, 20130403, published 10 November 2014 369 2014 Phil. Trans. R. Soc. B Sonja A. Kotz, Anika Stockert and Michael Schwartze mental model Cerebellum, temporal predictability and the updating of a References http://rstb.royalsocietypublishing.org/content/369/1658/20130403.full.html#related-urls Article cited in: http://rstb.royalsocietypublishing.org/content/369/1658/20130403.full.html#ref-list-1 This article cites 60 articles, 12 of which can be accessed free Subject collections (377 articles) cognition Articles on similar topics can be found in the following collections Email alerting service here right-hand corner of the article or click Receive free email alerts when new articles cite this article - sign up in the box at the top http://rstb.royalsocietypublishing.org/subscriptions go to: Phil. Trans. R. Soc. B To subscribe to on November 10, 2014 rstb.royalsocietypublishing.org Downloaded from on November 10, 2014 rstb.royalsocietypublishing.org Downloaded from

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
, 20130403, published 10 November 2014369 2014 Phil. Trans. R. Soc. B Sonja A. Kotz, Anika Stockert and Michael Schwartze mental modelCerebellum, temporal predictability and the updating of a
References
http://rstb.royalsocietypublishing.org/content/369/1658/20130403.full.html#related-urls Article cited in:
http://rstb.royalsocietypublishing.org/content/369/1658/20130403.full.html#ref-list-1
This article cites 60 articles, 12 of which can be accessed free
Subject collections (377 articles)cognition �
Articles on similar topics can be found in the following collections
Email alerting service hereright-hand corner of the article or click Receive free email alerts when new articles cite this article - sign up in the box at the top
http://rstb.royalsocietypublishing.org/subscriptions go to: Phil. Trans. R. Soc. BTo subscribe to
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
rstb.royalsocietypublishing.org
ResearchCite this article: Kotz SA, Stockert A,
Schwartze M. 2014 Cerebellum, temporal
predictability and the updating of a mental
model. Phil. Trans. R. Soc. B 369: 20130403.
http://dx.doi.org/10.1098/rstb.2013.0403
One contribution of 14 to a Theme Issue
‘Communicative rhythms in brain and
behaviour’.
Subject Areas:cognition
Keywords:cerebellum, lesion, timing, prediction,
forward model, event-related potentials
Author for correspondence:Sonja A. Kotz
e-mail: [email protected]
†These authors have contributed equally to
this work.
& 2014 The Author(s) Published by the Royal Society. All rights reserved.
Cerebellum, temporal predictability andthe updating of a mental model
Sonja A. Kotz1,2,†, Anika Stockert2,3 and Michael Schwartze1,†
1School of Psychological Sciences, University of Manchester, Brunswick Street, Manchester M13 9PL, UK2Department of Neuropsychology, Max Planck Institute for Human Cognitive and Brain Sciences, Stephanstrasse1a, 04103 Leipzig, Germany3Language and Aphasia Laboratory, University of Leipzig, Liebigstrasse 20, 04103 Leipzig, Germany
We live in a dynamic and changing environment, which necessitates that we
adapt to and efficiently respond to changes of stimulus form (‘what’) and
stimulus occurrence (‘when’). Consequently, behaviour is optimal when we
can anticipate both the ‘what’ and ‘when’ dimensions of a stimulus. For
example, to perceive a temporally expected stimulus, a listener needs to estab-
lish a fairly precise internal representation of its external temporal structure, a
function ascribed to classical sensorimotor areas such as the cerebellum. Here
we investigated how patients with cerebellar lesions and healthy matched con-
trols exploit temporal regularity during auditory deviance processing. We
expected modulations of the N2b and P3b components of the event-related
potential in response to deviant tones, and also a stronger P3b response
when deviant tones are embedded in temporally regular compared to irregu-
lar tone sequences. We further tested to what degree structural damage to the
cerebellar temporal processing system affects the N2b and P3b responses
associated with voluntary attention to change detection and the predictive
adaptation of a mental model of the environment, respectively. Results
revealed that healthy controls and cerebellar patients display an increased
N2b response to deviant tones independent of temporal context. However,
while healthy controls showed the expected enhanced P3b response to deviant
tones in temporally regular sequences, the P3b response in cerebellar patients
was significantly smaller in these sequences. The current data provide evi-
dence that structural damage to the cerebellum affects the predictive
adaptation to the temporal structure of events and the updating of a mental
model of the environment under voluntary attention.
1. IntroductionThe cerebellum has featured prominently in past and recent attempts to identify
the neural basis of temporal processing and to understand how the brain rep-
resents and uses temporal structure [1–5]. However, this line of research is one
of many contributing to a growing body of evidence that the cerebellum is
engaged in numerous cognitive functions including speech and music [6–9]
and the question arises how specific these functions are. One possible way to
delineate these functions is to have a closer look at cerebello-cortical connections
that may allow specification of their respective contribution to cognition. For
example, some of these connections manifest links between the cerebellum and
other brain areas associated with temporal processing such as the basal ganglia
or the supplementary motor area (SMA) [10–14]. Increasingly more fine-grained
anatomical differentiations corroborate assumptions of divergent, and also
shared, operations in motor (production) and non-motor (perception, cognition)
functions in brain areas such as the cerebellar dentate nucleus and its motor and
non-motor sub-compartments, the caudate/putamen of the basal ganglia or the
subdivisions of the SMA [15–18].
Temporal processing is considered fundamental to neurocognitive oper-
ations underlying motor and non-motor function(s) relevant in domains such
as speech and music to optimize overt and covert behaviour. Importantly, opti-
mal timing implies a form of predictive adaptation to the temporal structure of
events in the environment to circumvent exclusively reactive behaviour. It is
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
2
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
therefore not surprising that ‘prediction’ is another neurocog-
nitive operation that has been associated with the cerebellum
[19–21]. However, efficient prediction and adaptation in be-
haviour requires adequate internal representations of both
the formal structure (‘what’) and the temporal structure
(‘when’) of events in the environment. In other words, infor-
mation pertaining to the form or identity of events has to be
encoded alongside information that relates to the rhythm or
temporal locus of events [22]. Turning to concrete examples,
we can ask how musicians coordinate their play while creat-
ing music together. Recognizing the absence of sound in a
musical piece marks the start of a pause. Prior information
about the duration of a pause allows the musicians to predic-
tively adapt their behaviour and to resume playing together at
the right point in time. In the absence of an external reference,
such as the arm movement of a conductor, the behavioural
benefit granted by temporal prediction is determined byan indi-
vidual’s ability to exploit regular rhythms and temporal
relations such as estimating a pause’s duration. Similarly, we
can consider how two people communicate with each other.
Here, lengthening of a phrase’s final word or syllable, followed
by a pause, may (among other factors) indicate that one social
partner signals the opportunity for the other partner to take a
turn at talk. The combination of verbal and non-verbal temporal
cues as described above allows the communicative partners to
predictively adapt to each other and to take turns at the correct
point in time to ensure optimized communication flow.
