddition, many gram-negative bacilli

Extended spectrum Extended spectrum penicillins penicillins Cont…Cont…

Pharmacokinetics:Pharmacokinetics:
Acid resistantAcid resistant
Oral absorption is incomplete but adequateOral absorption is incomplete but adequate
Primary excretion is kidney, partly enterohepatic circulation Primary excretion is kidney, partly enterohepatic circulation
occursoccurs
Plasma half life is 1hrPlasma half life is 1hr
Uses:Uses:
UTI, RTI, Meningitis, GonorrhoeaUTI, RTI, Meningitis, Gonorrhoea, typhoid fever, bacillary dysentery, typhoid fever, bacillary dysentery, ,
Cholisystitis, Subacute bacterial endocarditis and SepticemiasCholisystitis, Subacute bacterial endocarditis and Septicemias
Extended spectrum Extended spectrum penicillins penicillins Cont…Cont…

Adverse effects:Adverse effects:
DiarrhoeaDiarrhoea
RashesRashes
HypersensitivityHypersensitivity
Interactions:Interactions:
Hydrocortisone –inactivates ampicillin if mixed in the I.V solutionHydrocortisone –inactivates ampicillin if mixed in the I.V solution
OC –failure of oral contraceptionOC –failure of oral contraception
Probenecid –retards renal excretionProbenecid –retards renal excretion
Extended spectrum Extended spectrum penicillins penicillins Cont…Cont…

Bacampicillin –ester prodrug of ampicillinBacampicillin –ester prodrug of ampicillin
Talampicillin, Pivampicillin and Hetacillin are other Prodrugs Talampicillin, Pivampicillin and Hetacillin are other Prodrugs
of ampicillinof ampicillin
Amoxicillin:Amoxicillin:
Close congener of ampicillin but not a prodrugClose congener of ampicillin but not a prodrug Similar to it in all aspects except:Similar to it in all aspects except:
Better oral absorptionBetter oral absorption Higher and sustained blood levels are producedHigher and sustained blood levels are produced Incidence of diarrhoea is lowerIncidence of diarrhoea is lower Less effective against Less effective against ShigellaShigella and and H. influenzaeH. influenzae
Extended spectrum Extended spectrum penicillins penicillins Cont…Cont…

2. Carboxypenicillins (Carbenicillin, Ticarcillin)2. Carboxypenicillins (Carbenicillin, Ticarcillin)
andand
3. Ureidopenicillins (Piperacillin)3. Ureidopenicillins (Piperacillin)
Extended spectrum Extended spectrum penicillins penicillins Cont…Cont…

These are called antipseudomonal penicillinsThese are called antipseudomonal penicillins
Piperacillin is more potent among thesePiperacillin is more potent among these
Carbenicillin is less effective against Carbenicillin is less effective against Salmonella, E. Coli Salmonella, E. Coli and and
enterobacter but not active against enterobacter but not active against KlebshiellaKlebshiella and gram- and gram-
positive coccipositive cocci
Piperacillin has good activity against Piperacillin has good activity against KlebshiellaKlebshiella, and is , and is
used mainly in neutropenic/ immunocompromised patients used mainly in neutropenic/ immunocompromised patients
having serious gram-negative infections and in burnshaving serious gram-negative infections and in burns
Extended spectrum Extended spectrum penicillins penicillins Cont…Cont…

Beta-lactamase inhibitorsBeta-lactamase inhibitors
Clavulanic acid, Sulbactam Clavulanic acid, Sulbactam
and Tazobactamand Tazobactam
They contain beta-lactam They contain beta-lactam
ring but themselves, do ring but themselves, do
not have significant not have significant
antibacterial activityantibacterial activity

Clavulanic acidClavulanic acid::
Obtained from Streptomyces clavuligerusObtained from Streptomyces clavuligerus
Called a suicide inhibitorCalled a suicide inhibitor
Pharmacokinetics matches amoxicillin with which it is usedPharmacokinetics matches amoxicillin with which it is used
Sulbactam:Sulbactam:
Semisynthetic beta-lactamase inhibitorSemisynthetic beta-lactamase inhibitor
Related chemically as well as in activity to clavulanic acidRelated chemically as well as in activity to clavulanic acid
It is also a progressive inhibitorIt is also a progressive inhibitor
Combined with ampicillinCombined with ampicillin
Beta-lactamase inhibitors Beta-lactamase inhibitors Cont…Cont…

Tazobactam:Tazobactam:
Similar to SulbactamSimilar to Sulbactam
Pharmacokinetics matches with Piperacillin with which it is Pharmacokinetics matches with Piperacillin with which it is
used for used in severe infections like peritonitis, used for used in severe infections like peritonitis,
pelvic/urinary/respiratory infectionspelvic/urinary/respiratory infections
However, the combination is not effective against piperacillin-However, the combination is not effective against piperacillin-
resistant Pseudomonasresistant Pseudomonas
Beta-lactamase inhibitors Beta-lactamase inhibitors Cont…Cont…

They are available only in fixed combinations They are available only in fixed combinations
with specific penicillins:with specific penicillins:
Ampicillin + Sulbactam (1g+0.5g I.V/I.M inj)Ampicillin + Sulbactam (1g+0.5g I.V/I.M inj)
Amoxycillin + Clavulanic acid (250mg+125mg Amoxycillin + Clavulanic acid (250mg+125mg
tab)tab)
Piperacillin + Tazobactam sodium (2g+0.25g Piperacillin + Tazobactam sodium (2g+0.25g
I.V/I.M inj)I.V/I.M inj)

Related Documents