Nestin expression in pancreatic exocrine cell lineages Alexandra Delacour a , Virginie Nepote a , Andreas Trumpp b , Pedro Luis Herrera a, * a Department of Morphology, room 5040, University of Geneva Medical School, 1 rue Michel-Servet, CH-1211 Geneva 4, Switzerland b Swiss Institute for Cancer Research (ISREC), Chemin des Boveresses 155, CH-1066 Epalinges, Switzerland Received 19 September 2003; received in revised form 20 November 2003; accepted 21 November 2003 Abstract Expression of nestin has been suggested to be a characteristic of pancreatic islet stem cells. To determine whether nestin is indeed expressed in such putative cells during embryonic development, or in the adult pancreas after injury, we performed a cell lineage analysis using two independent lines of transgenic mice encoding Cre recombinase under the control of rat nestin cis-regulatory sequences, each crossed with loxP-bearing R26R mice. F1 animals produced the reporter molecule b-galactosidase only upon Cre-mediated recombination, thus solely in cells using (or having used) the transgenic nestin promoter. In early pancreatic primordia, b-galactosidase was observed in mesenchymal and epithelial cells. At later developmental stages or in adults, vast clusters of acinar cells and few ductal cells were labeled, in addition to fibroblasts and vascular cells, but no endocrine cells were tagged by b-galactosidase. This correlated with the transient expression, observed with an anti-nestin antibody, of endogenous nestin in about 5% of epithelial cells during development (whether in cord-forming arrangements or in nascent acini), and in vascular and mesenchymal structures. After partial pancreatectomy, there was a transient increase of the number of anti-nestin-labeled endothelial cells, but again, no endocrine cells bore b-galactosidase. Together, these findings show that nestin is expressed in the pancreatic exocrine cell lineage, and suggest that consistent nestin expression is not a major feature of islet endocrine progenitor cells. q 2003 Elsevier Ireland Ltd. All rights reserved. Keywords: Pancreas; Islet; Development; Transgenic; Mouse; Cre; Cell tracing; Lineage; Nestin; Insulin; Progenitor; Stem cell 1. Introduction The isolation of islet or pancreas stem cells requires the identification of molecular markers characteristic of such lineage precursors. During murine embryogenesis, the pancreas begins to develop at embryonic day E9.5 from evaginations of the endoderm at the level of the anterior intestinal portal (AIP), forming two primordia (dorsal and ventral) capped by mesenchymal cells (Pictet et al., 1972; Wells and Melton, 1999), which are subsequently brought together to form a single gland. Proliferation of the pancreatic epithelial endoderm results in the formation of a tubular complex from which clusters of endocrine and exocrine cells progressively differentiate (Herrera et al., 1991; Slack, 1995). The four main pancreatic endocrine cell types (b, a, d and PP cells, which produce insulin, glucagon, somatostatin and pancreatic polypeptide, respectively) arise from epithelial progenitor cells that can be identified for their expression of transcription factor Ngn3 (Gu et al., 2002; Herrera et al., 2002). In adult mice, pancreatic stem cells seem to reside in the ducts and within the islets (Bonner-Weir and Sharma, 2002); nevertheless, it is not known whether they are Ngn3 þ , and no marker for such putative precursor/stem cell has been identified so far. Since pancreatic and intestinal endocrine cells share many markers with neuronal cells, namely: peptidic hormones, neuropeptide-processing enzymes, glucose transporters, transcription factors (Ngn3, Pax-4, Pax-6, Isl1, NeuroD/ Beta2, Nkx 2.2 and Nkx 6.1) (Ahlgren et al., 1997; Alpert et al., 1988; Gradwohl et al., 2000; Naya et al., 1997; Sander et al., 2000; Sosa-Pineda et al., 1997; Sussel et al., 1998; Turque et al., 1994), among others, it was thought that the Class IV intermediate filament nestin, a specific marker of neuronal stem cells (Cattaneo and McKay, 1990; Johansson et al., 1999; Lendahl et al., 1990), which is widely expressed during development but is very rare in adult organs, could be a marker of islet progenitor cells as well (Hunziker and Stein, 2000). Nestin has been detected in many proliferating regions of the central nervous system (CNS) and in 0925-4773/$ - see front matter q 2003 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.mod.2003.11.004 Mechanisms of Development 121 (2004) 3–14 www.elsevier.com/locate/modo * Corresponding author. Tel.: þ 41-22-379-5225; fax: þ41-22-379-5260. E-mail address: [email protected] (P.L. Herrera).

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Nestin expression in pancreatic exocrine cell lineages
Alexandra Delacoura, Virginie Nepotea, Andreas Trumppb, Pedro Luis Herreraa,*
aDepartment of Morphology, room 5040, University of Geneva Medical School, 1 rue Michel-Servet, CH-1211 Geneva 4, SwitzerlandbSwiss Institute for Cancer Research (ISREC), Chemin des Boveresses 155, CH-1066 Epalinges, Switzerland
Received 19 September 2003; received in revised form 20 November 2003; accepted 21 November 2003
Abstract
Expression of nestin has been suggested to be a characteristic of pancreatic islet stem cells. To determine whether nestin is indeed
expressed in such putative cells during embryonic development, or in the adult pancreas after injury, we performed a cell lineage analysis
using two independent lines of transgenic mice encoding Cre recombinase under the control of rat nestin cis-regulatory sequences, each
crossed with loxP-bearing R26R mice. F1 animals produced the reporter molecule b-galactosidase only upon Cre-mediated recombination,
thus solely in cells using (or having used) the transgenic nestin promoter. In early pancreatic primordia, b-galactosidase was observed in
mesenchymal and epithelial cells. At later developmental stages or in adults, vast clusters of acinar cells and few ductal cells were labeled, in
addition to fibroblasts and vascular cells, but no endocrine cells were tagged by b-galactosidase. This correlated with the transient expression,
observed with an anti-nestin antibody, of endogenous nestin in about 5% of epithelial cells during development (whether in cord-forming
arrangements or in nascent acini), and in vascular and mesenchymal structures. After partial pancreatectomy, there was a transient increase of
the number of anti-nestin-labeled endothelial cells, but again, no endocrine cells bore b-galactosidase. Together, these findings show that
nestin is expressed in the pancreatic exocrine cell lineage, and suggest that consistent nestin expression is not a major feature of islet
endocrine progenitor cells.
q 2003 Elsevier Ireland Ltd. All rights reserved.
