Earth and Planetary Science Letters, 100 (1990) 179-194 179 Elsevier Science Publishers B.V., Amsterdam [MK] Volcanism in the Sumisu Rift, I. Major element, volatile, and stable isotope geochemistry Alfred G. Hochstaedter a, James B. Gill a, Minoru Kusakabe b, Sally Newman c, Malcolm Pringle d, Brian Taylor e and Patty Fryer e a Board of Earth Sciences, University of California, Santa Cruz, CA 95064, U.S.A. h Institute for Study of the Earth's Interior, Okayama University, Misasa, Tottori-Ken 682-02, Japan c Division of Geological Sciences, California Institute of Technology, Pasadena, CA 91125, U.S.A. a U.S. Geological Survey, 345 MiddlefieldRd, Menlo Park, CA 94025, U.S.A. e Hawaii Institute of Geophysics, University of Hawaii, Honolulu, HI 96822, U.S.A. Received January 22, 1990; revised version accepted March 21, 1990 ABSTRACT A bimodal volcanic suite with K-Ar ages of 0.05-1.40 Ma was collected from the Sumisu Rift using ALVIN. These rocks are contemporaneous with island arc tholeiite lavas of the Izu-Ogasawara arc 20 km to the east, and provide a present day example of volcanism associated with arc rifting and back-arc basin initiation. Major element geochemistry of the basalts is most similar to that of basalts found in other, more mature back-arc basins, which indicates that back-arc basins need not begin their magmatic evolution with lavas bearing strong arc signatures. Volatile concentrations distinguish Sumisu Rift basalts from island arc basalts and MORB. H20 contents, which are at least four times greater than in MORB, suppress plagioclase crystallization. This suppression results in a more mafic fractionating assemblage, which prevents AI203 depletion and delays the initiation of Fe203,o, and TiO 2 enrichment. However, unlike arc basalts, Fe3+/•Fe ratios are only slightly higher than in MORB and are insufficient to cause magnetite saturation early enough to suppress Fe203,o,~ and TiO 2 enrichment. Thus, major element trends are more similar to those of MORB than arcs. H20, CO 2 and S are undersaturated relative to pure phase solubility curves, indicating exsolution of an H20-rich mixed gas phase. High H20/S, high 80, and low (MORB-like) 834S ratios are considered primary and distinctive of the back-arc basin setting. 1. Introduction Tholeiitic lavas erupted in intra-oceanic back- arc basins range in chemical composition from being indistinguishable from mid-ocean ridge basalts (MORB) to intermediate between MORB and tholeiitic island arc basalt (IAB) [1-3]. The intermediate compositions are defined as back-arc basin basalt (BABB). A1203, H20 , and fO2 are higher in BABB than in MORB, while trends of Fe203,,,. and TiO 2 vs. MgO are similar to those of MORB, but displaced slightly towards the lower Fe203,,oo and TiO z concentrations of IAB. Thus, BABB suites are dominated by basalt, but differ from MORB in major element trends that are similar to, but lesser in magnitude than IAB. For the purposes of this report, BABB is a chemical 0012-821X/90/$03.50 © 1990 - Elsevier Science Publishers B.V. definition of basalt commonly erupted in back-arc basins. In addition to discriminating BABB from MORB, volatiles play important roles in determin- ing the liquid line of descent in basaltic magmas. Specifically, H20 controls the relative stability of plagioclase, olivine, and pyroxene [4], and fO 2 controls the onset of titanomagnetite crystalliza- tion [5]. High H20 and fO 2 are often called upon to explain the differentiation trends of volcanic arc suites [4,5]. Because arc lavas commonly erupt subaerially and degas before solidification, their volatile composition is not preserved, and infor- mation relating liquid line of descent to volatile composition is lost. Submarine eruption of basalt, however, preserves volatiles in chilled glassy pil- low rims. The combination of major element trends

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Earth and Planetary Science Letters, 100 (1990) 179-194 179 Elsevier Science Publishers B.V., Amsterdam
[MK]
Volcanism in the Sumisu Rift, I. Major element, volatile, and stable isotope geochemistry
Alfred G. Hochs taedter a, James B. Gill a, Minoru Kusakabe b, Sally N e w m a n c, Malcolm Pringle d, Brian Taylor e and Pat ty Fryer e
a Board of Earth Sciences, University of California, Santa Cruz, CA 95064, U.S.A. h Institute for Study of the Earth's Interior, Okayama University, Misasa, Tottori-Ken 682-02, Japan
c Division of Geological Sciences, California Institute of Technology, Pasadena, CA 91125, U.S.A. a U.S. Geological Survey, 345 MiddlefieldRd, Menlo Park, CA 94025, U.S.A.
e Hawaii Institute of Geophysics, University of Hawaii, Honolulu, HI 96822, U.S.A.
Received January 22, 1990; revised version accepted March 21, 1990
ABSTRACT
A bimodal volcanic suite with K - A r ages of 0.05-1.40 Ma was collected from the Sumisu Rift using ALVIN. These rocks are contemporaneous with island arc tholeiite lavas of the Izu-Ogasawara arc 20 km to the east, and provide a present day example of volcanism associated with arc rifting and back-arc basin initiation. Major element geochemistry of the basalts is most similar to that of basalts found in other, more mature back-arc basins, which indicates that back-arc basins need not begin their magmatic evolution with lavas bearing strong arc signatures.
Volatile concentrations distinguish Sumisu Rift basalts from island arc basalts and MORB. H 2 0 contents, which are at least four times greater than in MORB, suppress plagioclase crystallization. This suppression results in a more mafic fractionating assemblage, which prevents AI203 depletion and delays the initiation of Fe203,o, and TiO 2 enrichment. However, unlike arc basalts, Fe3+/•Fe ratios are only slightly higher than in MORB and are insufficient to cause magnetite saturation early enough to suppress Fe203,o,~ and TiO 2 enrichment. Thus, major element trends are more similar to those of MORB than arcs.
H20, CO 2 and S are undersaturated relative to pure phase solubility curves, indicating exsolution of an H20-rich mixed gas phase. High H 2 0 / S , high 80 , and low (MORB-like) 834S ratios are considered primary and distinctive of the back-arc basin setting.
1. Introduction
Tholeiitic lavas erupted in intra-oceanic back- arc basins range in chemical composition from being indistinguishable from mid-ocean ridge basalts (MORB) to intermediate between MORB and tholeiitic island arc basalt (IAB) [1-3]. The intermediate compositions are defined as back-arc basin basalt (BABB). A1203, H20 , and fO2 are higher in BABB than in MORB, while trends of Fe203,,,. and TiO 2 vs. MgO are similar to those of MORB, but displaced slightly towards the lower Fe203,,oo and TiO z concentrations of IAB. Thus, BABB suites are dominated by basalt, but differ from MORB in major element trends that are similar to, but lesser in magnitude than IAB. For the purposes of this report, BABB is a chemical
0012-821X/90/$03.50 © 1990 - Elsevier Science Publishers B.V.
definition of basalt commonly erupted in back-arc basins.
In addition to discriminating BABB from MORB, volatiles play important roles in determin- ing the liquid line of descent in basaltic magmas. Specifically, H20 controls the relative stability of plagioclase, olivine, and pyroxene [4], and f O 2 controls the onset of titanomagnetite crystalliza- tion [5]. High H 2 0 and f O 2 a r e often called upon to explain the differentiation trends of volcanic arc suites [4,5]. Because arc lavas commonly erupt subaerially and degas before solidification, their volatile composition is not preserved, and infor- mation relating liquid line of descent to volatile composition is lost. Submarine eruption of basalt, however, preserves volatiles in chilled glassy pil- low rims. The combination of major element trends
Japa
n 15 N
~[
Sumisu Ri
30
Torish
Phi
lippi
,
25Ia
10'E
Io
25,N
~o
D
10°40'N
L 0°
40'N
139 °2-. ~
~u- 10'
E b
* J
¢ lO
km
'~
~ig.
1.
(a)
Loc
atio
n of
the
Sum
isu
Rif
t in
the
lzu
arc
. T
rian
gle
s ar
e vo
lcan
ic f
ront
vol
cano
es.
Das
hed
lin
e is
the
3 k
m b
ath
ym
etri
c co
ntou
r.