One way to investigate the operations underlying the ability
to use temporal structure in order to optimize behaviour is to
infer function from pathological dysfunction. Here we con-
sidered structural damage to the cerebellum, which should
affect precise event-based perceptual encoding of temporal struc-
ture [23,24]. Thus, structural damage to the cerebellum should
disrupt how one efficiently extracts and uses temporal regularity
to generate predictions about the temporal locus of an upcoming
event and may further lead to suboptimal performance in a
number of cognitive tasks. To explore this potential link between
temporal predictability and optimal performance, we tested how
temporal predictability influences auditory deviance processing
under voluntary attention in patients with cerebellar lesions and
healthy controls. Deviance processing provides a relatively
simple framework, which should make it possible to probe
how patients and healthy controls exploit temporal regularity
and the temporal predictability associated with it in optimal
tone perception.
Broadly speaking, deviance processing refers to a set of
neurocognitive operations associated with change. For example,
how does one perceive a change in tone frequency or duration,
while expecting a particular tone frequency or duration that has
been previously established? Deviance processing is classic
in event-related potential (ERP) research. Different stages of
deviance processing are reflected in early responses associated
with sensory processing and progressively later responses
associated with cognitive operations [25,26]. Critically, deviance
processing makes it possible to test the interaction of formal
and temporal structure by varying formal stimulus properties
(e.g. tone frequency) and temporal regularity (e.g. when a
tone occurs in a tone sequence) in one single experimental
setting. In addition, the impact of temporal regularity on
deviance processing (e.g. optimal detection of deviance) is
linked to a number of well-established ERP components.
Consequently, we set out to test patients with cerebellar
lesions and healthy, age-matched controls using an auditory
oddball paradigm, in which frequent standard and less frequent
deviant tones are presented in either a temporally regular or irre-
gular context. This particular approach is based on previous
work, in which pre-attentive deviance processing as indexed
by the P50, N1, mismatch negativity (MMN), P3a and re-
orienting negativity was contrasted with attentive deviance
processing as indexed by the P50, N1, N2b and P3b ERP
components [24,27]. Statistically indifferent ERP responses
obtained in the pre-attentive context suggest that pre-attentive
deviance processing is relatively robust against the manipu-
lation of temporal regularity [24,27], while amplitude
modulations in the P3 range for attentive deviance processing
may indicate an interaction of formal and temporal stimulus
properties at this processing stage [24]. Considering the active
experimental task (counting of deviant tones) and the centro-
parietal topographical distribution of the effect, this finding
was interpreted as an attention-dependent differentiation of
the P3b component. More specifically, deviant tones presented
in a temporally regular context evoked a larger P3b response
(amplitude enhancement) in comparison with physically identi-
cal deviant tones presented in a temporally irregular context.
Similar findings have been obtained with linguistic stimuli
[28], suggesting that the P3b enhancement in response to devi-
ant stimuli in temporally regular stimulus sequences may
reflect the impact of temporal structure and temporal predict-
ability on deviance processing. The fact that P3b enhancement
is reported for lower level auditory stimuli (tones) as well as
more complex linguistic stimuli suggests that these operations
in deviance processing apply to a number of cognitive functions
including speech and music. As a result, it is important to under-
stand how structural damage to the cerebellar temporal
processing system affects the precise encoding of the temporal
structure of successive events and how this may affect the
efficient adaptation to a dynamic environment.
In this study, participants listened attentively to tone
sequences, and counted deviant tones while their electro-
encephalogram (EEG) was recorded. We focused on two ERP
responses in this context. The fronto-centrally distributed
N2b response is typically associated with the detection of a
deviant event, whereas the predominant theoretical account
for the more centro-parietally distributed P3b links this com-
ponent to the updating of a mental model of the
environment in response to a deviant event [29–31]. The
exact nature of this mental model remains elusive at this
stage. However, the P3b is typically believed to instantiate a
neural signature of a change in this model, conceived as a gen-
eral schema of all available data in the environment and of the
stimulus context, in particular [29,31]. With respect to the odd-
ball paradigm used here this suggests that the model
established by frequent standard tones has to be transformed
(updated) when an infrequent deviant tone is encountered,
reflecting the mediation between the participants’ expectancies
and sensory input [32,33]. We expected that healthy controls
would show an N2b response to deviant tones in both types
of sequences and a further enhanced P3b response to deviant
tones in temporally regular compared with irregular tone
sequences [24] reflecting the beneficial effects of temporal regu-
larity on deviance processing and, in turn, effective updating of
a mental model of the environment. Patients with cerebellar
lesions, who are expected to be less sensitive to temporal regu-
larity owing to increased system noise and uncertainty in the
event-based encoding of temporal structure, should not show
a similarly beneficial effect of temporal regularity. Thus,
11
9
7
5
3
1
L ÍR
–53 –42 –33 –23 –12
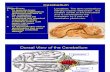
Figure 1. Cerebellar lesion distribution. Lesions were manually delineated on normalized T1-weighted magnetic resonance (MR) scans of each patient and super-imposed on a single subject template in Montreal Neurological Institute (MNI) space. MNI coordinates are displayed; the orthogonal view illustrates correspondingaxial planes. The colour bar specifies the number of patients with overlapping lesions in each voxel. Purple shades indicate voxels affected in at least one patient,whereas blue shades reflect maximal lesion overlap (maximum N ¼ 3). The respective lesions are distributed over both cerebellar hemispheres, extending to thevermis, cerebellar peduncles and the midbrain. L/R indicates left and right hemisphere. (Online version in colour.)
Table 1. Patient history. m/f, male or female; edu., education (in years); HI, handedness index according to Edinburgh Handedness Inventory [34]; TSL, timesince lesion (in months); vol., volume (in cc). Lesion aetiology: AVM, arteriovenous malformation; SCA, superior cerebellar artery; PICA, posterior inferiorcerebellar artery; ICH, intracranial haemorrhage. Descriptions of lesion locations refer to the MRI atlas nomenclature of the human cerebellum [35]; MCP, middlecerebellar peduncle; SCP, superior cerebellar peduncle.
