-
8/7/2019 Treatment of human pulmonary paragonimiasis with triclabendazole- clinical tolerance and drug efficacy
1/4
TRANSACTIONS OFTHEROYALSOCIETYOFTROPICALMEDICINEANDHYGIENE(1998)92,566-569
Treatment of human pulmonary paragonimiasis with triclabendazole: clinicaltolerance and drug efficacyManuel Calvopifia, Ronald H. Guderian, Wilson Paredes, Martha Chico and Philip J . Cooperment of Clinical Investigation, Hospital kzandes, Casilla 17 -17-691, Quito, Ecuador Depart-
AbstractAn open clinical trial to determine the effi cacy and tolerability of postprandial doses of triclabendazoleagainst Paragonhus mexicanus in 62 patients with pulmonary paragonimiasis from the Ecuadorian Ama-zon region was performed. Praziquantel was used as therapeutic control. Patients were allocated atrandom to the following 4 therapeutic regimens: triclabendazole, 5 mgikg once daily for 3 d (16 patients),10 mg/kg twice on one day (15 patients), and 10 mgikg in a single dose (16 patients), and praziquantel,25 mg/kg thrice daily for 3 d (15 patients). Clinical tolerance, based on the frequency and severity of ad-verse reactions, was superior in all 3 triclabendazole regimens to that of praziquantel. No alteration wasobserved in hepato-renal functions or haematological values.The clinical symptoms resolved at a compa-rable rate in all 4 treatment groups. A more rapid parasitological response to treatment, as determined bythe reduction in the average number of parasite eggs found in sputum, was seen in patients treated withtriclabendazole than with praziquantel. By day 90, 60 patients had no egg detected in their sputum; 2patients, treated with a single dose of 10 mgikg, had a few and were re-treated with triclabendazole (5 mgdaily for 3 d). On day 365, none of the patients had eggs in their sputum.Triclabendazole can be recom-mended as an alternative drug of choice for the treatment of pulmonary paragonimiasis; it is as effect iveas praziquantel in clearing infections and better tolerated.Keywords: paragonimiasis, Paragon&us mexicanus, chemotherapy, triclabendazole, tolerance, efficacy, Ecuador
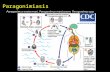
IntroductionPulmonary paragonimiasis is caused by zoonotictrematodes of the genus Paragonimus. Humans may be-come infected after ingesting raw or inadequatelycooked crustaceans which harbour Paragonimus meta-cercariae. The parasite has a tropism for lung tissueswhere adults become encgsted and cause disease. An es-timated 20 million people are currently infected in Afri-ca, Asia and the Americas (TOSCANO et al., 19941. Inrecent years, the infection has become recognizedas asignificant public health problem of increasing impor-tance due to ecological and environmental changes thatfavour transmission (WHO, 1993). In Ecuador, para-gonimiasis is endemic in 15 of the 22 provinces andone-fifth of the total population is at risk of infection.Based on serological epidemiological surveys, it is esti-mated that 500000 persons are infected with the para-site (R. H. Guderian, unpublished data).Drugs used for the treatment of human paragonimia-sis include bithional, emetine, niclofolan, praziquanteland, more recently, triclabendazole. The fir st 3 drugsare associated with significant adverse reactions and lowclinical ef fi cacy (COLEMAN &BARRY, ~~~~;JOHNSONet al., 1985a; CALVOPIfiA et al., 1993). Over the lastdecade, praziquantel has been recognized as the drug ofchoice for this infection with a cure rate of 90% to 100%at standard doses given over 3 d (JOHNSON et al.,1985b; ANONYMOUS, 1992). However, praziquantel isexpensive and the 3 d dosage regimen is, at times, toocumbersome for widespread treatment of isolated ruralcommunities where the disease tends to be endemic.Triclabendazole is a highly effic ient agent against acuteand chronic Fasciola hepatica infection in animals(COLE& 1986), and has been shown to be safe and ef-fec tive in human fascioliasis (APT et al., 1995). Tricla-bendazole is also effecti ve in treating rats experimentallyinfected with I? uterobilateralis (see WEBER et al., 1988),and patients infected with pulmonary paragonimiasis inCameroon were effect ive ly treated with a single dose-(F~PPERT etal., 1992).This renort describes the results of an ouen clinicaltrial comparing 3 different dosage regimens of tricla-bendazole and the standard praziquantel regimen in thetreatment of pulmonary paragonimiasis due to l? mexi-cams.Address for correspondence: Dr Manuel Calvopiiia H., De-partment of Clinical Investigat ion, Hospital Vozandes, HCJB,Casilla 17-17-69 1, Quito, Ecuador, South America.