Aft
er Y
amaz
aki
[13]
. (b
) S
eaM
AR
C
~at
hym
etri
c m
ap o
f th
e S
umis
u R
ift.
Co
nto
urs
are
lab
eled
in
hu
nd
red
s of
met
ers.
No
te l
oca
tio
ns
of S
had
ow
Mt
dive
189
1 an
d r
ift
wal
l di
ve 1
889.
Tri
ang
les
= A
tlan
tis
II d
red
ge
z
; sq
uare
s =
HIG
dre
dges
; d
iam
on
ds
= G
eolo
gica
l S
urve
y of
Jap
an d
redg
es.
The
en-
eche
lon
ridg
es a
re b
oxed
an
d s
ho
wn
in
Fig
. lc
. A
fter
Bro
wn
an
d T
aylo
r [1
4].
(c)
Sea
BE
AM
~at
hym
etri
c m
ap o
f th
e en
-ech
elon
rid
ges.
Fif
ty m
eter
co
nto
urs
are
lab
eled
in
hu
nd
red
s of
met
ers.
Div
e tr
acks
are
sh
ow
n a
s so
lid
line
s an
d s
amp
le l
ocal
itie
s m
enti
on
ed i
n th
e
text
are
sho
wn
as t
rian
gles
and
lab
eled
wit
h nu
mbe
rs.
Ex
amp
le:
the
9 al
on
g d
ive
1894
is
refe
rred
to
in t
he t
ext
as s
amp
le 1
894-
9.
> t-
V O L C A N I S M IN T H E S U M I S U R I F T , I 181
intermediate between MORB and IAB and the preserved volatile information in BABB provides opportunity to investigate their interrelationships.
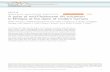
The Sumisu Rift (SR) is one of a series of grabens along the currently rifting Izu-Ogasawara arc (hereafter referred to as the Izu arc) [6] (Fig. la). Together, these grabens constitute an incipi- ent back-arc basin. The purpose of this report is to describe the major element and volatile composi- tion of the first magmatic products of this rifting, and to investigate the relationship between their volatile composition and differentiation trends. In a companion paper, we [7] report trace element and radiogenic isotope results from the SR.
The SR was investigated via the deep submersi- ble ALVIN and dredging aboard the Atlantis II during August, 1987. One hundred thirty samples were collected during eight ALVIN dives within the SR. Thirty of these samples have been analyzed for major and trace elements by XRF and nine have also been analyzed for volatile concentration and stable isotopic composition. The complete set of volatile data is reported here along with repre- sentative major elements (Tables 3 and 5). Ad-
ditional rocks used in this study (D962-1) were dredged from the region in 1987 by the Geological Survey of Japan. Ikeda and Yuasa [8] report re- sults of Japanese collections dredged prior to 1987. Fryer et al. [9] report results of volcanic rocks dredged by the Hawaii Institute of Geophysics (HIG).
2. Tectonic setting and physical volcanology
The SR is an 80 km long by 20-40 km wide rift graben located in the middle of the Izu arc at about 31°N (Fig. la). Its tectonic history is sum- marized in this volume [6], and was one objective of recent ODP drilling [10]. The 1 km deep rift lies about 20 km behind the active volcanic front between the volcanoes of Sumisujima and Torish- ima (Fig. lb). The age of initiation of rifting is between 1.1 and 3.6 Ma [10].
The SR is divided into North and South Basins by a series of three nor th-south trending en-eche- lon ridges which parallel the volcanic front and are the largest volcanic features in the rift. In this report, "outer rift" refers to the region of shal-
TABLE1
L~ationandpetrograp~cdata ~rSumisu ~ftlavas
Lava Type Locatity Petrography
Inner R i f t Nafic basatt East and west ridges,
base of central ridge
Dif ferentiated West ridge -- dive 1896 basalt eastern seamount -- D967
Rhyotite Central seamount dives 1888 and 1995
Nixed Base of west ridge dacite sampte 1896-2
Shadow Ht South Basin Dive 1891
Rift Watt South Basin Dive 1889
Outer Rift Outer Rift All dredge 2
Nearty aphyric with rare pt±aug phenocrysts 0.5-1.0mm pt+ot+aug microphenocrysts ~ith ot quench textures sheet, pittow and btocky tavas, 15-35~ vesictes
nearly aphyric ~ith rare pt±aug phenocrysts pt+ot+aug+opaque microphenocryts with ol quench textures blocky tavas, 15-]5~ vesictes
1-2~ pt<opx<opaque<aug phenocrysts <2mm, 30-40~ vesicles Two morphotogicat types: 1) grey to btack obsidian with large (1-10mm) vesictes found at tower etevations 2) microvesicutar, btocky fracture, found at higher elevations
grassy groudmass, anhedrat ot+hb sub- to euhedrat ptag+opx+aug phenocrysts, 15-25~ vesictes
3-8X <lmm phenocrysts ot>aug>pt quench textures tess common, 15-35~ vesicles
10-15~ 1-2mm phenocrysts pt>ot>aug 15-30~ vesictes
Same as Rif t Watt
182
2 .0 I I I I I
d ¢Q
1.5 S u m i s u
B i m o d a l
1.0
I
50
0 .5
o
Rif t S u i t e
0 . 0 I R i i 45 55 60 65 70 75
SiO 2 , wt%
Fig. 2. SiO 2 wt.% vs K20 wt.% variation diagram shows the bimodal suite that has erupted from the SR. Symbols are: six-sided stars=rhyoli tes; asterisk= mixed dacite (1896-2).
Basalt symbols are described in the caption of Fig. 4.
lower bathymetry to the west of the en-echelon ridges (Fig. lb). Seven dives were concentrated on the en-echelon ridges while the outer rift was sampled with a single dredge (dl-2). Fryer et al. [9] discuss the outer rift area in more detail.
In this section, the morphology and variety of rock types is discussed for each region. Petrogra- phy of the volcanic rocks, which have been di- vided by locality and rock type, is summarized in Table 1.
The en-echelon ridges are 5 -7 km long by 2 -3 km wide and 400 m high (Fig. lc). They originated as fissure eruptions in water depths of 1500-2000 m, and constitute a bimodal suite of basalt and rhyolite lavas (Fig. 2). The central ridge is largest and capped by rhyolite, while its lower slopes are basaltic (sample 1888-1). Similar rhyolite was dredged from both the eastern and western flanks of the western ridge by H I G [9, dredges 14 and 17]. The east and west ridges are composed of pillow and blocky basalt lava and coarse talus with negligible to thin sediment cover [11], Dif- ferentiated basalt ( < 5% MgO) occurs primarily as blocky lava while mafic basalt may be blocky or pillow. Thus, the factors causing the distinction between blocky and pillow lavas may be similar to those producing aa versus pahoehoe lavas: erup-
A.Q. H O C H S T A E D T E R ET AL.
tion rate, topography and gas content, as well as composition. Differentiated basalt was collected only on the western ridge (dive 1896) and a seamount east of the ridges (D967). Mafic basalt was collected on all ridges including the western ridge (dive 1890, 1896-7). A mixed dacite (1896-2) containing hornblende as well as anhedral olivine was collected by both ALVIN and HIG [9] amidst the differentiated basalts near the base of the western ridge at 1850 m.
Edifices in the outer rift consist of seamounts rather than ridges. In addition to basalt (dl-2), rare andesite and two-pyroxene dacite have been dredged from the outer rift [9,8,12]. Pumice was commonly dredged from the outer rift, eastern rift wall and arc carapace, but is notably absent on the en-echelon ridges. Shadow Mt. (dive 1891) is a 3 km long, 100 m high pillow ridge in the southern basin. The "r i f t wall" refers to the steep topogra- phy forming the faulted eastern boundary of the southern basin. Pumice covered much of the out- crop there, and the few basalts recovered were weathered and had m m thick Mn rinds.
3 . P e t r o g r a p h y
3.1. Basalts Abundant vesicles and a lack of thick glassy
rims physically differentiate SR basalt from MORB. Basalts usually contain 15-35% vesicles 0.25-5 mm in diameter despite eruption depths of 1500-2500 m. Neither their amount nor size vary with depth. Some basalts have thin, frothy, glassy rims a few m m thick, which contain microvesicles less than 1 m m in size as well as micropheno- crysts. These glass rims were analyzed for vola- tiles.