Keywords: Pancreas; Islet; Development; Transgenic; Mouse; Cre; Cell tracing; Lineage; Nestin; Insulin; Progenitor; Stem cell
1. Introduction
The isolation of islet or pancreas stem cells requires the
identification of molecular markers characteristic of such
lineage precursors. During murine embryogenesis, the
pancreas begins to develop at embryonic day E9.5 from
evaginations of the endoderm at the level of the anterior
intestinal portal (AIP), forming two primordia (dorsal and
ventral) capped by mesenchymal cells (Pictet et al., 1972;
Wells and Melton, 1999), which are subsequently brought
together to form a single gland. Proliferation of the
pancreatic epithelial endoderm results in the formation of
a tubular complex from which clusters of endocrine and
exocrine cells progressively differentiate (Herrera et al.,
1991; Slack, 1995). The four main pancreatic endocrine cell
types (b, a, d and PP cells, which produce insulin, glucagon,
somatostatin and pancreatic polypeptide, respectively) arise
from epithelial progenitor cells that can be identified for
their expression of transcription factor Ngn3 (Gu et al.,
2002; Herrera et al., 2002). In adult mice, pancreatic stem
cells seem to reside in the ducts and within the islets
(Bonner-Weir and Sharma, 2002); nevertheless, it is not
known whether they are Ngn3 þ , and no marker for such
putative precursor/stem cell has been identified so far. Since
pancreatic and intestinal endocrine cells share many
markers with neuronal cells, namely: peptidic hormones,
neuropeptide-processing enzymes, glucose transporters,
transcription factors (Ngn3, Pax-4, Pax-6, Isl1, NeuroD/
Beta2, Nkx 2.2 and Nkx 6.1) (Ahlgren et al., 1997; Alpert
et al., 1988; Gradwohl et al., 2000; Naya et al., 1997; Sander
et al., 2000; Sosa-Pineda et al., 1997; Sussel et al., 1998;
Turque et al., 1994), among others, it was thought that the
Class IV intermediate filament nestin, a specific marker of
neuronal stem cells (Cattaneo and McKay, 1990; Johansson
et al., 1999; Lendahl et al., 1990), which is widely expressed
during development but is very rare in adult organs, could
be a marker of islet progenitor cells as well (Hunziker and
Stein, 2000). Nestin has been detected in many proliferating
regions of the central nervous system (CNS) and in
0925-4773/$ - see front matter q 2003 Elsevier Ireland Ltd. All rights reserved.
doi:10.1016/j.mod.2003.11.004
Mechanisms of Development 121 (2004) 3–14
www.elsevier.com/locate/modo
* Corresponding author. Tel.: þ41-22-379-5225; fax: þ41-22-379-5260.
E-mail address: [email protected] (P.L. Herrera).
developing mesenchyme and blood vessels throughout the
embryo (Dahlstrand et al., 1995; Frojdman et al., 1997;
Kachinsky et al., 1994, 1995; Mokry and Nemecek, 1998a,b,
1999; Yang et al., 2000), where it could be involved in
epithelium–mesenchymal interactions (About et al., 2000)
and in cell migration (Doyle et al., 2001). Whether nestin is
a marker of a multipotent pancreatic stem cell population is
debated (Blyszczuk et al., 2003; Duvillie et al., 2003; Klein
et al., 2003; Lardon et al., 2002; Lumelsky et al., 2001;
Selander and Edlund, 2002; Zulewski et al., 2001). Nestin
has been reported in cells located within adult pancreatic
islets (Hunziker and Stein, 2000); when isolated, these
nestin-positive cells can be grown ex vivo into different
pancreatic cell types (Zulewski et al., 2001). Others reported
that ES cells selected for their expression of nestin can
differentiate in vitro into the four islet endocrine cell types
(Lumelsky et al., 2001). Taken together, these results have
suggested that nestin-positive cells represent a multipotent
pancreatic stem cell population, which could be used in
future cell replacement therapies to cure diabetes. However,
other experimental evidences have toned down the hope of
generating new b-cells from such hypothetical nestin-
positive precursors. Nestin has been shown by immuno-
histochemistry to be expressed in mesenchymal cells during
pancreatic development, but not in epithelial cells of the
developing mouse pancreas (Klein et al., 2003; Lardon et al.,
2002; Selander and Edlund, 2002), where the islet cells
form, whereas in the pancreas of adult mice nestin was only
found in stellate and endothelial cells (Lardon et al., 2002).
Other reports failed to reproduce the results obtained with
nestin-enriched ES cells (Rajagopal et al., 2003).
In this study we sought to determine whether islet
endocrine cells derive from nestin-expressing progenitors.
We thus have systematically analyzed in vivo, during
embryogenesis and in adulthood, the progeny of cells that
transcribe the nestin gene by marking them genetically
using the Cre/loxP approach (Gannon et al., 2000; Gu et al.,
2002, 2003; Herrera, 2000; Herrera et al., 1998, 2002;
Kawaguchi et al., 2002). Briefly, in doubly transgenic mice
(nestin-Cre/R26R), the cells transiently (or permanently)
expressing nestin also produce Cre and are genetically
tagged, at the Rosa26 locus (R26R mice, Soriano, 1999), so
that their progeny stably expresses the b-galactosidase
reporter. We found that nestin is expressed not only in
mesenchymal tissues but also in epithelial cells of the
exocrine lineage, and that islet endocrine cell progenitors do
not express this marker.
2. Results
1. Nestin is expressed in mesenchymal and epithelial
cells of the developing pancreas (Fig. 1). In order to
determine whether nestin is expressed during pancreas
development we used an anti-mouse nestin monoclonal
antibody. At the earliest stage analyzed, E10.5,
mesenchymal stained cells were abundant around the
pancreatic primordia (not shown). At E12.5, stained cells
were frequent in the mesenchyme surrounding the branch-
ing primordium, as described (Klein et al., 2003; Selander
and Edlund, 2002) (Fig. 1B,C); however, contrary to
previous reports (Hunziker and Stein, 2000; Klein et al.,
2003; Lardon et al., 2002; Selander and Edlund, 2002; Piper
et al., 2002), nestin-positive cells forming clusters within
the growing epithelial cords were also found: 5.7% of the
epithelial cells were nestin-positive (Figs. 1B,C,4G). At
E15.5 and E17.5, anti-nestin antibody clearly labeled
mesenchyme and vessels (Fig. 1E,F,H) as well as some
acini (Fig. 1E,F,H), but no staining was ever seen in nascent
islets (Fig. 1I). The proportion of nestin-expressing
epithelial cells decreased as differentiation progressed
(3.9% at E15.5, 0.2% at E17.5), so that after birth, nestin-
expressing epithelial cells were absent (Fig. 4G).
2. The expression pattern of the two nestin-Cre
transgenes accurately reflects that of endogenous nestin
gene (Figs. 2,3). Nestin-expressing cells and their progeny
were traced in vivo using the Cre/loxP system, as in
previous pancreatic cell lineage analyses (Gannon et al.,
2000; Gu et al., 2002; Herrera, 2000; Herrera et al., 1998,
2002; Kawaguchi et al., 2002) (Fig. 2). We used two
different transgenic strains of mice, encoding Cre under the
control of rat nestin cis-regulatory sequences, which were
generated independently in different laboratories (Tronche
et al., 1999; Trumpp et al., 1999). Both yielded the same
expression pattern, indicating that the phenotype was likely
not affected by the integration site and the number of copies
of the transgenes. Throughout development, doubly trans-
genic embryos displayed a fully penetrant expression of the
reporter b-galactosidase gene in the central nervous system
and in other organs such as the eyes, vessels and hair
follicles (Fig. 3A,B,D,E), where nestin expression has been
demonstrated by immunostaining (Kachinsky et al., 1995;
Li et al., 2003; Mokry and Nemecek, 1998b; Yang et al.,
2000). In early pancreatic primordia (E10.5–12.5), b-
galactosidase was found in cells that were stained with anti-
nestin antibody, indicating that the activity of nestin-Cre
transgenes is accurate (Fig. 3F,G). Nestin-expressing cells
were slightly more abundant than X-Gal þ cells (5.7 vs.