Materials and MethodsStudy site and patient selectionThe study was undertaken at the regional HealthMinistry Hospital Francisco de Orellana in Coca, Napoprovince, Ecuador, in an area known to be endemic forpulmonary paragonimiasis (AMUNARRIZ, 1980). Thestudy was approved by the institutional ethics commit-tee of Hospital Vozandes and the Ecuadorian Ministryof Public Health.Local hospital medical records of the past 5 yearswere reviewed and communities were identified fromwhich patients had been diagnosed with paragonimia-sis. These communities were visited by a medical teamand all inhabitants who had a history of ingesting raw orundercooked crustaceans and complaining of bloody orrust-coloured sputum were screened for the presence ofParagonimus eggs by direct examination of smears ofsputum samples. Informed consent was obtained fromall infected individuals who wished to enter the study.Those refusing to enter the study were offered a stand-ard course of praziquantel (25 mg/kg twice daily for 34. Sixty-two individuals (25 males and 37 females) withproven pulmonary paragonimiasis (Paragonimus eggspresent in sputum) were recruited for the study. Theirmean age was 27 years (range 12-60). Exclusion criteriafor the study were pregnancy, lactation, or severe con-current illness including sputum-positive tuberculosis.None of the participants had received previous treat-ment for trematode infections.Patient examination and laboratory testsAll patients were admitted to hospital before treat-ment and received a complete physical examination andchest X-ray, and clinical specimens were collected forhaematology (haemoglobin, haematocrit, red blood cellcount, white blood cell count, differential cell count);biochemistry (creatinine, blood urea nitrogen, alaninetransaminase, aspartate transaminase, alkaline phos-phatase, bilirubin [direct and indirect]); urine analysis(glucose, albumin, sediment); sputum and stool exami-nation (enumeration of Paragonimus eggs).Patients were kept in hospital for the first 7 d of thestudy and then followed as outpatients 30, 90 and 365days after treatment. Treatment was given on studydays 1, 2 and 3, depending on the treatment regimen(see below). Adverse drug reactions were assessed dailyfor the first 7 d after treatment. All laboratory tests, as
-
8/7/2019 Treatment of human pulmonary paragonimiasis with triclabendazole- clinical tolerance and drug efficacy
2/4
TREATM ENT OF PARAGONIMIASIS 567
listed above, were repeated on days 4,6,30,90 and 365afte r treatment. Chest X-rays were repeated on day 90after treatment.TreatmentTriclabendazole was supplied by Ciba-Geigy Labo-ratories (Easel, Switzerland). The treatment protocolwas developed- in consultation with the manufacturerand the Division of Control of Tronical Diseases. WorldHealth Organization, Geneva, Switzerland. The 62 patients were allocated at random to one ofthe following 4 treatment groups. A: triclabendazole, 5mgikg daily for 3 d (16 patients); B: triclabendazole, 10mgikg twice on one day (15 patients); C: triclabenda-zole, 10 mgikg in a single dose (16 patients); and D:praziquantel, 25 mgikg thrice daily for 3 d (15 patients).Parasite egg countsEarly morning sputum samples were collected fromeach patient to count the number of Parugonimus eggs.The exact volume was measured and the colour andconsistency of the sample were recorded. An equal vol-ume of 3% NaOH was added and the sample was vor-texed and allowed to stand for 20 min or until all themucus had been liquefied. The samples were then cen-trifuged at 400 g for 5 min. The supernatant was dis-carded and the sediment resuspended in 250 uL of0.9% saline. The number of eggs per mL of sputum wascalculated using a counting chamber under a low-powermicroscope objective.The Kato-Katz technique was used to count thenumber of Puragonimus eggs in stool samples (WHO,1991).Statistical analysisEgg counts were expressed as geometric means. Dif-ferences in drug clearance rates between the differenttreatment regimens were determined by calculating thearea under the curves of the graphs of percentage reduc-tion (compared to baseline counts) over time after treat-ment, using the trapezium rule. These values werelogarithmically transformed and group geometricmeans were compared using one-way analysis o f vari-ance.Table 1. Prevalence of adverse reactions in 62paragonimiasis patients treated with threediffe rent regimens of triclabendazole andpraziquantel