Petrographic information is summarized in Ta- ble 1. Phenocrysts are rare in most en-echelon ridge basalts. Rare plagioclase or clinopyroxene phenocrysts ( > 0.5 mm) occur in a few thin sec- tions. Plagioclase phenoerysts commonly contain sieved cores. SR basalts, regardless of differentia- tion, are triply saturated with olivine, plagioclase, and clinopyroxene microphenocrysts, which occur in glassy rims as well as pillow interiors. Olivine and clinopyroxene microphenocrysts commonly show quench morphologies. Neither orthopyrox- ene nor pigeonite was observed with the optical microscope. In differentiated basalts, olivine rni-
V O L C A N I S M IN T H E S U M I S U R I F T , I 183
crophenocrysts with quench morphologies occur with small magnetite grains (0.1 ram), indicating SR basalts become saturated with magnetite be- fore reaching the olivine-low-Ca pyroxene reaction point. Alteration in all SR basalts is limited to groundmass glass. In some samples the glass has started to become anisotropic, while in others, such as 1894-9, secondary minerals have formed.
3.2. Rhyolites Two morphological types of rhyolite were col-
lected from the central ridge. Microvesicular rhyolite, which is completely glassy and breaks along blocky fractures, was collected at the higher elevations of the central ridge (1888-10, [13]). Ob- sidian with large (1-10 mm), stretched vesicles was collected at lower elevations (1895-4). The obsidian is easily broken along curved fractures and is less dense than the blocky variety.
4. Analytical techniques
Samples for K - A r geochronology were chosen from pillow interiors of the most crystalline sam- ples and were analyzed at the U.S. Geological Survey in Menlo Park. These samples were fresh and contained less than 5% groundmass glass, except for the rhyolites. Partial alteration of these samples would lower K - A r dates. Approximately 500 g of sample were crushed and sized to 0.5-1 mm and ultrasonically washed with deionized water. K20 concentrations were measured on each of two powdered 10 g aliquot of the crushed sample by flame photometry after lithium metaborate fusion and dissolution [15]. Argon analyses were made in duplicate on each of two 15-20 g aliquots by isotope dilution [16]. Argon mass analyses were measured by multiple collector mass spectrometry [17]. Aliquots of atmospheric argon were measured between each sample; the reproducibility of the atmospheric 4°Ar/36Ar mea- surements was better than 0.2% (1 sigma). Errors are reported as an estimate of the standard devia- tion of analytical precision [18], except that the uncertainty in the mass discrimination has also been considered. Ages are calculated using decay constants and 4°K//Kt from Steiger and JSger [19]. Reported ages are mean ages, weighted by the inverse of the variances [20].
Volatiles were measured in glass from pillow rims of basalt and interiors of rhyolite. Separate splits were analyzed for H 2 0 and CO 2 concentra- tions at the California Institute of Technology (CIT), and for H20 , S, and FeO(tot ) concentra- tions, Fe3+/~Fe, and stable isotope ratios at Okayama University. At CIT H20 and CO 2 con- centrations were measured on doubly polished glass chips by Fourier transform infrared spec- trometry (FTIR) ([21,22]; appropriate extinction coefficients were used: HzO- -P . Dobson, S. New- man, and E. Stolper, unpubl.; CO 2 [23]). This microbeam technique measures only species dis- solved in unaltered glass. It avoids the effects of alteration and anhydrous crystals by polishing away surface layers where adsorbed H20 would reside and by focusing on unaltered 25-400 /~m regions away from crystals and altered surfaces. Accuracy and precision are discussed by Dixon et al. [24]. At Okayama University, 0.5-1.0 mm sized glass fragments were hand-picked to avoid pheno- crysts and alteration and then cleaned with 3N HC1 at 60 °C for 1 hour, followed by ultrasonic washing with distilled water. After heating to 200 °C to remove adsorbed H20, H 2 0 concentra- tions were measured manometrically following Suzuoki and Epstein [25]. Twenty analyses of a sericite standard gave H20 = 4.58 + 0.03 wt.%.
D / H ratios were measured by mass spectrome- try following the techniques of Suzuoki and Ep- stein [25] and are expressed using the 8D notation relative to the V-SMOW standard. Seventeen analyses of the same sericite standard gave 6D = - 59.7 _+ 1.6%o. S was extracted following the tech- niques of Ueda and Sakai [26] and converted to SO 2 for measuring concentration by manometric analysis and isotopic composition by mass spec-
• trometry. S isotopic compositions are expressed using the 834S notation relative to the CDT stan- dard. Accuracy of S concentration and 834S is estimated to be _+5% and _0.2%0, respectively. FeO(tot ) and Fe3+ /~Fe were determined colori- metrically following the techniques of Uchida et al. [27]. Six analyses of standard JB-2 gave FeO~tot ) = 10.19 + 0.15 wt.% and FeO = 12.96 _+ 0.41 wt.%. Both results are within 1% (relative) of the accepted values. Fe203,o,~ analyses of pillow interiors by XRF at UCSC are an average of 2.3 relative per cent higher than the glass results from Okayama University. Only UCSC data are plotted in Fig. 4,
] 84 A,G. HOCHSTAEDTER ET AL.
but Fe3+/EFe are based solely on the glass analyses.
Major element analyses are from nearly aphyric pillow interiors. Major elements except for Na20 were analyzed by XRF at U.C. Santa Cruz (UCSC) following the methods of Stork et al. [28]. Na20 contents were measured by AA at UCSC with estimated accuracy of + 3%. Major elements were also measured for comparison by DCP at Lamont-Doherty Geological Observatory. Agree- ment is within 1% for all elements.
5. Results
5.1. K - A r ages
K - A r ages of rocks collected within the SR are 600_+30 ka to 50_+25 ka ( lo) (Table 2). The analyzed samples are fresh, holocrystalline, and likely to have remained closed systems. In detail,
basalts from the eastern en-echelon ridge and rhyolite from the top of the central ridge (Fig. lc) are 143 to 276 _+ 30 Ka (Table 2). Rocks from Shadow Mt. are younger at < 100 ka (95% confi- dence level), and a sample collected from the outer rift (sample dl-2; Fig. lb) is older at 600 _+ 30 ka (Table 2). Rocks collected on dive 1889 along the rift wall (Fig. lb) are about 1.0-1.4 Ma. These rift wall samples are similar in age and composition to those drilled in the basin axis, but younger than the sediments drilled in the adjacent arc edifice [10]. Consequently, they are interpreted as early syn-rift lavas or sills.
5.2. Volatiles
H20 concentrations obtained by the two tech- niques, manometry and FTIR, agree well except in a few cases (Table 3). Where the manometry data are lower, especially for rhyolite 1888-13 and basalt
T A B L E 2
K - A r ages of whole rock samples f rom the Sumisu Rift
K20 ARGON SAMPLE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . AGE ± S.D.