4.8% in the epithelia, respectively—see below); since there
is a delay of several hours between Cre expression and
activation of the recombined reporter transgene (Davidson
et al., 2003), an early population of pancreatic nestin þ
precursor cells might not be X-Gal-labeled in the buds of
E10.5–12.5 transgenic embryos. Later and post-natally, the
proportion of b-galactosidase þ cells increased (see
below), concomitantly with a decrease to almost total
dismiss of anti-nestin-labeling, since b-galactosidase
expression, once triggered, is irreversible (Fig. 2).
3. The reporter b-galactosidase transgene is activated in
mesenchymal and epithelial cells of early pancreatic
primordia, but not in endocrine cells (Figs. 4,5). We started
the systematic analysis of X-Gal staining at E12.5. At this
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–144
stage, the blue labeling was observed in mesenchymal and
vascular cells, as well as in 4.8% of epithelial cells
(Fig. 4A,G), which correlates with the expression of endo-
genous nestin, as shown above. From E15.5 to adulthood, the
proportion of stained epithelial/acinar cells, which were
distributed within the entire gland, increased from 6.5 to
almost 20% (Figs. 4B–G,5D,6C). Frequently, within stained
acini, not all cells bore b-galactosidase (Fig. 4E). Excretory
ducts, mesenchymal and vascular cells were also stained
(Fig. 4). Within the islets, no staining was found (Fig. 4F). By
combining X-Gal staining and immunohistochemistry, we
performed a precise identification of traced cells. Islet
endocrine cells were not labeled (Fig. 5A–C). X-Gal þ cells
were stained with anti-amylase (acinar cells, Fig. 5D), anti-
cytokeratin (ducts), anti-vimentin (fibroblasts), and anti-
myosin or anti-pecam (blood vessels) antibodies (not
shown). That hormone-containing endocrine cells were
never X-Gal þ indicates that they had not been tagged by
Cre expression, and suggests that neither they nor their
progenitors express significant amounts of nestin.
4. Pancreas remodeling after partial pancreatectomy is
characterized by a transient over-expression of endothelial
nestin (Fig. 6). In order to determine whether expression of
nestin is a feature of the cells that generate new endocrine
cells during islet neogenesis in the adult pancreas, we
performed a partial pancreatectomy of about 60% of the
gland (Bonner-Weir et al., 1983) in four wild type mice and
in four nestin-Cre/R26R animals (two of each strain), and
the pancreatic remnants (which correspond to the duodenal
pancreas) were quantitatively analyzed either 1, 3 or 13 days
after the operation, or 1 month later. The nestin-Cre/R26R
transgenics were only analyzed 13 or 30 days post-surgery,
when the process of regeneration has been completed. In the
adult pancreas, occasional nestin-expressing endothelial
cells were observed (Fig. 6A). After partial pancreatectomy,
the number of cells stained with anti-nestin antibody in the
remaining pancreas increased dramatically starting 3 days
post-removal, both in islets and in the exocrine parenchyma
(Fig. 6A,B). Thirty days after pancreatectomy the anti-
nestin staining pattern was indistinguishable from that of
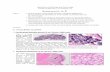
Fig. 1. Nestin is expressed in mesenchymal and epithelial cells of the developing pancreas. Nestin-positive cells were detected by immunohistochemistry at
different stages of mouse pancreas development with an anti-mouse nestin antibody. Negative controls (A,D,G) were sections incubated without the primary
anti-nestin antibody, and showed no background staining. At E12.5 (A–C), nestin (brown staining) was found in some epithelial cells, as well as in
mesenchymal and vascular cells. At E15.5 (D–F), nestin was detected in some epithelial and acinar cells of the developing pancreas. Nestin-positive cells in
mesenchyme and vessels were less conspicuous than at earlier stages (F). At E17.5 (G–I), nestin immunostaining was observed in some acinar cells (H). Very
few nestin-containing mesenchymal or vascular cells were detected at this stage, whereas no stained cells were seen in the growing islets (I). Dashed lines
indicate epithelial cords in B and C. Scale bars, 20 mm.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–14 5
non-operated pancreata (Fig. 6A), showing the transitory
character of the increase in nestin expression. The nestin-
expressing cells, whether within the islets or amongst the
acini, were revealed to be endothelial with anti-pecam
staining (Fig. 6B). In nestin-Cre/R26R mice, the proportion
of cells having expressed the nestin-Cre transgenes (i.e.
having b-galactosidase) after partial pancreatectomy was
similar to that in non-operated controls (Fig. 6C), and no
islet endocrine cell was stained using anti-b-galactosidase
antibody or the substrate X-Gal, indicating that cells having
expressed the nestin gene contribute to adult pancreas
remodeling to a similar extent as during embryonic
development.
3. Discussion
Tracking the progeny of a particular cell in transgenic
mice expressing Cre recombinase is an informative
approach with broad potential applications. The direct
pancreatic cell tracing experiments reported herein suggest
two main conclusions: first, about one-fifth of adult exocrine
cells descend from nestin-expressing progenitors, and
second, nestin is probably not a general marker of the
endocrine lineages. Furthermore, after partial pancreatect-
omy, nestin is transiently expressed in endothelial cells that
are probably involved in tissue remodeling.
Tracking the fate of cells in vivo: limitations of
transgenesis. In this study we used two different, previously
generated, nestin-Cre transgenic strains (Tronche et al.,
1999; Trumpp et al., 1999). The pattern of b-galactosidase
activity was the same in both cases, which strengthens the
conclusions supported by our observations. Nevertheless,
since the nestin regulatory elements used are incomplete,
Cre expression may not accurately recapitulate the entire
pattern of endogenous nestin expression in the embryo; in
the early developing pancreas, however, all X-Gal þ cells
were stained with anti-nestin antibody.
Fig. 2. Tracking the fate of cells in transgenic mice. General strategy used to irreversibly label cells using the nestin regulatory elements. In doubly transgenic
mice, if a progenitor cell expresses nestin, whether transiently or not, then it should also express the transgenic Cre, which would induce the irreversible
excision of the floxed STOP sequence at the R26R locus, thus allowing the expression of b-galactosidase in that particular cell, and in its progeny, and therefore
the tracing of its lineage.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–146
Fig. 3. The nestin cis-regulatory sequences-driven transgenes express Cre accurately. (A–C) X-Gal-staining in whole mount embryos. Cre activity corresponds
to nestin expression in early E10.5 nestin-Cre/R26R embryos, in which the encephalon and neural tube were deeply stained in the presence of X-Gal, as
described (Zimmerman et al., 1994) (A). At E15.5, the retina, hair follicles (double arrow) and blood vessels (arrow) were clearly stained (B), as well as many
cells in the pancreas (C). (D,E) Sagittal (D) and transverse (E) sections of the neural tube (developing spinal cord, E10.5) at the level indicated with a box in
panel A. b-Galactosidase activity (D) and anti-nestin antibody (E) marked the ependymal layer (e), which is very thick at this early stage. (F,G) Doubly
transgenic E12.5 pancreatic buds were double-stained using X-Gal (pale blue) and anti-nestin antibody (brown). In these sections, endogenous nestin and
b-galactosidase were detected in the same cells. An arteriole (F) and mesenchymal cells (G) are shown. Scale bars, 0.5 mm (A–C) and 20 mm (D–G). a,
amnion; c, central canal (neural lumen); d, duodenum; da, dorsal aorta; dp, dorsal pancreas; e, ependymal layer; m, muscle; vp, ventral pancreas.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–14 7
The main limitation of classical transgenesis by pro-
nuclear microinjection of DNA into zygotes is variegation,
i.e. reliability of transgene expression, which is site- and
copy number-dependent, even within the same litter.