SymptomsNo. of patients reporting symptomaRegimen ARegimen BRegimen CRegimen D
(n=16) (n=15) (n=16) (n=15)Dizziness 2(125%)2(125%) 3(2oqO%) 0 4(26.7%)Headache 2(13.3%)Abdominal oain 2121.5%) 1 (6.7%) 5(3:3%) 6(40.0%)Nausea - 0 0 0 4(26,7%jFever 1 (6.3%) 2(13.3%)Diarrhoea ii : 0 2(13.3%)aRegimens A-C: oral triclabendazole, 5 mgikg daily for 3 d(A), 10 mg/kg twice on one day (B), or 10 mg/kg as a singledose (C). Regimen D: oral praziquantel, 25 mgikg thrice dailyfor 3 d.
ResultsClinical toleranceTransitory adverse reactions were documented in all4 treatment-groups during the fir st 4 d after treatment(Table 1I. The clinical tolerance (based on the freauen-cy and severity of adverse reactions) of all 3 triclabenda-zole regimens studied was superior to that ofpraziquantel; regimen B was the best tolerated. Abdom-inal pain was the chief complaint of those treated withregimen C. Nausea and diarrhoea were reported only bypatients treated with praziquantel.No important change in the laboratory tests was doc-umented after therapy with all 4 drug regimens. All fol-low-up haematological, biochemical and urine analyseswere within normal limits.Chest X-rav before treatment revealed basal infiltra-tion in 46.6% of the subjects and cavitation in 48.3%,which were equally distributed among the 4 groups. At90 d following treatment, all pulmonary infiltrationshad resolved but 37.7% showed oersistent radionranhicevidence of pulmonary cavitation. No differenvce wasseen between the treatment groups.Ejjicacy of treatmentThe eff icacy of treatment was determined by moni-toring clinical response to treatment, sputum volumeand colour, egg count in sputum, and time required forpatients to cease excreting Paragonimus eggs in sputumand stool samples.The clinical response o f patients who presented withclinical symptoms, such as cough and expectoration,improved more effect ive ly with triclabendazole thanwith praziquantel. Clinical symptoms resolved at acomparable rate in all 3 triclabendazole treatmentgroups.All patients in all 4 groups presented an increased vol-ume of sputum at the morning collection before treat-ment. The majority of the sputum samples (66.1%)were rust coloured to brilliant red. The remaining sam-ples (33.9%) were greenish. Decrease in sputum vol-ume was recorded afte r treatment in all 4 groups.However, the decrease was more rapid in patients treat-ed with triclabendazole than in those given praziquan-tel. The colour of sputum returned to normal by day 6after treatment, while the sputum volume did so by day30. There was a reduction in the average number of para-site eggs found in sputum after treatment in all 4 groups(Table 2). A more rapid parasitological response totreatment was seen in patients treated with triclabenda-zole than in those receiving praziquantel; egg clearancewas more rapid in all 3 triclabendazole groups com-pared to praziquantel (Fig. l), though the differencewas not statistically significant. On day zero, however,the average egg count was higher in patients treatedwith praziquantel (108.7imL) than that in any of theother groups (76.6/mL). By day 30, the average eggcount had decreased by 94.2% in those treated with reg-imen A, 92.7% with regimen B, 93.2% with regimen C,and 8 1.8 % in those given praziquantel. By day 90 all ex-cept 2 patients were no longer excreting eggs in theirsputum; the exceptions were both receiving regimen C;they were re-treated with triclabendazole (5 mgid for 3Table 2. Numbers of Pa~agonimus eggs in sputum after treatment with three different regimens oftriclabendazole and praziquantel
Egg counts per mL of sputumbRegimena No. Day 0 Day 4 Day 6 Day 30 Day 90 Day 365A 16 97.8(22-235) 915(13-275) 63.9 (o-203) 9.2 (O-40) 0 0: 1516 63.4(12-187)82.3 (20-267) 46.4(10-186)53.3 (7-160) 32.629.9 (O-146)(5-105) 15.611.8 (O-75)(O-90) 5.4 (i-67) 00D 15 108.7(14400) 85.9(16-273) 65.3(15-293) 20.4 (O-53) 0 0aRegimens A-C: oral triclabendazole, 5 mg/kg daily for 3 d (A), 10 mg/kg twice on one day (B), or 10 mgikg as a single dose (C).Regimen D: oral praziquantel, 25 mg/kg thrice daily for 3 d.bMean (range in parenth eses).