WEIGHT S.D. 4OAr* 4OAr* (g) (mot/gm) (g) (Ha) (my)
01-2 0.407 ± 0.004 3,246X10 - I 3 7.1 0.554 ± 0.052 3 361X10 -13 7.4 0.574 + 0.060
" -1S 3.665X10 7.4 0.626 ± 0.056 3.923X10 -13 7.9 0.670 ± 0.065
1891-26 0.360 ± 0.005 2.584X10 -14 0.5 0.050 ± 0.047 2.561XI0 "14 0.5 0.049 ± 0.052 1.760X10 -14 0.3 0.034 ± 0.048 3.540XI0 -14 0.6 0.068 ± 0.052
. . . . . 10-13 1889-4 0.172 ± 0.001 ¢ . too^ 8 .8 1.121 ± 0.082 2 664X10 -13 8.5 1.078 ± 0.098 2~545X10 -13 6.5 1.031 ± 0.085 2.385X10 -13 6.1 0.966 ± 0.098
1889-11 0.227 ± 0.004 4 435X10 -13 14.0 1.354 ± 0.070 4"393X10 -13 13.8 1.342 ± 0.081 41590X10 -13 12.3 1.403 ± 0.071 4.747X10 -13 12.7 1.450 ± 0.081
1893-5 0.355 ± 0.003 1.016X10 -13 2.0 0.199 ± 0.055 1 125X10 -13 2.2 0.220 ± 0.062
" -13 1.835X10 1.3 0.359 ± 0.110 2.478X10 -13 1.8 0.485 ± 0.110
1893-10 0.315 ± 0.001 6.649X10 -14 1.2 0.147 ± 0.062 7.531X10 -14 1.4 0.166 ± 0.070 6.328X10 -14 1.0 0.140 ± 0.059 5.481XI0 "14 0 .9 0.121 ± 0.065
1888-13 1.787 ± 0.005 6.201X10 -13 1.7 0.241 ± 0.057 6 264X10 -13 1.7 0.243 ± 0.057 71894X10 -13 2.2 0.307 ± 0.056 8.029X10 -13 2.2 0.312 + 0.056
WEIGHTED ± S.D. AGE (Ma) (my)
0.601 ± 0.029
0.050 + 0.025
1.054 ± 0.046
1.386 ± 0.038
0.255 ± 0.044
0,143 ± 0.032
0,276 ± 0.028
VOLCANISM IN THE SUMISU RIFT, I
TABLE 3
Volatile and ferrous Fe glass analyses of Susimu Rift volcanic rock
185
En-echelon ridge basatts Shadow Mt. Rhyolite 1894-9 1896-7 1890-8 1890-I0 1891-3 1891-7 1891-19 1888-I0 1888-13
sul f ide (ppm) 167 217 184 250 125 139 135 1 0
su l fa te (ppm) 8 14 1 3 4 2 1 2 1 634S (vs CDT) -1.4 -0.1 -0.4 0.5 -0.9 -2.3 -1.0
co 2 (ppm) ~50 ~45 ~15 ~25 s22 ~15 ~50 180±30 c ~20
H20 (wt.~) a 1.23 0.97 1.00 0.85 1.30 1.16 1.30 0.20 1.90 H20 (wt.~) b 1.22 0.98 1.11 1.05 1.28 1.27 1.27 0.14 1.17 ~D (vs SMOW) -29.6 -35.8 -36.6 -38.6 -40.9 -44.3 -44.6 -74.0 -68.1
FeO(tot) (wt.%) 8.62 9.29 9.91 10.37 8.66 8.73 2.65 2.54
Fe3+/~Fe 0.071 0.121 0.118 0.233 o.1oi 0.104 0.264 0.253
Depth (meters) 1900 1670 1825 1780 2260 2250 2250 1550 1500
Ana[yzed by FTIR at ClT AnalyZed by ma~ometry at Okayama Universi ty
Cpresent as CO 3 -
1891-19, the difference may reflect the presence of microphenocrysts. The FTIR technique focuses on crystal-free region, whereas anhydrous crystals di- lute the volatile concentrations determined by manometry.
The concentrations and isotopic composition of volatiles are listed in Table 3 and compared to MORB, Mariana Trough basalt, and arc basalts in Table 4. H20 contents are higher than in MORB and similar to those for other BABB and Mariana arc andesites, but are undersaturated according to the solubility curve of Dudfis [29] (Fig. 3a). H20 concentrations correlate with both depth and K20 with the exception of sample 1894-9, which prob- ably contains excess H20 due to alteration (see Fig. 3b and discussion below). The H20-depth correlation parallels the H20 solubility curve. 6D values are also higher than in MORB and similar
to results for Mariana Trough basalts (Fig. 3c). H20 and 6D correlate along a steep negative trend, with Shadow Mt. basalts having higher H20 contents yet lower 8D values than do en- echelon ridge basalts.
CO 2 concentrations also are lower than in MORB and similar to those of most MaNana Trough basalts [24,30] (Table 4). CO 2 contents are undersaturated relative to the solubility curve for pure CO 2 [31]. S concentrations are substantially lower than in MORB and Mariana Trough basalts (Table 4) and undersaturated with respect to the solubility curves of Mathez [32]. 634S values are similar to results for MORB and Mariana Trough basalts, but lower than arc values (Table 4). S concentration and t~34S correlate positively, with Shadow Mt. basalts having lower 834S values and S concentrations (Fig. 3d).
TABLE4
Comparison o fvo l a t i l eda t ao fbasa l t s f rom differenttectonicsettings
SR N-MORB Mariana Trough
S (ppm) 125-250 500-1000 [37] 500-700 [34]
634S (vs CDT) 0 -1 t o +1 [37] -1 .5 t o 0 [34]
CO 2 (ppm) _~50 45-360 [24] 0-150 [30]
H20 (wt.~) 0.98-1.42 0 . 1 - 0 . 4 [24,35] 0 . 5 - 2 . 1 [30]
6D (vs SMOW) -30 t o -45 -75 t o -85 [36] -50 t o -30 [33]
Arcs
~35a,[ 39] +3 to +6 a'[39]
0 .8 -1 .S b'[38]
alzu Arc bMariana Arc
186 A . G . H O C H S T A E D T E R E T A L .
~ 4
2~ ~3
2
-25
-35
-45
;L -55
-65
-75
a ' ' ' i
0.5 1.o 115 2:o H g ) w t %
Hz0 wt% 2.0 1.0 0.5 0.4 0.3 0.2
500
400
300
200
100 ¢r
0.8
~'~ 0.6
% 0.4
b
0.2 ~
0.0 0.0
Scotia ~" ~t
! |
0.5 1.0 H2 0 wt%
1.5
.r..~
0
2
~ T ARCS d Up to 21 per rail
- 0 ©
O 1 8 9 4 - 9
-k
- 8 5 - 3 I I 0 1 2 3 4 5 0 500 1000 1500
l /H20 S, p p m
Fig. 3. Variation diagrams showing volatile data. MORB fields are from [35]. Labelled fields are: M TB = Mariana Trough basalt [38,33]; Scotia = East Scotia Sea [30]; MA = Mariana Arc [38]. Symbols are: circles = en-echelon ridge basalts; stars = Shadow Mt. basalts. (a) H20 wt.% vs. depth shows that SR basalts are undersaturated relative to the pure H20 solubility curve [29]. H 2 0 contents in SR basalts are similar to other BABB as well as the Mariana Arc. (b) H20 wt.% vs. K 2 0 wt.% shows a positive correlation. H 2 0 / K 2 0 ratios are higher than in MORB and similar to those of other BABB. (c) 6D vs. H20 wt.% shows negative correlation between 6D and H20. Ticked line shows mixing trend between en-echelon ridge basalt and Standard Mean Ocean Water (SMOW). Numbers indicate mass fraction of SMOW added to en-echelon ridge basalt. Sample 1894-9 plots along the mixing trend and is inferred to have gained excess seawater. Data sources are: N-MORB, [36]; E-MORB, [40]; Lau Basin and MTB, [33]. (d) S (in ppm) concentration vs. ~34S shows that S concentrations are substantially lower than in MORB, Kilauea, and MTB, while 634S values are
MORB-like, but lower than arcs. Data sources are: arc, [39]; MORB, [37]; Kilauea, [53]; MTB, [34].
The rhyolite samples are notably poor glass, making analysis difficult. In particular, 1888-13 is heterogeneous, with FTIR analyses ranging from 0.8 to 2.7 wt.% H20. The two SR rhyolites contain very different amounts of H20 (Table 3). The rhyolite with low water content contains signifi- cant CO 2- but no molecular CO 2, which is the
usual C-bearing species for rhyolites. The presence of CO 2- probably reflects alteration.
5.3. Fe;+/•Fe ratios Molar Fe3+/Y~Fe ratios of SR volcanic rocks
range from 0.071 to 0.253 (Table 3). Nearly the entire range occurs within SR basalts as Fe 3 +/EFe
VOLCANISM IN THE SUMISU RIFT, I 187
i i i i i
56 ~ Si02 54 ~ - 52
50 arc
4~iI / l \ l I I
1.0 /
I ~ ° l I ~ 2 4 6 8 10
" ~ o Na20
I I I I I
0.6 o K20 o
o d~
o arc []
0.4 ~ "Irtr'~t
2 4 6 8 10
Mg0, wt%
20
18
16
14
12
20
15
10
14
12
10
8
i
3
0 []
I 1 I
Fee0 a / \ \ \ ' \ \ / / '%
I I I I I 2 4 6 8 10 CaOe~, ~ ~ , ~ ' ~
4 6 8 10
MgO, wt%
I n n e r Rift
O u t e r Ri f t
~" S h a d o w Mr.