Transgene silencing (loss of expressivity) may result from
a number of alterations, whether genetic (intra-locus
recombination, when there is a high number of tandem
copies) or epigenetic (hypermethylation). For this reason,
Fig. 4. b-Galactosidase is expressed in exocrine and interstitial cells of the growing pancreas. (A) At E12.5, b-galactosidase expression, revealed by
histochemistry (X-Gal staining) on pancreatic sections from nestin-Cre/R26R mice, was detected in mesenchymal and endothelial cells, and in some epithelial
cells (arrows). (B) At E15.5, b-galactosidase activity was detected in some acini (arrow), ductal cells (d), mesenchymal cells, and in endothelial cells. Reporter
gene expression in E17.5 fetal (C,D) and P12 post-natal (E,F) pancreata was seen a fraction of acini (C,E,F), vessels (D), ducts and interstitial cells (E,F). In
labeled acini, not all acinar cells were stained (E). In islets, no stained endocrine cells were found (F). (G) Graph representing the proportions of nestin-
expressing cells and of X-Gal þ cells during pancreas development and in the adult. Scale bars, 20 mm. d, duct; e, epithelial cord; i, islet; v, blood vessel.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–148
this technique requires the production of large numbers of
founder mice; in most cases, these allow for the selection of
good strains in which there is reproducible transgene
expression, without aberrant activity.
More recently, new approaches, such as the use of very
large stretches of DNA in bacterial artificial chromosomes
(BACs) comprising entire enhancer/promoter sequences, or
the generation of knock-in mice, may help overcome the
problems of accuracy in transgene expression. In both cases,
homologous recombination is used to replace or modify, by
introducing the transgenic DNA, the transcribed region of
the gene whose regulatory sequences are to be used in the
transgenic animal. Even though the generation of knock-in
nestin-Cre mice would probably be the best solution to
address the Cre expression concerns, these techniques have
their own problems, as for classical transgenes, since genes
may have unknown internal regulatory elements that are
replaced, or split, or altered somehow by the inserted
foreign DNA. In other situations, which may not apply to
nestin, the generation of knock-in mice may not be the best
solution, such as for genes located on the X chromosome, or
parentally imprinted, or expressed monoallelically, or even
for those genes whose normal activity is only obtained when
both alleles are expressed.
Nestin is expressed in pancreatic epithelial cells. The
expression pattern of nestin in the pancreas is controversial.
The detection of nestin transcripts in early pancreatic bud
cells (E12.5 total RNA) was recently reported in gene
expression profiling analyses (Chiang and Melton, 2003).
The presence of nestin has been described, by immuno-
histochemistry, in islet cells that do not contain hormones
(Hunziker and Stein, 2000; Zulewski et al., 2001) or in some
ductal cells (Zulewski et al., 2001) or only in mesenchymal
cells surrounding the developing gland (Selander and
Edlund, 2002). Other studies on adult pancreata show that
pancreatic nestin is found in capillaries (Klein et al., 2003)
and also in pericytes after duct ligation, or during
development (Lardon et al., 2002). Our observations using
anti-mouse nestin antibody confirm that nestin is much more
abundant in the developing pancreas than in the adult, and is
mainly expressed in mesenchymal and vascular cells (Klein
et al., 2003; Lardon et al., 2002; Selander and Edlund,
2002). However, we show, together with the accompanying
paper by Esni and co-workers, that nestin is also expressed
in early epithelial cords and, later in development, in some
acini as well as in ductal cells. That nestin is expressed in
certain epithelial cells during development has been
demonstrated in other organs such as the mesonephros,
eye and hair follicles (Frojdman et al., 1997; Mokry and
Nemecek, 1998b). The difference with previous reports on
pancreatic primordia probably reflects the different sensi-
tivity of the antibodies or the immunohistochemical
techniques used.
We have reported that some 20% acinar (and ductal)
cells are labeled in the adult nestin-Cre/R26R mice.
Whereas Esni and colleagues (this issue) have obtained
very similar results with pancreatic primordia grown in
vitro, this observation is discordant with a recently
published report (Treutelaar et al., 2003) also using the
nestin-Cre mice generated by one of us (Trumpp et al.,
1999, which is one of the two strains used in this study). The
reason for this discrepancy is not clear at this time.
Between E15.5 and adult life, i.e. in a period where
nestin is not expressed, the proportion of epithelial cells that
express b-galactosidase increases from 6.5 to 20%. This
observation suggests that, for some reason, the progeny of
Fig. 5. Characterization of the progeny of nestin-expressing progenitor cells. E15.5 pancreata from nestin-Cre/R26R embryos were doubly stained with X-Gal
and with antibodies against islet hormones, as well as endothelial, epithelial or mesenchymal markers (see text). b-Galactosidase did not co-localize with
insulin or glucagon (A), somatostatin (B) or PP (C). The majority of the b-galactosidase þ cells were also stained with anti-amylase (acinar cells, D). Arrows,
arrowheads and dashed line indicate the same cells in every pair of consecutive panels. Scale bars, 20 mm.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–14 9
Fig. 6. Nestin expression is transiently upregulated after partial pancreatectomy. (A) Sections of pancreata from adult pancreatectomized (PX) non-transgenic
or double transgenic animals were incubated with anti-nestin antibody either 1 (PX-d1), 3 (PX-d3), 13 (PX-d13) or 30 days (PX-d30) after ablation. In control
mice and 1 day after PX (PX-d1), nestin-positive cells were rare. Three and 13 days post-surgery, nestin-expressing cells were, on the contrary, very abundant,
but 1 month after the operation (PX-d30) the nestin-expression pattern was as in non-operated animals. Scale bars, 20 mm. Dashed lines delimitate islets. (B)
Confocal images of pancreatic sections from Px-d13 animals stained with anti-nestin and anti-pecam antibodies revealed that nestin-expressing cells were
endothelial, whether within the islets (B) or among the acini (not shown). Scale bar, 20 mm. (C) Estimation of the area occupied by b-galactosidase-stained
cells in nestin-Cre/R26R mice, whether non-operated (control, n ¼ 2) or pancreatectomized (PX-d30, n ¼ 2). The distributions were not significantly different
ðP , 0:01Þ: An example of b-galactosidase staining pattern in nestin-Cre/R26R PX-d13 is shown on the right of the histogram (Scale bars, 20 mm).