no. m/f age HI edu. TSL vol. aetiology lesion location
1 m 25 þ100 8 93 48.00 AVM, ICH bilateral lobule VIIB, crus I/II, vermis (VI –
VIII)
2 m 30 þ100 8 108 30.12 SCA aneurysm, ICH left lobule III – VI, crus I, SCP, MCP
3 m 45 þ100 10 77 4.88 basilar artery aneurysm, SCA
infarction
right lobule III – VI, medial thalamus, vermis
(III – V) SCP, left crus I
4 m 35 þ50 12 120 9.00 cerebellopontine angle tumour
resection
right lobule IV – VIII, X, crus I/II, MCP
5 m 59 þ80 10 28 7.13 PICA infarction right lobule VIIB – IX, crus I/II
6 f 39 þ80 10 35 0.29 PICA infarction right crus II
7 f 59 þ100 8 36 5.67 SCA infarction right lobule IV – VI, crus I
8 f 45 þ100 12 71 7.85 PICA infarction left lobule VIIB – IX, crus II, right crus I/II
9 m 56 þ100 10 36 14.50 PICA infarction left lobule VIIB – IX, crus II
10 f 33 þ100 12 40 8.84 medulloblastoma resection right lobule VIIB – VIIIB, crus II, vermis (IV –
VIIIB), SCP
11 f 50 þ100 10 16 2.43 PICA infarction right lobule VIIIA – IX
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
3
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
while an N2b response to deviance per se may be comparable
with healthy controls, the P3b responses to deviant tones in
temporally regular and irregular tonal sequences should be
dissimilar, substantiating the idea that temporal predictability
cannot be efficiently applied to optimally update the mental
model. We therefore addressed two core questions in the cur-
rent experiment: (i) Does temporal predictability influence
deviance processing under voluntary attention in a similar
fashion in healthy ageing controls as previously observed in
young healthy participants? (ii) How does structural damage
to the cerebellum affect the potentially optimizing influence
of temporal predictability on deviance processing and the
updating of a mental model as indicated by N2b and P3b?
2. Material and methods(a) ParticipantsEleven patients with cerebellar lesions (figure 1 and table 1) and a
corresponding number of healthy controls participated in the
study. Healthy controls matched the patients in terms of gender
(five women), age (mean 43.3+11.9 years), handedness (all
right handed) and education (10+1.6 years). All participants
gave their informed written consent regarding the experiment
and received a compensatory fee. Patients were recruited through
the database of the Clinic for Cognitive Neurology at the Univer-
sity Hospital of Leipzig. Healthy controls were recruited from
the participant database of the Max Planck Institute for Human
Cognitive and Brain Sciences in Leipzig.
(b) Magnetic resonance imaging and lesion mappingHigh-resolution T1-weighted magnetic resonance (MR) scans were
obtained at 3 T with a Siemens TrioTim (Siemens Healthcare,
Erlangen, Germany) or a Bruker BioSpin (BioSpin GmbH, Rhein-
stetten, Germany) MR system with a 32-channel phased-array
head array coil using an MP-RAGE sequence [36]. The resulting
images were segmented and spatially normalized to the Montreal
Neurological Institute (MNI) space by means of the unified
segmentation approach [37] as implemented in SPM (SPM8,
Wellcome Department of Imaging Neuroscience, London, UK,
http://www.fil.ion.ucl.ac.uk/spm). The MRIcron software pack-
age [38] was used to manually delineate lesions on axial slices of
600 ms
600 versus 660 Hz (ratio 4 : 1)
200–1000 ms (600 ms on average)
(b)
(a)
Figure 2. Visualization of excerpts from the auditory stimulus sequences. Using a variant of the set-up implemented in Schwartze et al. [24], equidurational(300 ms, 10 ms rise and fall) sinusoidal standard (600 Hz, N ¼ 360) and deviant tones (660 Hz, N ¼ 90) are presented in two conditions that differ exclusivelyin their ISI. In the regular condition (a) the ISI are fixed to 600 ms. In the irregular condition (b) the ISI are randomly chosen from a range between 200 and1000 ms.
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
4
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
the normalized T1-weighted images and to create binary lesion
maps for each patient (average lesion volume 12.61 cc, s.d. 14.16
cc). To visualize overall lesion distribution (figure 1), the lesion
maps were superimposed on a scalp-stripped T1-weighted single
subject template in MNI space (Colin27_T1_seg_MNI.nii, available
at http://brainmap.org/ale/index.html).
(c) Stimulus sequences and experimental taskDuring the EEG recording, stimuli were delivered by Presentation
12.0 (Neurobehavioral Systems). Each oddball sequence (figure 2)
consisted of 450 tones in total, corresponding to 360 standard
(600 Hz, 300 ms, 10 ms rise and fall times) tones and 90 deviant
(660 Hz, 300 ms, 10 ms rise and fall times) tones (4 : 1 ratio). In
the regular condition, the inter-stimulus intervals (ISI) were fixed
to 600 ms, while the ISIs were randomly chosen from a range
between 200 and 1000 ms (600 ms on average) in the irregular con-
dition. Randomization constraints ensured that no more than two
deviants were presented in a row and that the first four tones of
each sequence were standards in order to allow participants to
establish a memory trace for these tones. The type of initial
sequence presented was counterbalanced across participants. A
short excerpt of this sequence was presented to the participants
in order to familiarize them with the different tone types. During
the experiment, participants listened to the tones presented via
loudspeakers and silently counted the number of deviant tones
embedded in a sequence. The counting task was employed in
order to make sure that the participants directed their attention
towards the stimulus sequence. This conforms to a cognitive task
with no motor aspect during the recording interval, in which
any brain potential confound owing to impaired motor control
in the cerebellar patients should be absent or at least minimal.
Participants were not informed about the nature of the temporal
context that tones could occur in. They reported one final count
at the end of each type of sequence to which they had listened.
A short pseudo-randomized sequence consisting of 12 additional
tones (7 standards and 5 deviants) was attached to the irregular
sequence to obtain different correct count values for the two con-
ditions (90 regular versus 95 irregular) in order to prevent
accurate task performance being achieved by simply reporting
the same value twice. These additional tones were disregarded
from all further EEG analyses.
(d) Electroencephalogram recording and analysisThe EEG recordings took place in a dimly lit sound-attenuated
booth. Participants sat in front of a computer screen, which dis-
played a fixation cross throughout the session. The EEG was
recorded at a sampling rate of 500 Hz from 25 Ag/AgCl scalp elec-
trodes mounted in an elastic cap and arranged according to the
standard 10–20 International System. Additional horizontal and
vertical electrooculography was recorded by means of four separ-
ate electrodes. The ground electrode was placed on the sternum
and electrodes placed on the left and right mastoids served as
reference during the recording.
Data pre-processing and ERP analyses were performed using
the EEP 3.2 software package (Max Planck Institute for Human
Cognitive and Brain Sciences, Leipzig, commercially available
as EEProbe, ANT Neuro). Raw data were re-referenced offline
to averaged mastoids. Epochs lasting from 2100 to 450 ms rela-
tive to stimulus onset were averaged for standards and deviants
for each condition per participant and across participants. All
epochs of tones that immediately followed the presentation of a
deviant tone were rejected. Automatic rejection was applied to
artefacts exceeding 30 mV at eye-channels or 40 mV at electrode
CZ. To increase the number of eligible trials for both groups, pro-
totypical artefacts reflecting eye blinks and saccades identified
via electrooculography were used to obtain propagation factors,
which were then used to compensate for similar artefacts in the
remaining trials via a regression algorithm (Electrooculogram
Epoch Classification), implemented in the EEP software.