+ Rif t Wall
Fig. 4. Major element variation diagrams show trends for SR basalts, MORB and Izu arc lavas. SR analyses include data from Table 5 and unpublished UCSC analyses. SR basalts from the eastern en-echelon ridge and adjacent seamount to the east are enclosed (see text). All data are in wt.%. Fields for the Izu arc lavas are from Zhang and Langmuir [43]. Fields for MORB enclose data for suites collected from depths of 0-400 m [44-49]. MORB fields for A1203, Fe203(,o0, Na20, and K20 exclude suites collected from depths
greater than 3000 m.
188 A.G. HOCHSTAEDTER ET AL.
20 10 2 ~ 4 0
0 Ol Si
o
()~ ~ ~ ,_~ O "
Fig. 5. Pseudo-ternary projections. Projection scheme and cotectic position from [57]. (a) Plagioclase projection shows most SR basalts plot within the olivine field. (b) Clinopyroxene projection shows that most SR basalts plot within the plagioclase field,
consistent with its contraction due to high Pn2o. Symbols as in Fig. 4.
increases with differentiation. Shadow Mt. basalts have higher Fe 3 +/52Fe ratios when basalts of simi- lar MgO content are compared ( - 0 . 1 0 vs. 0.07, Table 3). These values are considered minima because post-eruptive alteration may have oxidized the thin glass rims [42]. Fe3+/EFe for all SR basalts, except sample 1894-9, is higher than for MORB with similar MgO contents.
5.4. Major elements Whole rock pillow interiors were analyzed in
this study because glassy rims were rare. Because the suite is nearly aphyric, the analyses are inter- preted as liquid compositions and compared to glass data for MORB. Major element variation
diagrams are presented in Fig. 4, in which SR basalts are compared to MORB and Izu arc volcanic rocks. The basalts show a large range of differentiation, ranging from 8.5 to 3.3 wt.% MgO. They are tholeiitic; Fe203,o,, is high and increases with differentiation. K 2 0 is low throughout the suite, ranging from 0.25 wt.% in the basalts to
- 1.0 wt.% in the rhyolites. K 2 0 is also the most variable element, varying by a factor of two at constant MgO (Fig. 4). High K 2 0 basalts are typified at Shadow Mt, and are common in the outer rift [9], but rare in the en-echelon ridges. Basalts analyzed from the rift wall are similar to other en-echelon ridge basalts despite giving the oldest K - A r dates (Table 2). Basalts from the
TABLE 5
Major element composition of Susimu Rift lavas
Inner Rift Outer Shadow Rift Mixed Rhyolites Rift Mt Walt Dacite
sampte 1894-9 962-1 a 1888-1 1896-7 1890-8 1896-1 1896-3 d l -2 1891-7 1891-Z6 1889-11 1889-4 1896-2 1888-10 1888-13
SiO~ 50.50 48.09 50.33 50.12 50.46 51.27 51.54 50.79 49,85 50.33 48.91 48.08 67,30 72.01 70.50 Ti02 0.86 0.72 0.85 1.15 1.16 1.70 1.68 1.13 0.84 0.84 0.89 0.96 0.61 0.41 0.40 AEZO] 16.34 18.00 17.31 16.34 16.18 16.01 15.87 16.63 16.09 15.98 16.61 17.16 14.94 14.43 14.13 Fe203t 10.12 10.20 9.86 11.06 10.67 12.37 13.55 11.18 9.70 9.66 10.97 11.09 3.71 2.76 2.73 RlgO 8.12 7.35 7.27 7.04 6.36 4.62 4.53 6.76 8.46 7.83 8.00 7.00 1.34 0.37 0.32 CaO 12.96 13.31 13.21 10.66 12.47 9.56 9.82 10.68 12.15 12.55 11.85 11.85 3.22 1.88 2.03 lla20 2.42 1.74 2.19 2.80 2.89 3.33 3.26 2.68 2.44 2.44 2.05 2.21 4.00 6.20 5.62 K20 0.23 0.17 0.25 0.27 0.23 0.36 0.43 0.40 0.32 0.34 0.20 0.20 0.86 1.05 1.67 PZ05 0.08 0.09 0.10 0.15 0.14 0.34 0.25 0.20 0.10 0.11 0.11 0.11 0.17 0.06 0.06 SlIM 101.63 99.67 101.37 99.59 100.56 99.56 100.93 100.45 99.95 100.08 99.59 98.66 96.15 99.17 97.46
nCotlected by GSJ
V O L C A N I S M IN T H E S U M I S U R I F T , I 189
south end of the eastern ridge (Fig. lc; dive 1893, south of sample 10) and from a seamount east of the eastern ridge (Fig. lc; dive 1894, east of sam- ple 9) have lower SiO 2 and Na 2 ° and higher CaO than other SR basalts at similar MgO contents (Fig. 4). These samples, which plot off the main major element trends, are enclosed in Figs. 4 and 5.
In SR basalts, Fe203,o, and TiO 2 contents in- crease with differentiation, but at lower concentra- tions and trends less steep than in MORB (Fig. 4). This behavior contrasts with Izu arc trends, in which Fe203,oo and TiO 2 decrease with differenti- ation [43]. A1203 concentrations in SR basalts are higher and more constant than in MORB, but lower than in Izu arc lavas. SiO 2 concentrations increase with decreasing MgO along a trend simi- lar to MORB. Izu arc lavas evolve to high SiO 2 concentrations along trends steeper than in MORB. CaO, Na20 and K20 concentrations in SR basalt are all slightly higher than in MORB. Izu arc K20 and Na20 concentrations are less than or equal to those of MORB and SR basalts.
The rhyolites contain - 72% SiO z and 1% K20. The sample with heterogeneous H20 (1888-13) deviates from the norm and contains 1.67% K20 (Table 5).
6. Discussion
6.1. Volatiles We argue below that pre-eruption SR basalt
differed from MORB by having higher H20 con- tents, 8D, and H20//S ratios, and perhaps H20// CO 2 ratios; and differed from Izu arc magmas by having lower 834S. In order to assess the pre-erup- tion volatile concentrations and stable isotope compositions of SR basalts, the effects of alter- ation, degassing, and crystallization must first be addressed. Most of our results are unaffected by secondary alteration because FTIR analysis mea- sures only H20 structurally contained within pil- low rim glass. Only sample 1894-9 appears to have gained excess H20. This pillow basalt contains secondary alteration minerals within its interior and the glass rim contains 1.22 wt.% H20 (FTIR) and the suite's highest 6D value. Its high 6D lies on a mixing trend between the en-echelon ridge basalts containing 1.0 wt.% H20, and SMOW (Fig. 3c). Mixing calculations indicate that 1894-9
has gained - 0 . 2 wt.% H20, which agrees well with its measured H 2 0 concentration of 1.22 wt.% (Fig. 3c). The volatile concentrations of all other samples are considered pristine and unaffected by alteration because none plot on mixing trends similar to that defined by 1894-9. Indeed, samples from the en-echelon ridges and Shadow Mt. define a negative correlation between H20 and 8D if 1894-9 is ignored. Similarly, only the CO32-- bearing rhyolite appears to have exchanged C with seawater, and no samples seem to have exchanged S with seawater because sulfate contents and ~34S are uniformly low.
The effects of degassing and crystal fractiona- tion are more difficult to assess. SR basalts and even glass are vesicular, indicating that some de- gassing must have occurred even though all vola- tile contents are undersaturated according to solu- bility considerations based on pure gas phases. Consequently, measured volatile concentrations are considered pre-eruption minima. The CO 2 and S contents are as low as in subaerially erupted basalt despite the absence of even a subaerial summit for pre-emption degassing.
Addition of CO 2 to an H20 undersaturated magma decreases the solubility of H20, instigating volatile exsolution [50]. Indeed, thermodynamic calculations using solubilities for pure CO 2 [31] and pure H20 extrapolated to low pressure from Dudfis [29] and assuming ideal mixing of the dissolved volatile components, indicate suppres- sion of the solubility of either pure volatile when the other is also present [21]. Consequently, the observed positive correlation between U20 and depth probably is controlled by the pressure-de- pendent solubility curve of a mixed vapor phase (Fig. 3a). Exsolution of a mixed vapor phase also has left SR basalts with lower CO 2 contents than in MORB [23,24] and undersaturated relative to the pure CO 2 solubility curve [31]. The inferred pressure-controlled solubility of the mixed vapor phase is debatable, however, because the more H20-rich Shadow Mr. basalts that erupted at deeper depths also contain more K 2 0 than en- echelon ridge basalts (Fig. 3b). Thus, incompatible element enrichment is an alternative explanation for the higher H20 contents in Shadow Mt. basalts.