-
8/7/2019 Treatment of human pulmonary paragonimiasis with triclabendazole- clinical tolerance and drug efficacy
3/4
568 MANUELCALVOP~A ETAL.
0 0 4 6 30 90 365Tim e post-treatment (days)
Fig. 1. Reduction in the average numbers of Purugonimus eggsper mL of sputum collected after treatment of patients given 3different regimens of triclabendazole (A-C) and praziquantel(D): +, regime n A (n=16), w, regimen B (n=15); A, regimenC (n=16), and V, regime n D (n=15) (see text for det ails ofdosage).
10090
g 80$! 70201 605
-I
050:
pE 30a 20
100 -r0 90
Tim e post-treatment (days)Fig. 2. Cumulative cure rate (absence of eggs in sputum) ofparagonim iasis patients given 3 different regimens of triclaben-&E;le (A-C) and praziquantel (D) (see text for details of dos-
d) and all patients were egg-free one year after the initialtreatment.The parasite egg clearance time was much more rapidin those treated with triclabendazole than in those givenpraziquantel and the rate of egg clearance was more rap-id with the higher total doses of triclabendazole (regi-mens A and B, Fig. 2). All patients (except the 2receiving re.gimen C referred to above) were not excret-ing eggs-by day 90 after the first treatment.The overall cure rates of the different triclabendazoleregimens used in this study were 87.5% for a single doseof 10 mg/kg and 100% for the other 2 regimens.Only 5 patients had parasite eggs found in stool sam-ples, fewer than in sputum samples: 2 patients on regi-men A and one receiving each of the other regimens (B,C and D). No egg was detected in stools on or after day6 from any patient receiving triclabendazole. In the
praziquantel group (D), eggs were observed in the stoolof 2 patients on day 6 but subsequent examinations re-vealed no egg in any stool sample from this group.DiscussionThis study demonstrated that triclabendazole is a safeand highly effective drug for the treatment of pulmonaryparagonimiasis. All patients treated with the higher dos-age regimens of triclabendazole were cured of infectionwith a single course, as was also achieved with a stand-
ard 3 d course of praziquantel. In our trial, toleranceand the rate of clearance of eggs from the sputum withall regimens of triclabendazole were better than withpraziquantel. The single, low dose regimen of triclaben-dazole fa iled to clear 2 patients of infection, but bothwere subsequently cured with a second similar course ofthe drug. This study confirmed the observations madein Cameroon, where 10 individuals were successfullytreated with triclabendazole (RIPERT et al., 1992).Adverse reactions in patients treated with triclaben-dazole were much less common and less severe than inthose treated with praziquantel. The adverse reactionsexperienced by patients treated with praziquantel in thisstudy (see Table 1) were similar to those reported inother studies (SPITALNY et al., 1982; UDONSI, 1989).Adverse reactions after praziquantel treatment can per-sist for several weeks (SOH et al., 198 1). In our study, alladverse reactions had resolved by the fourth day aftertreatment whether the patients had been treated withpraziquantel or triclabendazole.The ef ficacy of triclabendazole, as shown by the clin-ical response and the clearance rate of parasite eggsfrom sputum, was impressive. The decrease in sputumvolume, an indicator of clinical response, was more rap-id in patients treated with triclabendazole than in thosereceiving praziquantel. Also, a greater proportion ofthose treated with any of the triclabendazole regimenscleared their infections rapidly than those taking prazi-quantel (Fig. 2). The parasite clearance time with