Chapter 18: Lipids Spencer L. Seager Michael R. Slabaugh www.cengage.com/chemistry/seager Jennifer P. Harris

Chapter 18: Lipids Spencer L. Seager Michael R. Slabaugh Jennifer P. Harris.
Jan 01, 2016
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Chapter 18:Lipids
Spencer L. SeagerMichael R. Slabaugh
www.cengage.com/chemistry/seager
Jennifer P. Harris
IMPORTANT FUNCTIONS OF LIPIDS
• Protective wax coatings found on some plants• Energy-rich compounds with low densities• Storage form of energy for plants and animals• Structural components, especially in cellular membrane
formation
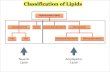
LIPID CLASSIFICATION (continued)
• Saponifiable lipids contain an ester that can undergo basic hydrolysis.• Triglycerides, waxes, phospholipids, and sphingolipids• Simple lipids contain a fatty acid and alcohol.• Triglycerides and waxes
• Complex lipids contain a fatty acid, alcohol, and other components.• Phospholipids and sphingolipids
• Nonsponifiable lipids do not contain an ester and cannot be hydrolyzed.• Steroids and prostaglandins
FATTY ACIDS• Fatty acids:• are the building blocks of many lipids.• are long chain carboxylic acids.• have long nonpolar tails responsible for fatty/oily
characteristics.• The carboxyl group is very hydrophilic under conditions of
physiological pH (exists as –COO-).
FATTY ACIDS (continued)• In water, fatty acids will form spherical clusters called
micelles.• Micelles are important for biological functions, like the
transport of insoluble lipids in the blood.
FATTY ACIDS (continued)• Fatty acids are:• usually straight chains (no branching).
• usually from C10 to C20.
• usually an even number of carbons.• either saturated (no C=C bonds) or unsaturated (has C=C
bonds, usually in the cis configuration). • Examples of saturated, monounsaturated, and
polyunsaturated fatty acids containing 18 carbon atoms include:
FATTY ACIDS (continued)• Fatty acids with C=C bonds
cannot pack closely together because of shape. This leads to decreased intermolecular attractions and lower melting points.
• Fatty acid melting points decrease as the number of C=C bonds increases.
• Most unsaturated fatty acids are liquids at room temperature.
FATTY ACIDS (continued)• Essential fatty acids are those needed by the body, but not
synthesized within the body in adequate amounts.
• For humans, linoleic and linolenic acid are essential, but easily obtainable from plant and fish oils.
FATTY ACIDS (continued)• Linoleic (an omega-3 fatty acid) and linolenic (an omega-6
fatty acid) acids:• are used to produce hormonelike substances that regulate
a wide range of functions and characteristics, including:• e.g. blood pressure, blood clotting, blood lipid levels,
the immune response, and the inflammation response to injury and infection.
• can be converted to other omega-3 and omega-6 fatty acids.
STRUCTURE OF FATS AND OILS• Fats are:• usually from animal sources.• solids at room temperature.• usually composed to a high degree of saturated fatty
acids.
• Oils are:• usually from plant and fish sources.• liquids at room temperature.• usually composed of more unsaturated fatty acids than
fats.
STRUCTURE OF FATS AND OILS (continued)
• Fats and oils are triglycerides (triacylglycerols) which are triesters of glycerol.
STRUCTURE OF FATS AND OILS (continued)
• Fatty acid components in naturally occurring triglyceride molecules are rarely identical.
• Natural triglycerides are usually mixtures of different triglyceride molecules.
STRUCTURE OF FATS AND OILS (continued)
• Excessive fat in the diet:• is recognized as a risk factor influencing the development
of chronic disease.• is a concern because of its role in raising blood cholesterol
levels.• High cholesterol is a risk factor in the development of
coronary heart disease (leading cause of death in Americans every year).
REACTIONS OF FATS AND OILS• Hydrolysis is important for fat and oil digestion.• Enzymes (lipases) can catalyze the hydrolysis process.
REACTIONS OF FATS AND OILS (continued)
• Saponification is the commercial production of the salts of fatty acids (soaps).
REACTIONS OF FATS AND OILS (continued)
• Soaps depend on the base used for saponification.• Sodium salts (hard salts) found in cake soap.• Potassium salts (soft soaps) found in shaving creams and
liquid soap preparations.
• Traditional soap making:• uses animal fat is the source of triglycerides.• uses lye (crude NaOH) or an aqueous extract of wood
ashes is the source of the base.• was lost with fall of Roman Empire.• The soapless centuries AD 500-1500 resulted in
devastating plagues in an unsanitary western Europe.
REACTIONS OF FATS AND OILS (continued)
• Hydrogenation decreases the degree of unsaturation and is used to make margarines from oils.
• Complete hydrogenation results in a hard and waxy product.• Partial hydrogenation results in a smooth, creamy product
that is desired by consumers.• Isomerization of cis to trans fatty acids will occur during this
process.• Current dietary advice includes reducing the consumption of
saturated and trans fatty acids.
WAXES• Waxes are esters of fatty acids and long chain alcohols (12-
32 carbon atoms). They are:• water insoluble and not easily hydrolyzed.• often found in protective coatings.• used commercially to make cosmetics, candles,
ointments, and protective polishes.
PHOSPHOGLYCERIDES• A phosphoglyceride is a phospholipid that contains glycerol,
fatty acids, phosphoric acid, and an alcohol (usually an amino alcohol).
PHOSPHOGLYCERIDES (continued)
• Lecithins:• contain the amino alcohol choline.• are an important cell membrane component.• are emulsifying and micelle-forming agents.
• Cephalins:• contain ethanolamine or serine as the alcohol.• are found in most cell membranes, especially brain tissue
and blood platelets (role in blood-clotting process).
Related Documents