Although the inferred equilibration with a mixed gas phase, as well as the small range in volatile element concentrations, complicate esti-
190 A.G. H O C H S T A E D T E R El" AL.
mation of pre-emption volatile concentrations and isotopic composition, we believe the high H20 / CO 2 and H 2 0 / S ratios, 634S values, and high 6D are primary for two reasons. First, co-variation of volatile concentrations in SR basalts show little effect of degassing. Closed system degassing creates a positive correlation between CO 2 and H 20 [21]. Open system degassing creates a wide range of CO 2 but little change in H20 because CO 2 is much less soluble [23,24]. Consistently low CO 2 concentrations in SR basalts indicate that the magmas are not variably degassed as in MORB, but leave pre-eruption absolute CO 2 concentra- tions unconstrained. However, if the saturation curves of Mysen [50] are correct, then under- saturation in CO 2 implies high pre-eruption H 2 0 / C O 2 because only in an HzO-rich system is the solubility of CO 2 decreased. Thus, the high degree of vesiculation (15-35%) at 1500-2500 m is due more to H20 than CO 2 exsolution, in contrast to the situation for MORB or Hawaiian basalts. (Alternatively, lower CO 2 solubility may reflect lower eruption temperature than for MORB, but the temperature dependence remains controversial [24].) Relatedly, H20 and S degas in relatively equal proportions at pressures > 30 bars, creating a positive correlation between H20 and S, which allows estimation of pre-eruption H20/S [51]. In SR basalts, H20 and S correlate negatively (Table 3), indicating that the relative concentrations of H20 and S are not dominated by degassing. Thus, high H20/S ratios in SR basalts probably ap- proximate pre-emption values.
Second, co-variation of volatile element con- centrations and their isotopic compositions also show little control by degassing. H20 and 6D values correlate negatively in SR basalts (Fig. 3c), whereas degassing results in a positive correlation [52]. Similarly, the steep positive trend between S content and 834S (Fig. 3d) is inexplicable via degassing unless fractionation factors as high as 1.003 between SO 2 and melt are used to explain the isotopic fractionation, which is unreasonable [53].
Volatile saturated crystal fractionation may also increase H20/CO 2 ratios. Because of the solubil- ity difference, CO 2 preferentially exsolves to the gas phase during fractionation while H20 prefer- entially remains in the melt, increasing in con-
centration. A negative correlation between H20 and CO 2 in Mariana Trough basalts suggests this process [30]. This does not explain the higher H20/CO 2 in SR basalts than in MORB, however, because mafic SR basalts with 8% MgO also have low CO 2 contents.
The 6D in basalts from the Mariana and Lau back-arc basins plot along a mixing trend between MORB and water with a 6D of about - 2 0 to -25%o [33]. Because, as a group, SR basalts plot along this mixing trend (Fig. 3c), we infer that the high water content in SR basalts also is slab-de- rived.
Small differences in volatile concentration and isotopic composition between Shadow Mt. and en-echelon ridge basalts probably preceded erup- tion and reflect source characteristics. Shadow Mt. basalts have higher H 2 0 / S , and lower 634s and 6D ratios; isotopically they are less arc-like.
6.2. Fe 3 +/)~Fe ratios Variation in Fe3+/b~Fe in magma is controlled
by f O 2, temperature, and composition [54]. At constant composition, higher Fe3+ /~Fe results from higher f O 2 at constant temperature or from lower temperature at constant f O 2. In either case, because f O 2 buffers define positive correlations between f O 2 and temperature, higher Fe3+ /~Fe implies higher relative f O 2 buffers at constant composition. Thus, the higher Fe 3 + /ZFe observed in SR basalts compared to MORB, and in Shadow Mt. basalts compared to en-echelon ridge basalts, are probably due to equilibration at a higher fO 2 buffer because the compositional effects are small. That differences in non-volatile composition can be ignored is shown by the compositional factor in Sack et al. [54], which is similar for SR basalts (en-echelon ridge: Zd iXi=05 .2 -0 .59 ; Shadow Mr.: )ZdiX~ = 0.55) and MORB (~d~X i = 0.53 _+ 0.04). If both SR basalts and MORB were erupted at 1200 o C, then using the equations of Sack et al [54] and the revised constants of Kilinc et al. [55], logl0fO 2 values are - 9.64 to - 8.48 for SR basalts. These values lie just below the QFM buffer at 1200°C and are about one log~0fO 2 unit more oxidized than MORB [42]. If SR basalt quenched at lower temperature than MORB due to higher water contents, the relative difference in fO 2 would be even greater.
V O L C A N I S M IN T H E S U M I S U R I F T , I 191
6.3. Differentiation In order to assess the effect of volatiles and
f O 2 on SR basalt differentiation trends, dif- ferences in trends predicted by increased H20 and f O 2 in MORB are compared to differences in trends between SR basalt and MORB. High H20 concentration in magma suppresses plagioclase crystallization to lower MgO contents and reduces the proportion of plagioclase in the fractionating assemblage [1,4,56]. The higher A1203 and lower Fe203,o~, and TiO 2 in SR basalts than in MORB (Fig. 4) are consistent with suppressed plagioclase stability due to increased PH2o. Delayed plagio- clase crystallization allows olivine and augite fractionation to proceed to lower MgO contents before plagioclase crystallization triggers an in- crease in Fe203,o,~ and TiO2, and a decrease in A1203 relative to MgO. A reduced proportion of plagioclase in the fractionating assemblage allows A1203 to remain high and TiO 2 and Fe203,o~ trends to be less steep than in MORB, thus preventing extreme Fe203,o, and TiO 2 enrichment. Overall, differentiation proceeds more quickly to lower MgO contents because of the relatively greater proportion of mafic phases in the fractionating assemblage. SR basalt trends are consistent with these predictions (Fig. 4).
On pseudo-ternary projections, SR basalts plot in the olivine field, but parallel to the 1 atm pl-ol-cpx cotectic when projected from plagio- clase, and above the same cotectic in the plagio- clase field when projected from clinopyroxene (Fig. 5). The clinopyroxene projection may be used as a geohygrometer because the plagioclase field con- tracts relative to olivine with increased PH2o [4]. Thus, the trend on this diagram is consistent with the measured H 2 0 contents of the basalts. The cause for SR basalts plotting parallel to, but be- low, the 1 atm cotectic on the plagioclase projec- tion (Fig. 5a) is currently under investigation. Elevated total pressure is not favored because the rocks contain clinopyroxene microphenocrysts with quench textures, indicating equilibration with clinopyroxene at low rather than high pressure. In addition, samples that plot below the 1 atm cotectic on Fig. 5a do not become more differenti- ated towards the silica apex.
The differences in differentiation trends be- tween SR basalts and N-MORB are similar, though greater in magnitude, to differences between E-
MORB and N-MORB [56]. Because E-MORB is invariably enriched in H 2 0 as well as other in- compatible elements [45], the cause of the dis- placed differentiation trends in E-MORB, SR basalts, and other BABB all may be their enrich- ment in H 2 0 and subsequent suppression of plagioclase crystallization.
Fe3+ /~Fe and f O 2 control the crystallization of magnetite. Sharp increase in SiO 2 and lack of Fe203,o~ enrichment with differentiation are both characteristic of IAB and often attributed to early magnetite precipitation [58]. Flattening and small decreases in the TiO 2 and Fe203,ot, vs. MgO trends as well as occurrence of small opaque grains in SR differentiated basalts signal magnetite precipita- tion in SR basalts at about MgO = 4.0-4.5 wt.% and before precipitation of Ca-poor pyroxene. MORB begins magnetite crystallization at similar MgO contents, although at significantly higher Fe203,o. and TiO 2. Thus, SR basalts demonstrate that high f O 2 is not a necessary consequence of high PH2o- The lack of significantly higher f O 2 despite high PH2o indicates that other factors are responsible for their common conjunction in IAB.