The ERPs of interest were analysed in four regions of interest
(ROIs) to assess both the typical fronto-central and centro-parietal
scalp distributions of the N2b and P3b components as well as
potential hemispheric differences of component distribution as a
result of cerebellar pathology. These ROIs covered left-anterior
(F7, F3, FT7, FC3), right-anterior (F8, F4, FT8, FC4), left-posterior
(T7, C3, CP5, P3) and right-posterior (T8, C4, CP6, P4) electrode
positions. A 2 � 2 � 2 � 2 � 2 ANOVA with a between-factor
group (patients versus controls) and within-factors temporal struc-
ture (regular versus irregular), formal structure (standard versus
deviant), hemisphere (left versus right) and region (anterior
versus posterior) was conducted using SAS 9.3 (SAS Institute
Inc.) for a 50 ms time-window lasting from 198 to 248 ms (N2b)
and a 130 ms time-window lasting from 298 to 428 ms (P3b) rela-
tive to the stimulus onset, respectively. These time-windows
were selected after visual inspection of the data and included the
peak of each component across groups and conditions. Thus,
N2b was analysed from the transition into a negative-going deflec-
tion until the earliest crossing into positive voltages (which was
found in response to regular deviants in controls; figure 3), while
P3b was analysed from the transition into a positive-going deflec-
tion until the latest transition into a negative-going deflection
(found in response to regular deviants in patients).
3. Results(a) Behavioural resultsBoth controls (regular mean value reported 89.36, s.d. 1.29,
range 87–91; irregular mean 94.55, s.d. 0.82, range 93–96)
and patients (regular mean 88.82, s.d. 2.18, range 86–92; irregu-
lar mean 94.45, s.d. 0.82, range 93–96) performed well in the
counting task, which is also indicated by comparably high
regular standard
irre
gula
rre
gula
rir
regu
lar
regu
lar
regular deviantirregular standardirregular deviant
–8.0 +8.0µV
CZ
CZ
N2 198–248 ms
450 ms
P3
N2
N1
–100 ms
–8 µV
8
P3 298–428 ms
CZ
cont
rols
patie
nts
Figure 3. Group-averaged EEG data for healthy controls and patients. Data recorded from a representative electrode (CZ) are shown on the left side. A 7 Hz low-passfilter was applied offline for graphical display only. Topographical maps on the right side illustrate the distribution of the corresponding N2b and P3b effects, i.e.voltage differences obtained by subtracting responses to standard events from responses to deviant events in each experimental condition. (Online version in colour.)
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
5
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
percentages of accuracy for controls (regular 99%, s.d. 1.1; irre-
gular 99%, s.d. 0.7) and patients (regular 98%, s.d. 1.4; irregular
99%, s.d. 0.7). One-sampled t-tests were conducted to test for
differences between the values provided by the participants
and the actual number of deviants embedded in each sequence
(90 regular, 95 irregular). The results were non-significant in the
control group (regular: t(10)¼ 21.64, p¼ 0.132; irregular:
t(10)¼ 21.84, p ¼ 0.096) and in the patient group, even
though there was a strong trend in the irregular context (regular:
t(10)¼ 21.80, p¼ 0.103; irregular: t(10)¼ 22.21, p¼ 0.052).
Taken together the behavioural results confirm that both healthy
controls and cerebellar patients paid attention to the task
requirements and executed the task appropriately.
(b) Event-related potential resultsVisual inspection of the averaged group ERP data confirmed
the typical component evolution associated with attentive
deviance processing, and also suggested qualitative com-
ponent differences between the two groups (figures 3 and 4).
While the N2b and P3b responses (components of empirical
interest) to standard tones seemed to be comparable between
both groups independent of temporal context, the responses
to deviant tones seemed to vary as a function of temporal con-
text. The N2b response to deviants embedded in irregular
temporal structure appeared similar across groups, but the
difference between regular and irregular temporal structure
seemed to be more pronounced in the patient than the control
group. The P3b was generally larger for deviants embedded in
regular temporal structure, but was much reduced in the
patient group compared with the control group.
Further, in comparison to controls, the patient group also
appeared to show more pronounced differences between
standards and deviants already in the N1 range, with
highly similar responses for standards embedded in regular
and irregular temporal structure. N1 responses in controls
followed a similar pattern as previously reported for younger
healthy participants [27]. However, complementary statistical
analyses in the N1 range did not confirm any significant
group differences.1
Likewise, the repeated-measures ANOVA of the N2b
time-window did not fully confirm the initial visual inspec-
tion. Results showed a strong trend towards a main effect
of temporal structure (F1,20 ¼ 4.30, p ¼ 0.051), while
there was a significant main effect of formal structure
(F1,20 ¼ 8.63, p , 0.01). There was a significant interaction
of temporal structure � hemisphere (F1,20 ¼ 6.12, p , 0.03),
indicating a global effect of temporal structure over right
(F1,20 ¼ 7.49, p , 0.02) but not left hemisphere electrode
sites (F1,20 ¼ 1.18, p ¼ 0.288). However, the three-way
3.00
2.00
1.00
0
–1.00
–2.00
–3.00
7.00
6.00
5.00
4.00
3.00
2.00
1.00
0
–1.00
–2.00P3b 95% Cl lower upper
N2b 95% Cl lower upper
PAT
_RE
G_D
EV
PAT
_RE
G_S
TA
PAT
_IR
R_D
EV
PAT
_IR
R_S
TA
CO
N_R
EG
_DE
V
CO
N_R
EG
_STA
CO
N_I
RR
_DE
V
CO
N_I
RR
_STA
–1.00
2.00
–0.74 –0.73–0.49 –0.41
2.51
1.31
4.47
0.62
–0.30
0.77
–0.31
0.78
0.05
1.30
Figure 4. Ninety-five percentage confidence intervals for N2b and P3b responses in both participant groups. REG, regular condition; IRR, irregular condition; PAT,patients; CON, controls; STA, standards; DEV, deviants. (Online version in colour.)
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
6
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
interaction of temporal structure � formal structure �hemisphere approached statistical significance (F1,20 ¼ 4.06,
p ¼ 0.058) in accordance with the initial visual inspection.
Of note is the lack of a significant main effect of group
(F1,20 ¼ 0.54, p ¼ 0.473) or interaction involving the factor
group (all p . 0.20). In order to explore a potential relation
between the extents of structural damage (i.e. lesion
volume) and the electrophysiological response, individual
lesion volumes were correlated with individual mean N2b
amplitudes obtained at electrode CZ for patients in all four
conditions. However, this procedure yielded no significant
result for regular standards (r ¼ 0.06, p ¼ 0.87) or deviants
(r ¼ 0.12, p ¼ 0.72) nor for irregular standards (r ¼ 0.12, p ¼0.73) or deviants (r ¼ 0.22, p ¼ 0.53). Thus, the current findings
verify the presence of a typical N2b response to deviant tones
in both groups independent of temporal context.