prazi-quantel was slow. Other studies have shown that eggspersist in sputum for 3 or 4 months after treatment withpraziquantel (SOH et al., 1981; JOHNSON et al., 1985b).Triclabendazole may have a more rapid killing ef fect onthe adult parasites than praziquantel; however, its modeof action is unknown. Studies in vitro on F. hepaticashowed that triclabendazole can penetrate the tegumentand damage components of the cellular cytoskeleton(BENNET T & KHER, 1987).The low single dose of triclabendazole failed to clearinfection in 2 patients. Factors that may have contribut-ed to this inadequate response include (i) dose inade-quate to kill all adult parasites; (ii) poor drugabsorption; (iii) insufficient penetration of the drug tothe parasite (e.g., the adults may have been in a situa-tion with poor vascularization); (iv) poor uptake of thedrug by the adult parasite (a reflection of its metabolicstatus) ; (v) the patients may have been exposed to infec-tion at the time of treatment and the drug may not beeffecti ve against early stages of the parasite; and (vi) re-infection occurred afte r treatment.Macroscopic monitoring of the volume and colour ofsputum afte r treatment was a good indicator of responseto treatment. In all patients who responded posit ively totreatment, the sputum volume decreased and the colourreverted to normal. In the 2 patients whose sputum stillcontained parasite eggs on day 90, the sputum volumeremained high (data not shown).Triclabendazole can thus be recommended as an al-ternative drug for the treatment of pulmonary para-gonimiasis. The low frequency of side effects and effec -tiveness in clearing the sputum of eggs compared topraziquantel support its usefulness in large scale, com-munity-based treatment programmes. Additional clini-cal trials are encouraged in dif ferent geographical areasto determine the ef ficacy of the single day treatment reg-imen.Clinical cure rates were high with triclabendazole andits cost-effect iveness needs to be evaluated. In commu-nity-based programmes, which include health educa-tion and domestic nutrition counselling, relapsedinfections or reinfections can be detected by subsequentsurveillance and re-treated successfully with triclaben-dazole.AcknowledgementsWe are indebted to Dr A. A. Poltera, Ciba-Geigy, Basel,
-
8/7/2019 Treatment of human pulmonary paragonimiasis with triclabendazole- clinical tolerance and drug efficacy
4/4
TREA TMEN T OF PARAGONIMIASIS
Switzerland and Dr S. Cascante, Department of Medicine, Ci-ba-Geigy Ecuatoriano SA, Quito, Ecuador, for their invalua-ble support during this study. We thank the med ical staff of theHospital Francisco de Orellana for their technic al assista ncethroughout the study. We acknowledge the helpful com mentson the manuscript of Dr Ken E. Mott (deceased), Chief, Schis-tosom iasis Control Unit, Division of Control of Tropica l Dis-eases, WHO, Geneva.ReferencesAmunBrriz, M. (1980). Paragonim iasis en el nororiente Ecua-toriano. Revista Ecuatoriana de Higiene y Medicina Tropical,33,33-50.Anonymous (1992). Drugs for parasitic infe ctions. The MedicalLetter on Drugs and Therapeu tics, 34, 17-26.Apt, W., Aguilera, X., Vega, F., Miranda, C., Zulantay, Y., Pe-rez, C., Gabor, M. & Apt, I? (1995). Treatm ent of huma nchronic fasci olias is with triclabendazole: drug efficacy andserologic response. American Journal of Tropica l Medicine andHygiene, 52, 532-535.Bennett, J. L. & Kher, l? (1987). Fasciola hepatica: action in vit-ro of triclabendazole on immature and adult stages. Experi-mental Parasitology, 63,48-57.Calvopifia, M., Paredes, W ., Guderian, R. H. & Poltera, A. A.