6.4. Melting parameters SR basalts agree well with Klein and Langmuir's
[59] depth correlations for N a2 0 and CaO, as do other BABB. Fe203.o,, on the other hand, plots off the MORB-dep th correlations, as do other BABB, possibly because of increased H20 contents. Be- cause the SR has not yet developed an oceanic crustal structure, the agreement may be fortuitous. However, if not fortuitous, melting parameters which can be inferred by calibrating to MORB [59] are 13-15% melting at a mean pressure of about 10kbar, or deeper than the estimated bot- tom of the crust [60].
The enclosed group of samples in Figs. 4 and 5 plot off the main trends in a manner consistent with derivation from primary melts generated from greater degrees of melting (lower Na20, higher CaO) and greater pressure (lower SiO2) [59]. In Fig. 5a, these samples plot near the olivine-di- opside join, which is consistent with greater de- grees of melting of a common source a n d / o r mean melt segregation occurring at higher pres- sures. These rocks may also be younger than other en-echelon ridge basalts; sample 1893-10 may be about 0.1 Myr younger than sample 1893-5 (Table
192 A.G. HOCHSTAEDTER ET AL.
2). If these inferences are correct then the per cent fusion and depth of melting have increased with time beneath the SR en-echelon ridges.
7. Conclusions
(1) BABB has erupted from the SR since its inception (1.1-1.4 Ma) and has continued within the rift at 1.1 Ma [10]. BABB has continued to erupt within the SR despite contemporaneous eruption of geochemically dissimilar IAB less than 20 km away from the rift axis.
(2) Volatiles distinguish SR basalt from both MORB and IAB. Although degassing must have o c c u r r e d , H 2 0 / S and perhaps H 2 0 / C O 2 c o n -
c e n t r a t i o n ratios, and their isotopic compositions, are not dominated by degassing, alteration, or fractional crystallization. H20 remains one of the best discriminants between BABB and MORB, while CO 2 and S do not.
(3) SR basalts fall on a mixing curve between MORB and H20 containing 6D values of - 2 0 to -25%0. The water endmember is inferred to be slab-derived [32].
(4) Although 634S shows a dramatic difference between the absence of slab-derived S in the rift axis versus its presence 20 km away in the volcanic arc, slab-derived water contents remain high in the rift where H 2 0 / S and H z O / K 2 0 ratios are higher than in MORB.
(5) High H20 contents suppress plagioclase, which imparts some aspects of IAB differentiation onto SR basalts. Suppressed plagioclase fractiona- tion leads to a more mafic fractionating assem- blage which delays the initiation of Fe203,o, and TiO 2 enrichment and produces Fe203.~,,, and TiO 2 trends that are less steep and lower in concentra- tion than in N-MORB.
(6) Slightly higher Fe3+/F~Fe and inferred higher f O 2 in SR basalts than in MORB do not influence magnetite saturation. Inflection points in TiO 2 and Fe203,o,,, interpreted as the onset of magnetite fractionation, remain similar to those of N-MORB.
Acknowledgements
M. Yuasa provided us with preliminary petro- logical information on the Sumisu Rift and with dredge samples collected immediately after the
dive program. S. Mayeda measured the volatile concentrations and isotopic ratios reported here. Jim Carolan prepared the samples for geochemical analysis. The manuscript was improved by con- versations with Q. Williams and reviews by A. Ewart and R.S. Harmon. This work was supported by NSF grants OCE-8512829 to Gill, OCE- 8410605 to Taylor, and INT-8415165 to Taylor, Gill and Craig.
References
1 J.M. Sinton and P. Fryer, Mariana Trough lavas from 18 ° N: Implications for the origin of back arc basin basalts, J. Geophys. Res. 92, 12782-12802, 1987.
2 A.D. Saunders and J.T. Tarney, The geochemistry of basalts from a back-arc spreading center in the East Scotia Sea, Geochim. Cosmochim. Acta 43, 555-572, 1979.
3 J.W. Hawkins and J.T. Melchior, Petrology of Mariana Trough and Lau Basin basalts, J. Geophys. Res. 90, 11431- 11468, 1985.
4 D.R. Baker and D.H. Eggler, Composit ions of anhydrous and hydrous melts coexisting with plagioclase, augite, and olivine or low-Ca pyroxene from 1 arm to 8 kbar: Applica- tion to the Aleutian volcanic center of Atka, Am. Mineral. 72, 12-28, 1987.
5 T. Sekine, T. Katsura and S. Aramaki, Water-saturated phase relations of some andesites with application to the estimation of the initial temperature and water pressure at the time of eruption, Geochim. Cosmochim. Acta 43, 1367- 1376, 1979.
6 B. Taylor, G. Brown, P. Fryer, J.B. Gill, A.G. Hochstaedter, H. Hotta, C.H. Langmuir, M. Leinen, A. Nishimura and T. Urabe, ALViN-SeaBeam studies of the Sumisu Rift, I zu- Bonin Arc, Earth Planet. Sci. Lett. 100, 127-147 (this volume).
7 A.G. Hochstaedter, J.B. Gill and J.D. Morris, Volcanism in the Sumisu Rift, II. Subduction and non-subduction related components, Earth Planet. Sci. Lett. 100, 195-209 (this volume).
8 Y. lkeda and M. Yuasa, Volcanism in nascent back-arc basins behind the Shichito Ridge and adjacent areas in the Izu-Ogasawara arc, northwest Pacific: evidence for mixing between E-type MORB and island arc magmas at the initiation of back-arc rifting, Contrib. Mineral. Petrol. 101, 377-393.
9 P. Fryer, B. Taylor, C.H. Langmuir and A.G. Hochstaedter, Petrology and geochemistry of lavas from the Sumisu and Torishima backarc rifts, Earth Planet. Sci. Lett. 100, 161- 178 (this volume).
10 ODP Leg 126 Shipboard Scientific Party, Volcanism and rifting in the Izu-Bonin forearc and backarc, Nature, in press.
11 J.R. Smith, B. Taylor, A. Malahoff and L. Petersen, Sub- marine volcanism in the Sumisu Rift, I zu-Bonin arc: sub- mersible and deep-tow camera results, Earth Planet. Sci. Lett. 100, 148-160 (this volume).
V O L C A N I S M IN T H E S U M I S U R I F T , l 193
12 A. Nishimura, T. Yamazaki, M. Yuasa, N. Mita and S. Nakao, Bottom sample and heat flow data of Sumisu and Torishima Rifts, Izu-Ogasawara Arc, Geol. Surv. Jap., Mar. Geol. Map Ser. 31, 1988.
13 T. Yamazaki, Heat flow in the Sumisu Rift, Izu-Ogasawara (Bonin) Arc, Bull. Geol. Sure. Jap. 39, 63-70, 1988.
14 G. Brown and B. Taylor, Sea-floor mapping of the Sumisu Rift, Izu-Ogasawara (BoNn) island arc, Bull. Geol. Sure. Jap. 39, 23-38, 1988.
15 C.O. Ingamells, Lithium metaborate flux in silicate analyses, Anal. Chim. Acta 52, 323-334, 1970.
16 G.B. Dalrymple and M.A. Lanphere, Potassium-Argon Dating, Freeman, New York, N.Y., 258 pp, 1969.
17 J.S. Stacey, N.D. Sherrill, G.B. Dalrymple, M.A. Lanphere and N.V. Carpenter, A five-collector system for the simul- taneous measurement of argon isotope ratios in a static mass spectrometer, Int. J. Mass Spectron. Ion Phys. 39, 167-180, 1981.
18 A. Cox and G.B. Dalrymple, Statistical analysis of geomag- netic reversal data and the precision of potassium-argon dating, J. Geophys. Res. 72, 2603-2614, 1967.
19 R.H. Steiger and E. J~iger, Subcommission on geochronol- ogy: Convention on the use of decay constants in geo- and cosmochronology, Earth Planet. Sci. Lett. 36, 359-362, 1977.
20 J.R. Taylor, An Introduction to Error Analysis, University Science Books, Mill Valley, CA, 1982.
21 A.T. Anderson, Jr., S. Newman, S.N. Williams, T.H. Druitt, C. Skitius and E. Stolper, H20, CO2, C1, and gas in plinian and ash-flow Bishop rhyolite, Geology 17, 221-225, 1989.
22 S. Newman, E.M. Stolper and S. Epstein, Measurement of water in rhyolite glasses: calibration of an infrared spectro- scopic technique, Am. Mineral. 71, 1527-1541, 1986.