Analysis of the P3b time-window revealed significant main
effects of temporal structure (F1,20 ¼ 20.66, p , 0.001), formal
structure (F1,20 ¼ 38.26, p , 0.0001) and region (F1,20¼ 11.71,
p , 0.01) but not of group (F1,20 ¼ 2.04, p ¼ 0.169). However,
there was a significant interaction of group � temporal
structure � formal structure (F1,20 ¼ 7.59, p , 0.02). Informed
by our hypotheses, a subsequent step-down analysis by the
factor formal structure revealed an interaction of group � tem-
poral structure for deviant (F1,20¼ 5.25, p , 0.04) but not for
standard tones (F1,20¼ 0.08, p ¼ 0.785). Further resolution
of the two-way interaction for deviant tones by the factor
temporal structure confirmed that the two groups differed
with regard to their deviance response in the regular tempo-
ral context (F1,20¼ 4.81, p ¼ 0.04) but not in the irregular
context (F1,20 ¼ 1.07, p ¼ 0.312). Patients showed a smaller
P3b response (2.0 mV) relative to healthy controls (4.5 mV).
This difference was confirmed by a paired-samples t-test
(t(10) ¼ 22.78, p ¼ 0.02). To further validate this finding
and to preclude any disproportional influence of individual
patients on the statistical group comparison, identical t-tests
were conducted with individual patient–control pairings
excluded one at a time (N-1 method). However, the result
remained significant in each of these cases (minimum
t ¼ 22.37, p ¼ 0.042). Pursuant to the ANOVA analysis, the
P3b amplitude was correlated with the extent of lesion size.
Similar to the non-significant N2b correlation results, this
P3b correlation was also non-significant for regular standards
(r ¼ 0.15, p ¼ 0.65) and deviants (r ¼ 0.06, p ¼ 0.86), as well
rstb.royalsocietypublishing.orgPhil.T
7
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
as for irregular standards (r ¼ 0.21, p ¼ 0.54) and deviants
(r ¼ 0.15, p ¼ 0.66). Thus, independent of lesion size, these
P3b results provide evidence for the significant impact of
cerebellar pathology on the use of temporal predictability
and the updating of a mental model of the environment as
reflected in the P3b. Moreover, the fact that deviance pro-
cessing was most affected in temporally regular, but not
irregular context substantiates initial assumptions that struc-
tural damage to the cerebellum leads to imprecise encoding
and use of temporal structure, and accordingly to the encod-
ing and use of temporal regularity. This, in turn, affects
optimized deviance processing in the case of maximal
temporal predictability as seen in the control group.
rans.R.Soc.B369:201304034. DiscussionTesting the proposed role of the cerebellum in precise event-
based temporal processing [4,14,39] and attempting to repli-
cate previous findings in healthy young participants [24] in
a healthy aging control cohort, this study investigated the
impact of cerebellar lesions on the interaction of formal struc-
ture (varying stimulus form) and temporal structure (varying
temporal regularity in tone sequences) in auditory deviance
processing. Both cerebellar lesion patients and healthy aging
controls detected deviant tones, as evident in the behavioural
and in the N2b results. However, cerebellar patients showed
a qualitatively different P3b response to deviant tones
embedded in temporally regular sequences as confirmed by
statistically significant group differences. Healthy controls
showed the expected enhanced P3b deviance response to
tones in regular compared with irregular temporal contexts.
These data suggest that healthy aging participants display
optimized deviance processing with full temporal predictabil-
ity under voluntary attention. By contrast, while patients with
cerebellar pathology detect formal stimulus deviance, they are
less capable of using temporal predictability, and presumably,
to exploit this information to optimally update a mental
model of the environment.
The current results substantiate previous findings on how
temporal regularity influences basic neurocognitive operations
linked to deviance processing. In earlier reports, deviance pro-
cessing under voluntary attention was evident in N2b and P3b
responses, but deviance processing in the P3b range varied as a
function of temporal context [24]. While a strong statistical
trend (three-way interaction of temporal structure, formal
structure and hemisphere) in the N2b time-window may indi-
cate that temporal regularity impacts deviance processing at
this earlier processing stage, the current results remain incon-
clusive. Clearly, further research has to confirm whether a
potential interaction of temporal and formal structure in
deviance detection (N2b) is prone to individual differences,
as previous results have not reported a similar trend in compar-
able experimental set-ups (i.e. [24]). Awaiting further evidence,
we can therefore only carefully conclude that patients with cer-
ebellar lesions detect deviance comparably to healthy controls
as evidenced in a similar N2b response to deviant tones
independent of temporal context.
However, we report clear group differences in the P3b
response to deviant tones, which confirms that patients with
cerebellar lesions cannot benefit in a similar way as healthy
controls from temporal predictability. Considering the ‘almost
unquestionable’ [33] agreement of P300 theories on the function
of this component as an index for the interplay of stimulus
expectancies and actual sensory input, it seems appropriate to
interpret this finding as an instance of impaired updating of a
mental model of the environment. This result substantiates
the critical role of the cerebellum in event-based temporal
encoding [4,14,39] and the optimized adaptation to change in
the environment (e.g. [29–31]).
The current findings further suggest that damage to the
cerebellum alters how temporal regularity optimizes the quality
of auditory deviance processing. In this context, deviance
processing under voluntary attention is but one example of a
perceptual process. It remains to be shown to what extent the
current findings generalize to other perceptual (e.g. visual, mul-
tisensory) or even higher-level cognitive (e.g. natural and
emotional speech) processes [14,40,41] and temporally more
complex stimulus qualities such as meter and rhythm in
speech or artistic forms of language such as poetry or rhetorical
speech [40,42].
Importantly, cerebellar lesions diminish the response to
deviants in temporally regular tone sequences, whereas no
such reduction was found in the irregular tone sequences.
While this dissociation of temporal processing as a function
of temporal regularity does not speak against the role of the
cerebellum in event-based encoding of temporal structure
per se, it substantiates assumptions concerning a cerebellar
contribution to perceptual processing in those cases in which
predictive adaptation is possible. However, most likely, the
cerebellar temporal processing system does not perform this
function in isolation, but in concert with other structures impli-
cated in temporal processing, namely the basal ganglia and the
SMA, as part of an integrative temporal processing network.
Importantly, the respective anatomical and functional connec-
tions may provide a means to compensate for dysfunction in
each structure [13,43,44]. While cerebellar lesions may lead to
imprecise encoding of temporal structure, thus reducing the
effect of full temporal predictability, the intact basal ganglia
temporal processing system may partially compensate for
this ‘noisy’ encoding, e.g. by increasing tolerance against tem-
poral variability in the processing of sequences with a regular
beat. However, such mechanisms are most likely highly depen-
dent on the specific temporal characteristics of the input, and
compensation for cerebellar dysfunction may be more difficult
for the timing of the absolute duration of single intervals [45].