(1993). Efica cia de1 triclabendazole en paragonim iasis pul-monar humana refractaria a la emetina, bithionol y praziqu-antel. Parasitologia al Dia, 17,44-46.Coleman, D. L. & Barry, M. (1982). Relapse of Faragonimuswestermani lung infection after bithional therapy. AmericanJournal of Tropica l Medicine and Hygiene, 31,71-74.Coles, G. C. (1986). Antihelm inthic activity of triclabenda-zole. Journal of Helminthology, 60,2 1 O-2 12.Johnson, R. J., Dunnig, S. B., Minshew, B. H. & Jong, E. C.(1985a). Succe ssful praziquantel treatment of paragonimia-sis following bithionol failure: a case report. American Jour-nal of Tropica l Medicine and Hygiene, 32, 1309-l 3 11.Johnson, R. J., Jong, E . C., Dunnig, S. B., Carberry, W. L. &
569
Minshew, B. H. (1985b). Paragonim iasis: diagno sis and theuse of praziquantel in treatment. Reviews of Infectious Diseas-es. 7.200-204.Rip&t; C., Couprie, B., Moyou, R., Gaillard, F., Appriuo, M.& Tribouley-Duret, J. (1992). Th erapeutic effect of tricla-bendazole in patients with paragonim iasis in Cameroon: apilot study. Transa ctions of the Royal Society of Tropica l Medi-cine and Hygiene, 86,4 17.Soh, C.T.,Ahn,Y. K., Bae, K. H. & Park, C.Y. (1981). Prazi-quantel in the treatment of paragonim iasis. Yonsei Reports onTropica l Medicine, 12, 22-32.Spitalny, K. C., Senft, A. W., Meglio, F. D., Moran, J. & Peters,G. (1982). Treatment of pulmonary paragonim iasis with anew broad-spectrum antihelm intic, praziquantel. Journal ofPediatrics, 101, 144-146.
Toscan o, C., Sen Hai,Y., Nunn, I. & Mott, K. E. (1994). Para-gonim iasis and Tubercu losis-Diagno stic Confusion: a Review ofthe Literature. Geneva: World Health O rganization, mimeo-graphed document WHO/SCHIST0/94.110.Udonsi, J. K. (1989). Clinic al field trials of praziquantel in pul-monary parago nimiasis due to Paragon&us uterobilateralis inendem ic populations of the Igwun B asin, Nigeria. Tropica lMedicine and Parasitology, 40, 65-68.Weber, I?, Biischer, G. & Biittner, D.W. (1988).The effects oftriclabendazole on the lung fluke, Paragonimus uterobilateralisin the experimental host Sigmodon hispidu s. Tropica l Medicineand Parasitology, 39, 322-324.WHO (199 1) Faecal specime ns. In: Bas ic Laboratory Methodsin Medical Parasitology. Geneva: World Health Organization,pp. 25-28.WHO (1993). Report of the WHO Study Group o n the Control ofFood-borne Trematode Infections. Manila 18-26, 1993. Gene-va: World Health Organization.Received 28 May 1998; accepted for publicatio n 16 June1998
1 Announcement /
Top ics in International HealthTop ics in. InternationaZ lieaZth is a series of tutorials produced as interactive CD-ROMs by the Wellcom e Trust
and marketed by CAB International.The following topics are already available: Trachoma, Malaria, Sexually transm itted disease s, and Sickle cell
disease; Leprosy, Tub erculosis, Diarrhoeal disease s, and Schist osom iasis will be available in September 1998,and HIV and AIDS and Nutrition in 1999.Further details and prices can be obtained from CAB International at the following addresses: Wallingford,Oxfordshire, OX10 SDE, UK (phone +44 (0)1491 832111, fax +44 (0)1491 826090, e-m ail publishing@c a-bi.org) or 10 East 40th Street, NewYork, NY 10016, USA (phone +I 212 453 2670 or toll-free +I 800 5284841, fax +l 212 686 7993, e-mail [email protected]).