23 G. Fine and E. Stolper, Dissolved carbon dioxide in basaltic glasses: concentrations and speciation, Earth Planet. Sci. Lett. 76, 263-278, 1986.
24 J.E. Dixon, E. Stolper and J.R. Delaney, Infrared spectro- scopic measurements of CO 2 and H20 in Juan de Fuca basaltic glasses, Earth Planet. Sci. Lett. 90, 87-104, 1988.
25 T. Suzuoki and S. Epstein, Hydrogen isotope fractionation between OH-bearing minerals and water, Geochim. Cosmo- chim. Acta 40, 1229-1240, 1976.
26 A. Ueda and H. Sakai, Simultaneous determinations of the concentration and isotope ratio of sulfate- and sulfide-sulfur and carbonate-carbon in geological samples, Geochem. J. 17, 185-196, 1983.
27 T. Uchida, M. Mitsumatsu, I. Kojima and C. Iida, Rapid spectrophotometric determination of iron (II, II) in silicates with 1, 10-phenanthroline (in Japanese), Bunseki Kagaku 35, 42-46, 1986.
28 A.L. Stork, D.K. Smith and J.B. Gill, Evaluation of geo- chemical reference standards by X-ray fluorescence analy- sis, Geostandards. Newsl. 11, 107-113, 1987.
29 F.(). Dudfis, The effect of volatile content on the vesicula- tion of submarine basalts, Econ. Geol. Monogr. 5, 134-141, 1983.
30 S. Newman, Water and carbon dioxide contents in basaltic glasses from the Mariana Trough, EOS 70, p. 1387, 1989.
31 E. Stolper and J.R. Holloway, Experimental determination
of the solubility of carbon dioxide in molten basalt at low pressure, Earth Planet. Sci. Lett. 87, 397-408, 1988.
32 E.A. Mathez, Sulfur solubility and magmatic sulfides in submarine basalt glass, J. Geophys. Res. 81, 4269-4275, 1976.
33 R. Poreda, Helium-3 and deuterium in back-arc basalts: Lau Basin and the Mariana Trough, Earth. Planet. Sci. Lett. 73, 244-254, 1985.
34 M. Kusakabe, S. Mayeda and E. Nakamura, S, O and Sr isotope systematics of active vent materials from the Mariana backarc basin spreading axis at 18°N, Earth Planet. Sci. Lett. 100, 275-282 (this volume).
35 P.J. Michael, The concentration, behavior and storage of H20 in the suboceanic mantle: implications for mantle metasomatism, Geochim. Cosmochim. Acta 52, 555-566, 1988.
36 H. Craig and J.E. Lupton, Primordial neon, helium and hydrogen in oceanic basalts, Earth Planet. Sci. Lett. 31, 369-385, 1976.
37 H. Sakai, D.J. Des Marais, A. Ueda and J.G. Moore, Concentrations and isotope ratios of carbon, nitrogen and sulfur in ocean-floor basalts, Geochim. Cosmochim. Acta 48, 2433-2441, 1984.
38 M.O. Garcia, N.W.K. Liu and K.W. Muenow, Volatiles in submarine volcanic rocks from the Mariana island arc and trough, Geochim. Cosmochim. Acta 43, 305-312, 1979.
39 A. Ueda and H. Sakai, Sulfur isotope study of Quaternary volcanic rocks from Japanese Island Arc, Geochim. Cosmo- claim. Acta 48, 1837-1848, 1984.
40 D.W. Muenow, N.W.K. Liu, M.O. Garcia and A.D. Saunders, Volatiles in submarine volcanic rocks from the spreading axis of the East Scotia Sea back-arc basin, Earth Planet. Sci. Lett. 47, 272-278, 1980.
41 R. Poreda, J-G. Schilling and H. Craig, Helium and hydro- gen isotopes in ocean-ridge basalts north and south of Iceland, Earth Planet. Sci. Lett. 78, 1-17, 1986.
42 D.M. Christie, I.S.E. Carmicliael and C.H. Langmuir, Oxidations states of mid-ocean ridge basalt glasses, Earth Planet. Sci. Lett. 79, 397-411, 1986.
43 Y. Zhang and C.H. Langmuir, Petrology and geochemistry of Torishima and Sumisujima, Izu arc, in prep.
44 A.P. leRoex, H.J.B. Dick, A.M. Reid, F.A. Frey, A.J. Erlank and S.R. Hart, Petrology and geochemistry of basalts from the American-Antarctic Ridge, Southern Ocean: im- plications for the westward influence of the Bouvet mantle plume, Contrib. Mineral. Petrol 90, 367-380, 1985.
45 D.M. Christie and J.M. Sinton, Major element constraints on melting, differentiation and mixing of magmas from the Galapagos 95.5°W propagating rift system, Contrib. Mineral. Petrol. 94, 274-288, 1986.
46 C.D. Byers, D.W. Muenow and M.O. Garcia, Volatiles in basalts and andesites from the Galapagos Spreading Center, 85 ° to 86 o W, Geochim. Cosmochim. Acta 47, 1551-1558, 1983.
47 G.R. Byerly, W.G. Melson and P.R. Vogt, Rhyodacites, andesites, ferro-basalts and ocean tholeiites from the galapagos spreading center, Earth Planet. Sci. Lett. 30, 215-221, 1976.
48 J.G. Schilling, M. Zajac, R. Evans, T. Jonston, W. White,
194 A.G. H O C H S T A E D T E R E T AL.
J.D. Devine and R. Kingsley, Petrologic and geochemical variations along the Mid-Atlantic Ridge from 29°N to 73°N, Am. J. Sci. 283, 510-586, 1983.
49 J.F. Bender, C.H. Langmuir and G.N. Hanson, Petrogene- sis of basalt glasses from the Tamayo region, East Pacific Rise, J. Petrol. 25, 213-254, 1984.
50 B.O. Mysen, The role of volatiles in silicate melts: solubility of carbon dioxide and water in feldspar, pyroxene and feldspathoid melts to 30 kbar and 1625 ° C, Am. J. Sci. 276, 969-996, 1976.
51 T.M. Gerlach, Exsolution of H20, CO 2 and S during eruptive episodes at Kilauea volcano, Hawaii, J. Geophys. Res. 91, 12, 177-12, 185, 1986.
52 S. Newman, S. Epstein and E. Stolper, Water, carbon dioxide, and hydrogen isotopes in glasses from the ca. 1340 A.D. eruption of the Mono Craters, California: constraints on degassing phenomena and initial volatile content, J. Volcanol. Geotherm. Res. 35, 75-96, 1988.
53 H. Sakai, T.J. Casadevall and J.G. Moore, Chemistry and isotope ratios of sulfur in basalts and volcanic gases at Kilauea Volcano, Hawaii, Geochim. Cosmochim. Acta 48, 2433-2441, 1984.
54 R.O. Sack, I.S.E. Carmichael, M. Rivers and M.S. Ghiorso,
Ferric-ferrous equilibria in natural silicate liquids at 1 bar, Contrib. Mineral. Petrol. 75,369-376, 1980.
55 A. Kilinc, I.S.E. Carmichael, M.L. Rivers and R.O. Sack, The Ferric-ferrous ratio of natural silicate liquids equi- librated in air, Contrib. Mineral. Petrol. 83, 136-140, 1983.
56 P.J. Michael and R.L. Chase, The influence of primary magma composition, H20 and pressure on Mid-Ocean Ridge basalt differentiation, Contrib. Mineral. Petrol. 96, 245-263, 1987.
57 D. Walker, T. Shibita and S.F. DeLong, Abyssal tholeiites from the Oceanographer Fracture Zone II: Phase equi- librium and mixing, Contrib. Mineral. Petrol. 70, 111-125, 1979.
58 J.B. Gill, Orogenic Andesites and Plate Tectonics, 389 pp., Springer, Berlin, 1981.
59 E.M. Klein and C.H. Langmuir, Global correlations of ocean ridge basalt chemistry with axial depth and crustal thickness, J. Geophys. Res. 92, 8089-8115, 1987.
60 E. Honza and K. Tamaki, Bonin Arc, in: The Ocean Basins and Margins, Vol. 7, The Pacific Ocean, A.E.M. Narin and S. Uyeda, eds., Plenum, New York, N.Y., pp. 459-487, 1985.
Related Documents