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–1410
nestin þ cells has a proliferative advantage as compared
with that of epithelial cells not expressing nestin. It is
noteworthy that, in the majority of X-Gal þ acini, only a
fraction of acinar cells were stained, suggesting that in vivo,
acini, as islets (Deltour et al., 1991), have a polyclonal
origin (Percival and Slack, 1999); alternatively, a nestin-
negative early acinar progenitor may give rise to nestin þ
and nestin 2 progeny in some acini. The biological
significance of these acinar and ductal cell populations
derived from nestin-expressing progenitors is not clear at
this time, and they do not appear to contribute to adult
regeneration differently than non-stained acini. Indeed,
whether these tagged exocrine cells represent a versatile
population of cells that, by transdifferentiation, could
directly contribute to the rise of new endocrine cells during
regeneration after injury seems unlikely, since our results
using a model of partial pancreatic ablation suggest the
contrary.
Whether the patchiness of the acinar b-galactosidase
staining is due to a lack of fully expressivity of the Cre
transgenes is unlikely, the proportions of nestin- and
b-galactosidase-expressing epithelial cells are very similar
in early pancreatic primordia, where b-galactosidase and
nestin colocalize. Therefore, the acinar cells bearing
b-galactosidase post-natally probably derive from early
epithelial cells, which expressed nestin transiently (as we
have shown with an anti-nestin antibody), further illustra-
ting an heterogeneity of the exocrine pancreas that is not
associated with a distinct arrangement or distribution of the
X-Gal-stained acini (for instance, ‘peri-‘ vs. ‘teleinsular’,
Adelson and Miller, 1989).
The expression of a neuronal progenitor marker such as
nestin represents a convergence between neurons and
acinar cells; in this perspective, the reported expression of
acinar cell markers such as Mist1 and p48 in various
neuronal cell types (Hewes et al., 2003; Obata et al., 2001)
is noteworthy.
Our results also indicate that endocrine progenitor cells
do not appear to express nestin. Esni and co-workers
provide a similar conclusion in an elegant accompanying in
vitro study, and Treutelaar and colleagues also report that
nestin is not expressed in islet endocrine cells (Treutelaar
et al., 2003); furthermore, studies using primary human
embryonic pancreatic cells in vitro suggest similar
conclusions (Humphrey et al., 2003). If true, this fact
would represent an important divergence between nerve
cells and islet endocrine cells. However, several consider-
ations must be addressed. It may be that endocrine cells
arise from a fraction of nestin þ cells in which nestin
expression results from cis-regulatory elements not present
in the transgenes used by us and Treutelaar et al.
Furthermore, Cre expression may be insufficient to tag
cells in which nestin is transcribed at very low levels or for
a short period of time, or both. Nevertheless, taken
together, our results suggest that there is no major
contribution of cells expressing nestin to the formation of
the endocrine cells of the islets of Langerhans.
What may be the role of nestin-expressing cells?
Previous in vitro studies suggest that nestin-expressing
cells are precursors to endocrine cells: cultures of nestin-
containing islet-derived cells (Zulewski et al., 2001), or ES
cells selected for the expression of nestin (Lumelsky et al.,
2001), generated islet-like structures in vitro; since no direct
cell tracing analyses were performed in those experiments,
nestin þ cells might act indirectly (i.e. non-cell autono-
mously) in such differentiation protocols. Whether different
mechanisms (involving different cell types and different
gene expression pathways) are implicated in the production
of new b-cells in different situations, such as during
development, or in the adult in pathological conditions, or
in experimental protocols in vitro from ES cells (Blyszczuk
et al., 2003; Lumelsky et al., 2001) or from other
undifferentiated cells (Zulewski et al., 2001), needs to be
further explored.
Anti-nestin staining confirmed that nestin-expressing
cells are rare in the adult pancreas. After partial
pancreatectomy, however, this is transitorily different, for
there is a massive increase in the numbers of nestin-
expressing endothelial cells. Others reported similar results
after duct ligation, another experimental model of pan-
creatic injury that triggers regeneration: nestin was shown
in new capillaries and in peri-acinar stellate cells,
indicating that nestin is an angiogenic marker (Lardon
et al., 2002). In nestin-Cre/R26R transgenics, the tempor-
ary rise of pancreatic endothelial nestin-expressing cells
after the partial ablation of the gland was not followed by a
subsequent increase of the proportion of cells stained with
either X-Gal or anti-b-galactosidase antibody. This indi-
cates that the transient nestin-expressing endothelium does
not represent a specific pool of islet progenitor cells, but
rather a population of cells that die without leaving any
labeled progeny.
Stellate cells, which are more abundant in the regenerat-
ing pancreas and liver (Kalinichenko et al., 2003; Zimmer-
mann et al., 2002), are a source of NGF and neurotrophins
(Cassiman et al., 2001), factors that have previously been
implicated in the regulation of islet neogenesis (Scharf-
mann, 1997). Nestin expression is a feature of de-
differentiation: it is re-expressed during repair processes in
a variety of organs such as liver (stellate cells, Friedman,
1999; Messing, 1999; Niki et al., 1999), teeth (odontoblasts,
About et al., 2000), muscle (myoblasts, Vaittinen et al.,
2001), or the CNS (astrocytes and endothelia, Duggal et al.,
1997; Ha et al., 2002). Expression of nestin may therefore
be viewed as a trait of cells involved mainly indirectly in the
genesis of new cells during regeneration, and possibly
during development, not only in the pancreas but also in
other organs. This could be via stellate or endothelial cells,
or both, and should be of relevance in devising new
protocols for cell replacement therapies in a variety of
diseases.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–14 11
4. Materials and methods
4.1. Transgenic mice
Two different nestin-Cre mice were generated indepen-
dently in two different laboratories (Tronche et al., 1999;
Trumpp et al., 1999). The transgenics produced by Klein
(Tronche et al., 1999) were purchased from The Jackson
Laboratories (strain: B6.Cg (SJL)-TgN(NesCre)1Kln; stock
#003771). Both strains bear a Cre recombinase gene under
the control of the rat nestin 50Flanking (5.8-kb) and 2nd
intron (1.8-kb) sequences, as described (Zimmerman et al.,
1994). The ROSA26R ubiquitous reporter mice (R26R)
(Soriano, 1999) were obtained from The Jackson Labora-
tories (strain: B6.129S4-Gtrosa26tm1Sor; stock #003474).
Doubly transgenic animals were genotyped by PCR and/or
directly with X-Gal or anti-b-galactosidase staining. The
oligonucleotides used as primers yielded two fragments of
300 and 500 bp for Cre recombinase and Lac Z genes,
respectively; their sequences were: 50-ATGCTTC-
TGTCCGTTTGCCG-30/50-CCTGTTTTGCACGTTCAC-
CG-30 for Cre, and 50-ACGGCAAACGACTGTCCTGG-30/
50-CGTGACTGGGAAAACCCTGG-30 for Lac Z, using a
standard 30-cycle PCR profile.