We now need to consider what classical functions of the
cerebellum (i.e. motor behaviour) and deviance processing in
perception may have in common. These functions may con-
verge in a common operation such as an, at least, two-fold
response to changes in the environment (e.g. initial deviance
detection in the form of an error response when expecting a
particular tone quality in a tonal sequence, followed by the
updating of a mental or internal model of the environment
via adaptation of the expected tone quality in a tonal sequence
to a new tone quality induced by the change and initial error
response to such change [31,46–48]). Accordingly, precise
encoding and subsequent use of temporal structure to optimize
performance should be critical in both the motor and non-
motor domains when updating of a mental model is required.
This line of thinking may therefore be related to the updating of
a forward model of cerebellar information processing [47,48].
A forward model receives its input in the form of (efference)
copies of motor commands, which are used to predict the
sensory consequences of an ideally executed movement,
backed up by a comparator, which identifies discrepancies
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
8
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
between the predicted and the actual consequences and signals
a corresponding error signal in order to adjust the accuracy of
the model [49]. Analogously, the mental model of the environ-
ment receives its input in the form of sensory events and may
implement a form of error detection and updating in response
to unpredicted events. While the forward model relies on cer-
ebellar connections to central motor and somatosensory brain
areas, one may speculate that a mental model of expected
events in the environment may rely on connections between
the cerebellum and sensory areas (e.g. the temporal cortices
[50,51]). However, whether implemented at the neural,
motor, or non-motor (sensory, cognitive) level, any efficient
model of the environment needs to be dynamic in nature.
Accordingly, temporal structure should contribute to the con-
tinuous generation of hypotheses about the environment by
the nervous system as indexed by the P3b [52]. In this context,
one of the functional roles of the cerebellum may be the precise
encoding of temporal structure, which subsequently is used
to generate specific temporal predictions [46]. Kahneman &
Tversky [53] remarked: ‘Before an event there are expec-
tations—after an event there may be surprise’. What remains
to be resolved is whether the underlying operations of cerebel-
lar function in temporal processing, prediction, or surprise as
indexed in the P3b response [54] share common ground as
they appear to be intimately tied to each other. Moreover,
whether prediction and surprise represent facets of more
global cerebellar computation(s) serving a predisposition
towards optimal timing and adaptation in motor and non-
motor behaviour alike, remains to be addressed in future
studies beyond lower level auditory processes.
Finally, the current results raise a more fundamental
question about how the component evolution from early to
late ERPs relates to formal and temporal stimulus dimensions
on the one hand, and the operations speculated to underlie
optimization of behaviour on the other. Typically, an ERP sup-
pression effect (i.e. an amplitude reduction) in early ERP
components such as the P50 or N1 is considered to reflect effi-
cient sensory gating or successful temporal prediction, while
ERP enhancement seems to indicate qualitative processing
differences in later components such as the P3b [24,28,55].
Morphologically, this component evolution changes at approxi-
mately 200 ms after stimulus onset. In other words, temporal
predictability seems to lead to suppression of early components
as opposed to an enhancement of late components. While there
are indications of reduced N1 suppression effects in cerebellar
patients [56], no significant effects were observed in the current
set-up. However, the absence of a similar finding does not
necessarily contradict these results or imply intact function, as
differences in the paradigms used may result in qualitative
and quantitative ERP differences, e.g. due to the use of a
motor response.
Of note is that early suppression effects reflect tempo-
ral predictability, while later enhanced effects are driven
by an interaction of temporal and formal structure. We
may therefore consider that a change from suppression to
enhancement may result from the differential engagement of
operations such as successful encoding of temporal properties
and the efficient updating/adaptation of a mental model of
the environment in the quest to optimize behaviour. Further,
while a classical dissociation of exogenous and endogenous
ERP components [57] could support such a differentiation
of operations, this conceptualization of ERP components
seems inappropriate as both so-called ‘pre-attentive responses’
(MMN) and early ‘exogenous’ components are sensitive to
attentional (i.e. cognitive) manipulations (e.g. [58]).
In conclusion, we set out to answer two critical questions
regarding the functional role of the cerebellum in temporal
predictability, deviance detection and the updating of a
mental model of the environment. The present results suggest
that structural damage to the cerebellum, apparently inde-
pendent of the extent (across anterior/posterior dimensions)
or the lateralization (left, right, bilateral) of the lesion, critically
affects temporal predictability and its impact on perceptual
deviance processing involving both an error response and
updating a mental model of the environment to achieve opti-
mal behaviour. Clearly, more studies with (i) larger patient
sample sizes and (ii) temporally more complex stimulus
aspects such as meter and rhythm in speech and music (for rel-
evant neuroimaging evidence see [35,39,59–61]) are needed to
further delineate whether specific sub-compartments of the
cerebellum contribute to these operations in a function-specific
or a domain-general manner.
Ethics statement. The local ethics committee at the University of Leipzigapproved the study.
Acknowledgements. The authors thank Anne-Kathrin Franz, Heike Boetheland Ingmar Brilmayer for their support during data acquisition.
Funding statement. This work was support by a DFG KO 2268/6-1 grantto S.A.K.
Endnote1To validate the impression of potential early group difference in theN1, identical analyses as for the later components were conducted ina 50 ms time-window (88 to 138 ms). This analysis yielded statisticallysignificant main effects of temporal structure (F1,20¼ 13.42, p , 0.01),formal structure (F1,20¼ 8.66, p , 0.01) and region (F1,20¼ 4.45, p ,
0.05), as well as a significant interaction of formal structure � region(F1,20¼ 4.84, p , 0.04). Resolving this interaction by the factor regionyielded significant effects of formal structure in anterior (F1,20¼ 10.32,p , 0.01) and posterior regions (F1,20¼ 5.91, p , 0.03). However, therewas no significant main effect of group (F1,20¼ 1.68, p ¼ 0.209) or anyinteraction involving the factor group (all p . 0.11), and only a non-sig-nificant trend for an interaction of temporal structure � formal structure(F1,20¼ 3.20, p ¼ 0.089).
References
1. Ivry RB, Keele SW. 1989 Timing functions of thecerebellum. J. Cogn. Neurosci. 1, 136 – 152. (doi:10.1162/jocn.1989.1.2.136)
2. Buhusi CV, Meck WH. 2005 What makes us tick?Functional and neural mechanisms of intervaltiming. Nat. Rev. Neurosci. 6, 755 – 765. (doi:10.1038/nrn1764)
3. Ivry RB, Schlerf JE. 2008 Dedicated and intrinsicmodels of time perception. Trends Cogn. Sci. 12,273 – 280. (doi:10.1016/j.tics.2008.04.002)
4. Koch G, Massimiliano O, Caltagirone C. 2009 Neuralnetworks engaged in milliseconds and seconds timeprocessing: evidence from transcranial magneticstimulation and patients with cortical or subcortical
dysfunction. Phil. Trans. R. Soc. B 364, 1907 – 1918.(doi:10.1098/rstb.2009.0018)
5. Spencer RMC, Ivry RB. 2013 Cerebellum and timing.In Handbook of the cerebellum and cerebellardisorders (eds M Manto, DL Grual, JD Schmahmann,N Koibuchi, F Rossi), pp. 1201 – 1219. Dordrecht,The Netherlands: Springer.
rstb.royalsocietypublishing.orgPhil.Trans.R.Soc.B
369:20130403
9
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
6. Schmahmann JD. 1991 An emerging concept: thecerebellar contribution to higher function. Arch.Neurol. 48, 1178 – 1187. (doi:10.1001/archneur.1991.00530230086029)
7. Stoodley CJ, Schmahmann JD. 2009 Functionaltopography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44,489 – 501. (doi:10.1016/j.neuroimage.2008.08.039)
8. Alexander MP, Gillingham S, Schweizer T, Stuss DT.2012 Cognitive impairments due to focal cerebellarinjuries in adults. Cortex 48, 980 – 990. (doi:10.1016/j.cortex.2011.03.012)
9. Strick PL, Dum RP, Fiez JA. 2010 Cerebellum andnonmotor function. Annu. Rev. Neurosci. 32, 413 –434. (doi:10.1146/annurev.neuro.31.060407.125606)
10. Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL.2005 The cerebellum communicates with the basalganglia. Nat. Neurosci. 8, 1491 – 1493. (doi:10.1038/nn1544)
11. Akkal D, Dum RP, Strick PL. 2007 Supplementarymotor area and presupplementary motor area:targets of basal ganglia and cerebellar output.J. Neurosci. 27, 10 659 – 10 673. (doi:10.1523/JNEUROSCI.3134-07.2007)
12. Bostan AC, Strick PL. 2010 The cerebellum and basalganglia are interconnected. Neuropsychol. Rev. 20,261 – 270. (doi:10.1007/s11065-010-9143-9)
13. Schwartze M, Rothermich K, Kotz SA. 2012Functional dissociation of pre-SMA and SMA-properin temporal processing. Neuroimage 60, 290 – 298.(doi:10.1016/j.neuroimage.2011.11.089)
14. Kotz SA, Schwartze M, Schmidt-Kassow M. 2009Non-motor basal ganglia functions: a review andproposal for a model of sensory predictability inauditory language perception. Cortex 45, 982 – 990.(doi:10.1016/j.cortex.2009.02.010)
15. Dum RP, Strick PL. 2003 An unfolded map of thecerebellar dentate nucleus and its projections to thecerebral cortex. J. Neurophysiol. 89, 634 – 639.(doi:10.1152/jn.00626.2002)
16. Draganski B, Kherif F, Kloppel S, Cook PA, AlexanderDC, Parker GJM, Deichmann R, Ashburner J,Frackowiak RSJ. 2008 Evidence for segregated andintegrative connectivity patterns in the basalganglia. J. Neurosci. 28, 7143 – 7152. (doi:10.1523/JNEUROSCI.1486-08.2008)
17. Bostan AC, Dum RP, Strick PL. 2013 Cerebellarnetworks with the cerebral cortex and basalganglia. Trends Cogn. Sci. 17, 241 – 254. (doi:10.1016/j.tics.2013.03.003)
18. Picard N, Strick PL. 2001 Imaging the premotorareas. Curr. Opin. Neurobiol. 11, 663 – 672. (doi:10.1016/S0959-4388(01)00266-5)
19. Courchesne E, Allen G. 1997 Prediction andpreparation, fundamental functions of thecerebellum. Learn. Mem. 4, 1 – 35. (doi:10.1101/lm.4.1.1)
20. O’Reilly JX, Mesulam MM, Nobre AC. 2008 Thecerebellum predicts the timing of perceptualevents. J. Neurosci. 28, 2252 – 2260. (doi:10.1523/JNEUROSCI.2742-07.2008)
21. Roth JM, Synofzik M, Lindner A. 2013 Thecerebellum optimizes perceptual predictions about
external sensory events. Curr. Biol. 23, 930 – 935.(doi:10.1016/j.cub.2013.04.027)
22. Schwartze M, Tavano A, Schroger E, Kotz SA. 2012Temporal aspects of prediction in audition: cortical andsubcortical neural mechanisms. Int. J. Psychophysiol.83, 200 – 207. (doi:10.1016/j.ijpsycho.2011.11.003)
23. Ivry RB. 1996 The representation of temporalinformation in perception and motor control. Curr.Opin. Neurobiol. 6, 851 – 857. (doi:10.1016/S0959-4388(96)80037-7)
24. Schwartze M, Rothermich K, Schmidt-Kassow M,Kotz SA. 2011 Temporal regularity effects on pre-attentive and attentive processing of deviance. Biol.Psychol. 87, 146 – 151. (doi:10.1016/j.biopsycho.2011.02.021)
25. Slabu L, Escera C, Grimm S, Costa-Faidella J. 2010Early change detection in humans as revealed byauditory brainstem and middle-latency evokedpotentials. Eur. J. Neurosci. 32, 859 – 865. (doi:10.1111/j.1460-9568.2010.07324.x)
26. Horvath J, Winkler I, Bendixen A. 2008 Do N1/MMN,P3a, and RON form a strongly coupled chainreflecting the three stages of auditory distraction?Biol. Psychol. 79, 139 – 147. (doi:10.1016/j.biopsycho.2008.04.001)
27. Schwartze M, Farrugia N, Kotz SA. 2013 Dissociationof formal and temporal predictability in earlyauditory evoked potentials. Neuropsychologia 51,320 – 325. (doi:10.1016/j.neuropsychologia.2012.09.037)
28. Otterbein S, Abel C, Heinemann LV, Kaiser J,Schmidt-Kassow M. 2012 P3b reflects periodicity inlinguistic sequences. PLoS ONE 7, e51419. (doi:10.1371/journal.pone.0051419)
29. Linden DEJ. 2005 The P300: where in the brain is itproduced and what does it tell us? Neuroscientist11, 563 – 576. (doi:10.1177/1073858405280524)
30. Polich J, Criado JR. 2006 Neuropsychology andneuropharmacology of P3a and P3b.Int. J. Psychophysiol. 60, 172 – 185. (doi:10.1016/j.ijpsycho.2005.12.012)