4.2. X-Gal staining
Total embryos or pancreatic buds at different develop-
mental stages, and adult pancreata, were harvested in cold
phosphate buffer (pH 7.4) and fixed for 1 h at 4 8C in 4%
PFA. Tissues were then incubated overnight at 37 8C in
X-Gal substrate (5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6,
2 mM MgCl2, 0.01% Na deoxycholate, 0.02% NP40,
1 mg/ml X-Gal in phosphate buffer, pH 7.4). Subsequently,
stained samples were embedded in paraffin, sectioned, and
counterstained with hemalun, for high-resolution analyses
of b-galactosidase activity.
4.3. Immunohistochemistry
Embryos at different developmental stages, and adult
pancreata, were harvested in cold PBS and fixed in 4% PFA
overnight at 4 8C. Then, the tissues were embedded in
paraffin and 5 mm-thick tissue sections were mounted on
adhesive-coated slides.
For detection of nestin and b-galactosidase, the slides
were dewaxed, rehydrated and soaked in 10 mM citrate
buffer (pH 6) in a microwave oven at 700 W for 10 min, and
then an additional 20 min at room temperature (‘antigen
retrieval’ procedure). When diaminobenzidine (DAB) was
used to reveal the immunostaining, sections were pre-
treated with 3% H2O2 (in PBS) for 10 min, in order to
quench endogenous peroxydases. Sections were sub-
sequently incubated with blocking reagent (5% donkey
serum, 0.1% Tween, free biotin—Vector Laboratories—in
PBS) for 30 min and then overnight at 4 8C with either
mouse anti-rat nestin (Chemicon, clone 401, dilution 1/100)
or rabbit anti-b-galactosidase (Abcam, dilution 1/250)
antibody in blocking reagent (free biotin was replaced by
free streptavidin), and then incubated for 1 h with
biotinylated anti-mouse or anti-rabbit (Jackson Immuno-
research, dilution 1/200) antibody, respectively. Immuno-
staining was revealed by either immunofluorescence with
streptavidin-Alexa 488 (for nestin) or 568 (for b-galacto-
sidase) (Molecular Probes, dilution 1/100, during 1 h at
room temperature), or with streptavidin-hrp (Jackson
Immunoresearch, dilution 1/300, 1-h)/DAB.
The surface occupied by epithelial cells stained with
X-Gal or anti-nestin antibody was measured on pancreatic
sections from two embryos per developmental stage
(i.e. E12.5, E15.5 and E17.5) by outline/integration using
the NIH’s Image J software (version 1.25).
All other antibodies were used on paraffin sections of
pancreata previously stained with X-Gal. For PanK and
myosin, antigen retrieval was required as well. In these
protocols, non-specific binding sites were blocked by
treating the sections for 30 min with 3% BSA, 0.1%
Tween in PBS. Primary antibodies were then incubated
for 1 h in blocking reagent. The antibodies and their dilution
used in the present analysis were as follows: guinea pig anti-
porcine insulin (Dako, dilution 1/400), mouse anti-porcine
glucagon (Sigma, dilution 1/1000), rabbit anti-human
somatostatin (Sigma, dilution 1/200), rabbit anti-human
PP (Bachem, 1/200), mouse anti-human PanK (Sigma,
dilution 1/100), goat anti-human vimentin (Sigma, dilution
1/20), rabbit anti-PECAM (kind gift of Dr Aurrand-Lyons,
University of Geneva Medical School, dilution 1/500) and
mouse anti-myosin (kind gift of Dr Piallat-Bochaton,
University of Geneva Medical School, at 1/20). Then,
sections were washed and incubated with specific secondary
antibodies coupled to Cy3 (Jackson Immunoresearch).
The specificity of the different immunostainings was
confirmed by controls in which primary antibodies were
omitted. Sections were examined and photographed with a
Nikon epifluorescence microscope (Eclipse TE200)
equipped with a Nikon CoolPix 990 digital camera, and
when indicated, the specimens were analyzed using a Zeiss
LSM510 confocal microscope.
4.4. Partial pancreatectomy
Partial (60%) pancreatectomy (PX) was performed in
wild type C57/B6xCBA/J mice (2 month-old, Iffa Credo)
for the analysis of endogenous nestin expression and on two
males of each strain of nestin-Cre/R26R mice. Briefly, the
pancreas was removed by gentle abrasion with cotton
applicators (Q-tips), leaving intact the major blood vessels
supplying other organs, as described in rats (Bonner-Weir
et al., 1983). The residual pancreas was easily defined as the
tissue within 1–2 mm of the common pancreatic duct that
extends to the first part of the duodenum. Littermates of the
same sex were not operated, and used as controls.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–1412
One, 3, 13 (for wild type mice) and 30 days (for the
nestin-Cre/R26R) after pancreatectomy, pancreas of con-
trols and pancreatectomized mice were collected and fixed
overnight in 4% PFA, at 4 8C. Then, the tissues were
embedded in paraffin and sections of 5 mm of thickness
were performed and mounted on coated slides for the
analysis of nestin and b-galactosidase expression, as
described above.
The surface occupied by cells having b-galactosidase
was measured using sections of pancreas from PX and
sham-operated nestin-Cre/R26R mice that were stained
using X-Gal or anti-b-galactosidase antibody. Four sections
per pancreas were randomly chosen and photographed with
the digital camera. Nestin- and b-galactosidase-stained
areas were quantified using the NIH’s Image J software. The
distributions were compared using a non-parametric Mann–
Whitney test.
Acknowledgements
We are grateful to Drs P. Vassalli, J.-D. Vassalli,
I. Rodriguez, K.A. Johansson, Y. Dor and S. Leach for
carefully reading the manuscript. We thank Mr Nicolas
Steiner, Mr Olivier Fazio, and Mr Angelo Gradia for their
skillful technical assistance. P.L.H. is supported by grants of
the Juvenile Diabetes Research Foundation, the Swiss
National Science Foundation and the NIH/NIDDK. A.T. is
supported by the Swiss National Science Foundation, the
Swiss Cancer League and the Leenards Foundation. A.T. is
a member of the EMBO Young Investigator Programme.
This study was part of the Geneva Programme for Metabolic
Disorders, and of the Frontiers in Genetics National Center
for Competence in Research.
References
About, I., Laurent-Maquin, D., Lendahl, U., Mitsiadis, T.A., 2000. Nestin
expression in embryonic and adult human teeth under normal and
pathological conditions. Am. J. Pathol. 157, 287–295.
Adelson, J.W., Miller, P.E., 1989. Heterogeneity of the exocrine pancreas.
Am. J. Physiol. 256, G817–G825.
Ahlgren, U., Pfaff, S.L., Jessell, T.M., Edlund, T., Edlund, H., 1997.
Independent requirement for ISL1 in formation of pancreatic
mesenchyme and islet cells. Nature 385, 257–260.
Alpert, S., Hanahan, D., Teitelman, G., 1988. Hybrid insulin genes reveal a
developmental lineage for pancreatic endocrine cells and imply a
relationship with neurons. Cell 53, 295–308.