31. Polich J. 2007 Updating P300: an integrativetheory of P3a and P3b. Clin. Neurophysiol. 118,2128 – 2148. (doi:10.1016/j.clinph.2007.04.019)
32. Donchin E, Coles GH. 1988 Is the P300 component amanifestation of context updating? Behav. Brain Sci.11, 357 – 374. (doi:10.1017/S0140525X00058027)
33. Kotchoubey B. 2006 Event-related potentials,cognition, and behavior: a biological approach.Neurosci. Biobehav. Rev. 30, 42 – 65. (doi:10.1016/j.neubiorev.2005.04.002)
34. Oldfield RC. 1971 The assessment and analysis ofhandedness: the Edinburgh inventory.Neuropsychologia 9, 97 – 113. (doi:10.1016/0028-3932(71)90067-4)
35. Schmahmann JD et al. 1999 Three-dimensional MRIatlas of the human cerebellum in proportionalstereotaxic space. Neuroimage 10, 233 – 260.(doi:10.1006/nimg.1999.0459)
36. Mugler III JP, Brookeman JR. 1990 Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn. Reson. Med.15, 152 – 157. (doi:10.1002/mrm.1910150117)
37. Friston KJ, Ashburner J, Frith CD, Poline J-B, HeatherJD, Frackowiak RSJ. 1995 Spatial registration andnormalization of images. Hum. Brain Mapp. 2,165 – 89. (doi:10.1002/hbm.460030303)
38. Rorden C, Brett M. 2000 Stereotaxic display of brainlesions. Behav. Neurol. 12, 191 – 200. (doi:10.1155/2000/421719)
39. Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB.2003 Disrupted timing of discontinuous but notcontinuous movements by cerebellar lesions. Science300, 1437 – 1439. (doi:10.1126/science.1083661)
40. Kotz SA, Schwartze M. 2010 Cortical speechprocessing unplugged: a timely subcortico-corticalframework. Trends Cogn. Sci. 14, 392 – 399. (doi:10.1016/j.tics.2010.06.005)
41. Kotz SA, Kalberlah C, Bahlmann J, Friederici AD,Haynes JD. 2013 Predicting vocal emotionexpressions from the human brain. Hum. BrainMapp. 34, 1971 – 1981. (doi:10.1002/hbm.22041)
42. Obermeier C, Menninghaus W, von Koppenfels M,Raettig T, Schmidt-Kassow M, Otterbein S, Kotz SA.2013 Aesthetic and emotional effects of meter andrhyme in poetry. Front. Psychol. 4, 10. (doi:10.3389/fpsyg.2013.00010)
43. Koziol LF et al. 2013 Consensus paper: the cerebellum’srole in movement and cognition. Cerebellum 13,151 – 177. (doi:10.1007/s12311-013-0511-x)
44. Kotz SA, Schwartze M. 2011 Differential input of thesupplementary motor area to a dedicated temporalprocessing network: functional and clinicalimplications. Front. Integr. Neurosci. 5, 86. (doi:10.3389/fnint.2011.00086)
45. Grube M, Lee K, Griffith TD, Barker AT, Woodruff PW.2010 Transcranial magnetic theta-burst stimulation ofthe human cerebellum distinguishes absolute,duration-based from relative, beat-based perceptionof subsecond time intervals. Front. Psychol. 1, 171.(doi:10.3389/fpsyg.2010.00171)
46. Schwartze M, Kotz SA. 2013 A dual-pathway neuralarchitecture for specific temporal prediction.Neurosci. Biobehav. Rev. 37, 2587 – 2596. (doi:10.1016/j.neubiorev.2013.08.005)
47. Wolpert DM, Miall RC, Kawato M. 1998 Internalmodels in the cerebellum. Trends Cogn. Sci. 2,338 – 347. (doi:10.1016/S1364-6613(98)01221-2)
48. Ito M. 2008 Control of mental activities by internalmodels in the cerebellum. Nat. Rev. Neurosci. 9,304 – 314. (doi:10.1038/nrn2332)
49. Ramnani N. 2006 The primate cortico-cerebellarsystem: anatomy and function. Nat. Rev. Neurosci. 7,511 – 522. (doi:10.1038/nrn1953)
50. Pastor MA, Vidaurre C, Fernandez-Seara MA,Villanueva A, Friston KJ. 2008 Frequency-specificcoupling in the cortico-cerebellar auditory system.J. Neurophysiol. 100, 1699 – 1705. (doi:10.1152/jn.01156.2007)
51. Perales M, Winer JA, Prieto JJ. 2006 Focalprojections of cat auditory cortex to the pontinenuclei. J. Comp. Neurol. 497, 959 – 980. (doi:10.1002/cne.20988)
52. Duncan-Johnson CC, Donchin E. 1977 Onquantifying surprise: the variation of event-related potentials with subjective probability.
rstb.royalsocietypublishing.orgPhil.T
10
on November 10, 2014rstb.royalsocietypublishing.orgDownloaded from
Psychophysiology 14, 456 – 467. (doi:10.1111/j.1469-8986.1977.tb01312.x)
53. Kahneman D, Tversky A. 1982 Variants ofuncertainty. Cognition 11, 143 – 157. (doi:10.1016/0010-0277(82)90023-3)
54. Donchin E. 1981 Surprise! . . . surprise?Psychophysiology 18, 493 – 513. (doi:10.1111/j.1469-8986.1981.tb01815.x)
55. Bendixen A, SanMiguel I, Schroger E. 2012Early electrophysiological indicators forpredictive processing in audition: a review.Int. J. Psychophysiol. 83, 120 – 131. (doi:10.1016/j.ijpsycho.2011.08.003)
56. Knolle F, Schroger E, Baess P, Kotz SA. 2012The cerebellum generates motor-to-auditorypredictions: ERP lesion evidence. J. Cogn.Neurosci. 24, 698 – 706. (doi:10.1162/jocn_a_00167)
57. Picton TW, Stuss DT. 1980 The component structureof the human event-related potentials. Prog. BrainRes. 54, 17 – 48. (doi:10.1016/S0079-6123(08)61604-0)
58. Naatanen R, Kujala T, Winkler I. 2011 Auditoryprocessing that leads to conscious perception: aunique window to central auditory processingopened by the mismatch negativity and related
responses. Psychophysiology 48, 4 – 22. (doi:10.1111/j.1469-8986.2010.01114.x)
59. Chen JL, Penhune VB, Zatorre RJ. 2008 Listening tomusic rhythms recruits motor regions of the brain. Cereb.Cortex 18, 2844 – 2854. (doi:10.1093/cercor/bhn042)
60. Grahn JA, Rowe JB. 2009 Feeling the beat: premotorand striatal interactions in musicians and non-musicians during beat perception. J. Neurosci. 29,7540 – 7548. (doi:10.1523/JNEUROSCI.2018-08.2009)
61. Teki S, Grube M, Kumar S, Griffith TD. 2011 Distinctsubstrates of duration based and beat-basedauditory timing. J. Neurosci. 31, 3805 – 3812.(doi:10.1523/JNEUROSCI.5561-10.2011)
ra ns. R.Soc.B369:20130403
Related Documents