Blyszczuk, P., Czyz, J., Kania, G., Wagner, M., Roll, U., St-Onge, L.,
Wobus, A.M., 2003. Expression of Pax4 in embryonic stem cells
promotes differentiation of nestin-positive progenitor and insulin-
producing cells. Proc. Natl Acad. Sci. USA 100, 998–1003.
Bonner-Weir, S., Sharma, A., 2002. Pancreatic stem cells. J. Pathol. 197,
519–526.
Bonner-Weir, S., Trent, D.F., Weir, G.C., 1983. Partial pancreatectomy in
the rat and subsequent defect in glucose-induced insulin release. J. Clin.
Investig. 71, 1544–1553.
Cassiman, D., Denef, C., Desmet, V.J., Roskams, T., 2001. Human and rat
hepatic stellate cells express neurotrophins and neurotrophin receptors.
Hepatology 33, 148–158.
Cattaneo, E., McKay, R., 1990. Proliferation and differentiation of neuronal
stem cells regulated by nerve growth factor. Nature 347, 762–765.
Chiang, M.K., Melton, D.A., 2003. Single-cell transcript analysis of
pancreas development. Dev. Cell 4, 383–393.
Dahlstrand, J., Lardelli, M., Lendahl, U., 1995. Nestin mRNA expression
correlates with the central nervous system progenitor cell state in many,
but not all, regions of developing central nervous system. Brain Res.
Dev. Brain Res. 84, 109–129.
Davidson, B.P., Tsang, T.E., Khoo, P.L., Gad, J.M., Tam, P.P., 2003.
Introduction of cell markers into germ layer tissues of the mouse
gastrula by whole embryo electroporation. Genesis: J. Genet. Dev. 35,
57–62.
Deltour, L., Leduque, P., Paldi, A., Ripoche, M.A., Dubois, P., Jami, J.,
1991. Polyclonal origin of pancreatic islets in aggregation mouse
chimaeras. Development 112, 1115–1121.
Doyle, K.L., Khan, M., Cunningham, A.M., 2001. Expression of the
intermediate filament protein nestin by sustentacular cells in mature
olfactory neuroepithelium. J. Comp. Neurol. 437, 186–195.
Duggal, N., Schmidt-Kastner, R., Hakim, A.M., 1997. Nestin expression in
reactive astrocytes following focal cerebral ischemia in rats. Brain Res.
768, 1–9.
Duvillie, B., Attali, M., Aiello, V., Quemeneur, E., Scharfmann, R., 2003.
Label-retaining cells in the rat pancreas: location and differentiation
potential in vitro. Diabetes 52, 2035–2042.
Friedman, S.L., 1999. Cytokines and fibrogenesis. Semin. Liver Dis. 19,
129–140.
Frojdman, K., Pelliniemi, L.J., Lendahl, U., Virtanen, I., Eriksson, J.E.,
1997. The intermediate filament protein nestin occurs transiently
in differentiating testis of rat and mouse. Differentiation 61,
243–249.
Gannon, M., Herrera, P.L., Wright, C.V., 2000. Mosaic Cre-mediated
recombination in pancreas using the pdx-1 enhancer/promoter. Genesis
26, 143–144.
Gradwohl, G., Dierich, A., LeMeur, M., Guillemot, F., 2000. Neurogenin3
is required for the development of the four endocrine cell lineages of the
pancreas. Proc. Natl Acad. Sci. USA 97, 1607–1611.
Gu, G., Dubauskaite, J., Melton, D.A., 2002. Direct evidence for the
pancreatic lineage: NGN3 þ cells are islet progenitors and are distinct
from duct progenitors. Development 129, 2447–2457.
Gu, G., Brown, J.R., Melton, D.A., 2003. Direct lineage tracing reveals the
ontogeny of pancreatic cell fates during mouse embryogenesis. Mech.
Dev. 120, 35–43.
Ha, Y., Choi, J.U., Yoon, D.H., Cho, Y.E., Kim, T.S., 2002. Nestin and
small heat shock protein expression on reactive astrocytes and
endothelial cells in cerebral abscess. Neurosci. Res. 44, 207–212.
Herrera, P.L., 2000. Adult insulin- and glucagon-producing cells
differentiate from two independent cell lineages. Development 127,
2317–2322.
Herrera, P.L., Huarte, J., Sanvito, F., Meda, P., Orci, L., Vassalli, J.D.,
1991. Embryogenesis of the murine endocrine pancreas: early
expression of pancreatic polypeptide gene. Development 113,
1257–1265.
Herrera, P.L., Orci, L., Vassalli, J.D., 1998. Two transgenic approaches to
define the cell lineages in endocrine pancreas development. Mol. Cell.
Endocrinol. 140, 45–50.
Herrera, P.L., Nepote, V., Delacour, A., 2002. Pancreatic cell lineage
analyses in mice. Endocrine 19, 267–277.
Hewes, R.S., Park, D., Gauthier, S.A., Schaefer, A.M., Taghert, P.H., 2003.
The bHLH protein Dimmed controls neuroendocrine cell differentiation
in Drosophila. Development 130, 1771–1781.
Humphrey, R.K., Bucay, N., Beattie, G.M., Lopez, A., Messam, C.A.,
Cirulli, V., Hayek, A., 2003. Characterization and isolation of
promoter-defined nestin-positive cells from the human fetal pancreas.
Diabetes 52, 2519–2525.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–14 13
Hunziker, E., Stein, M., 2000. Nestin-expressing cells in the pancreatic
islets of Langerhans. Biochem. Biophys. Res. Commun. 271, 116–119.
Johansson, C.B., Momma, S., Clarke, D.L., Risling, M., Lendahl, U.,
Frisen, J., 1999. Identification of a neural stem cell in the adult
mammalian central nervous system. Cell 96, 25–34.
Kachinsky, A.M., Dominov, J.A., Miller, J.B., 1994. Myogenesis and the
intermediate filament protein, nestin. Dev. Biol. 165, 216–228.
Kachinsky, A.M., Dominov, J.A., Miller, J.B., 1995. Intermediate filaments
in cardiac myogenesis: nestin in the developing mouse heart.
J. Histochem. Cytochem. 43, 843–847.
Kalinichenko, V.V., Bhattacharyya, D., Zhou, Y., Gusarova, G.A., Kim,
W., Shin, B., Costa, R.H., 2003. Foxf1 þ /2 mice exhibit defective
stellate cell activation and abnormal liver regeneration following CCl4
injury. Hepatology 37, 107–117.
Kawaguchi, Y., Cooper, B., Gannon, M., Ray, M., MacDonald, R.J.,
Wright, C.V., 2002. The role of the transcriptional regulator Ptf1a in
converting intestinal to pancreatic progenitors. Nat. Genet. 32,
128–134.
Klein, T., Ling, Z., Heimberg, H., Madsen, O.D., Heller, R.S., Serup, P.,
2003. Nestin is expressed in vascular endothelial cells in the adult
human pancreas. J. Histochem. Cytochem. 51, 697–706.
Lardon, J., Rooman, I., Bouwens, L., 2002. Nestin expression in pancreatic
stellate cells and angiogenic endothelial cells. Histochem. Cell Biol.
117, 535–540.
Lendahl, U., Zimmerman, L.B., McKay, R.D., 1990. CNS stem cells
express a new class of intermediate filament protein. Cell 60, 585–595.
Li, L., Mignone, J., Yang, M., Matic, M., Penman, S., Enikolopov, G.,
Hoffman, R.M., 2003. Nestin expression in hair follicle sheath
progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 100, 9958–9961.
Lumelsky, N., Blondel, O., Laeng, P., Velasco, I., Ravin, R., McKay, R.,
2001. Differentiation of embryonic stem cells to insulin-secreting
structures similar to pancreatic islets [erratum appears in Science 2001
Jul;293(5529):428]. Science 292, 1389–1394.
Messing, A., 1999. Nestin in the liver—lessons from the brain [comment].
Hepatology 29, 602–603.
Mokry, J., Nemecek, S., 1998a. Angiogenesis of extra- and intraembryonic
blood vessels is associated with expression of nestin in endothelial cells.
Folia Biol. 44, 155–161.
Mokry, J., Nemecek, S., 1998b. Immunohistochemical detection of
intermediate filament nestin. Acta Medica (Hradec Kralove) 41, 73–80.
Mokry, J., Nemecek, S., 1999. Cerebral angiogenesis shows nestin expression
in endothelial cells. Gen. Physiol. Biophys. 18 (Suppl. 1), 25–29.
Naya, F.J., Huang, H.P., Qiu, Y., Mutoh, H., DeMayo, F.J., Leiter, A.B.,
Tsai, M.J., 1997. Diabetes, defective pancreatic morphogenesis, and
abnormal enteroendocrine differentiation in BETA2/neuroD-deficient
mice. Genes Dev. 11, 2323–2334.
Niki, T., Pekny, M., Hellemans, K., Bleser, P.D., Berg, K.V., Vaeyens, F.,
et al., 1999. Class VI intermediate filament protein nestin is induced
during activation of rat hepatic stellate cells [comment]. Hepatology 29,
520–527.
Obata, J., Yano, M., Mimura, H., Goto, T., Nakayama, R., Mibu, Y., et al.,
2001. p48 subunit of mouse PTF1 binds to RBP-Jkappa/CBF-1, the
intracellular mediator of Notch signalling, and is expressed in the neural
tube of early stage embryos. Genes Cells 6, 345–360.
Percival, A.C., Slack, J.M., 1999. Analysis of pancreatic development using
a cell lineage label. Exp. Cell Res. 247, 123–132.
Pictet, R.L., Clark, W.R., Williams, R.H., Rutter, W.J., 1972. An
ultrastructural analysis of the developing embryonic pancreas. Dev.
Biol. 29, 436–467.
Piper, K., Ball, S.G., Turnpenny, L.W., Brickwood, S., Wilson, D.I., Hanley,
N.A., 2002. Beta-cell differentiation during human development does not
rely on nestin-positive precursors: implications for stem cell-derived
replacement therapy. Diabetologia 45, 1045–1047.
Rajagopal, J., Anderson, W.J., Kume, S., Martinez, O.I., Melton, D.A.,
2003. Insulin staining of ES cell progeny from insulin uptake. Science
299, 363.
Sander, M., Sussel, L., Conners, J., Scheel, D., Kalamaras, J., Dela Cruz, F.,
et al., 2000. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the
major pathway of beta-cell formation in the pancreas. Development
127, 5533–5540.
Scharfmann, R., 1997. Neurotrophin and neurotrophin receptors in islet
cells. Horm. Metab. Res. 29, 294–296.
Selander, L., Edlund, H., 2002. Nestin is expressed in mesenchymal and not
epithelial cells of the developing mouse pancreas. Mech. Dev. 113,
189–192.
Slack, J.M., 1995. Developmental biology of the pancreas. Development
121, 1569–1580.
Soriano, P., 1999. Generalized lacZ expression with the ROSA26 Cre
reporter strain [letter]. Nat. Genet. 21, 70–71.
Sosa-Pineda, B., Chowdhury, K., Torres, M., Oliver, G., Gruss, P., 1997.
The Pax4 gene is essential for differentiation of insulin-producing beta
cells in the mammalian pancreas. Nature 386, 399–402.
Sussel, L., Kalamaras, J., Hartigan-O’Connor, D.J., Meneses, J.J., Pedersen,
R.A., Rubenstein, J.L., German, M.S., 1998. Mice lacking the
homeodomain transcription factor Nkx2.2 have diabetes due to arrested
differentiation of pancreatic beta cells. Development 125, 2213–2221.
Treutelaar, M.K., Skidmore, J.M., Dias-Leme, C.L., Hara, M., Zhang, L.,
Simeone, D., et al., 2003. Nestin-lineage cells contribute to the
microvasculature but not endocrine cells of the islet. Diabetes 52,
2503–2512.
Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P.C.,
et al., 1999. Disruption of the glucocorticoid receptor gene in the
nervous system results in reduced anxiety. Nat. Genet. 23, 99–103.
Trumpp, A., Depew, M.J., Rubenstein, J.L., Bishop, J.M., Martin, G.R.,
1999. Cre-mediated gene inactivation demonstrates that FGF8 is
required for cell survival and patterning of the first branchial arch.
Genes Dev. 13, 3136–3148.
Turque, N., Plaza, S., Radvanyi, F., Carriere, C., Saule, S., 1994. Pax-QNR/
Pax-6, a paired box- and homeobox-containing gene expressed in
neurons, is also expressed in pancreatic endocrine cells. Mol.
Endocrinol. 8, 929–938.
Vaittinen, S., Lukka, R., Sahlgren, C., Hurme, T., Rantanen, J., Lendahl, U.,
et al., 2001. The expression of intermediate filament protein nestin as
related to vimentin and desmin in regenerating skeletal muscle.
J. Neuropathol. Exp. Neurol. 60, 588–597.
Wells, J.M., Melton, D.A., 1999. Vertebrate endoderm development. Annu.
Rev. Cell Dev. Biol. 15, 393–410.
Yang, J., Bian, W., Gao, X., Chen, L., Jing, N., 2000. Nestin expression
during mouse eye and lens development. Mech. Dev. 94, 287–291.
Zimmerman, L., Parr, B., Lendahl, U., Cunningham, M., McKay, R.,
Gavin, B., et al., 1994. Independent regulatory elements in the nestin
gene direct transgene expression to neural stem cells or muscle
precursors [erratum appears in Neuron 1994 Jun;12(6):following 1388].
Neuron 12, 11–24.
Zimmermann, A., Gloor, B., Kappeler, A., Uhl, W., Friess, H., Buchler,
M.W., 2002. Pancreatic stellate cells contribute to regeneration early
after acute necrotising pancreatitis in humans. Gut 51, 574–578.
Zulewski, H., Abraham, E.J., Gerlach, M.J., Daniel, P.B., Moritz, W.,
Muller, B., et al., 2001. Multipotential nestin-positive stem cells
isolated from adult pancreatic islets differentiate ex vivo into pancreatic
endocrine, exocrine, and hepatic phenotypes. Diabetes 50, 521–533.
A. Delacour et al. / Mechanisms of Development 121 (2004) 3–1414
Related Documents