Biogeosciences, 17, 3815–3835, 2020 https://doi.org/10.5194/bg-17-3815-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. The Southern Annular Mode (SAM) influences phytoplankton communities in the seasonal ice zone of the Southern Ocean Bruce L. Greaves 1 , Andrew T. Davidson 2,3 , Alexander D. Fraser 3,1 , John P. McKinlay 2 , Andrew Martin 1 , Andrew McMinn 1 , and Simon W. Wright 1,2,3 1 Institute for Marine and Antarctic Studies, University of Tasmania, Private Bag 129, Hobart, Tasmania 7001, Australia 2 Australian Antarctic Division, Department of the Environment and Energy, 203 Channel Highway, Kingston, Tasmania 7050, Australia 3 Antarctic Climate & Ecosystems Cooperative Research Centre (ACE CRC), University of Tasmania, Private Bag 80, Hobart, Tasmania 7001, Australia Correspondence: Bruce L. Greaves ([email protected]) Received: 3 October 2019 – Discussion started: 21 October 2019 Revised: 18 May 2020 – Accepted: 27 May 2020 – Published: 23 July 2020 Abstract. Ozone depletion and climate change are causing the Southern Annular Mode (SAM) to become increasingly positive, driving stronger winds southward in the Southern Ocean (SO), with likely effects on phytoplankton habitat due to possible changes in ocean mixing, nutrient upwelling, and sea ice characteristics. This study examined the effect of the SAM and 12 other environmental variables on the abundance of siliceous and calcareous phytoplankton in the seasonal ice zone (SIZ) of the SO. A total of 52 surface- water samples were collected during repeat resupply voyages between Hobart, Australia, and Dumont d’Urville, Antarc- tica, centred around longitude 142 ◦ E, over 11 consecutive austral spring–summer seasons (2002–2012), and spanning 131 d in the spring–summer from 20 October to 28 February. A total of 22 taxa groups, comprised of individual species, groups of species, genera, or higher taxonomic groups, were analysed using CAP analysis (constrained analysis of prin- cipal coordinates), cluster analysis, and correlation. Overall, satellite-derived estimates of total chlorophyll and measured depletion of macronutrients both indicated a more positive SAM was associated with greater productivity in the SIZ. The greatest effect of the SAM on phytoplankton commu- nities was the average value of the SAM across 57 d in the previous austral autumn centred around 11 March, which ex- plained 13.3 % of the variance in community composition in the following spring–summer. This autumn SAM index was significantly correlated pair-wise (p< 0.05) with the relative abundance of 12 of the 22 taxa groups resolved. A more positive SAM favoured increases in the relative abun- dance of large Chaetoceros spp. that predominated later in the spring–summer and reductions in small diatom taxa and siliceous and calcareous flagellates that predominated earlier in the spring–summer. Individual species belonging to the abundant Fragilariopsis genera responded differently to the SAM, indicating the importance of species-level observation in detecting SAM-induced changes in phytoplankton com- munities. The day through the spring–summer on which a sample was collected explained a significant and larger pro- portion (15.4 %) of the variance in the phytoplankton com- munity composition than the SAM, yet this covariate was a proxy for such environmental factors as ice cover and sea sur- face temperature, factors that are regarded as drivers of the extreme seasonal variability in phytoplankton communities in Antarctic waters. The impacts of SAM on phytoplankton, which are the pasture of the SO and principal energy source for Antarctic life, would have ramifications for both carbon export and food availability for higher trophic levels in the SIZ of the SO. Published by Copernicus Publications on behalf of the European Geosciences Union.

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Biogeosciences 17 3815ndash3835 2020httpsdoiorg105194bg-17-3815-2020copy Author(s) 2020 This work is distributed underthe Creative Commons Attribution 40 License
The Southern Annular Mode (SAM) influences phytoplanktoncommunities in the seasonal ice zone of the Southern OceanBruce L Greaves1 Andrew T Davidson23 Alexander D Fraser31 John P McKinlay2 Andrew Martin1Andrew McMinn1 and Simon W Wright123
1Institute for Marine and Antarctic Studies University of TasmaniaPrivate Bag 129 Hobart Tasmania 7001 Australia2Australian Antarctic Division Department of the Environment and Energy203 Channel Highway Kingston Tasmania 7050 Australia3Antarctic Climate amp Ecosystems Cooperative Research Centre (ACE CRC) University of TasmaniaPrivate Bag 80 Hobart Tasmania 7001 Australia
Correspondence Bruce L Greaves (bruceonariagmailcom)
Received 3 October 2019 ndash Discussion started 21 October 2019Revised 18 May 2020 ndash Accepted 27 May 2020 ndash Published 23 July 2020
Abstract Ozone depletion and climate change are causingthe Southern Annular Mode (SAM) to become increasinglypositive driving stronger winds southward in the SouthernOcean (SO) with likely effects on phytoplankton habitatdue to possible changes in ocean mixing nutrient upwellingand sea ice characteristics This study examined the effectof the SAM and 12 other environmental variables on theabundance of siliceous and calcareous phytoplankton in theseasonal ice zone (SIZ) of the SO A total of 52 surface-water samples were collected during repeat resupply voyagesbetween Hobart Australia and Dumont drsquoUrville Antarc-tica centred around longitude 142 E over 11 consecutiveaustral springndashsummer seasons (2002ndash2012) and spanning131 d in the springndashsummer from 20 October to 28 FebruaryA total of 22 taxa groups comprised of individual speciesgroups of species genera or higher taxonomic groups wereanalysed using CAP analysis (constrained analysis of prin-cipal coordinates) cluster analysis and correlation Overallsatellite-derived estimates of total chlorophyll and measureddepletion of macronutrients both indicated a more positiveSAM was associated with greater productivity in the SIZThe greatest effect of the SAM on phytoplankton commu-nities was the average value of the SAM across 57 d in theprevious austral autumn centred around 11 March which ex-plained 133 of the variance in community compositionin the following springndashsummer This autumn SAM indexwas significantly correlated pair-wise (p lt 005) with the
relative abundance of 12 of the 22 taxa groups resolved Amore positive SAM favoured increases in the relative abun-dance of large Chaetoceros spp that predominated later inthe springndashsummer and reductions in small diatom taxa andsiliceous and calcareous flagellates that predominated earlierin the springndashsummer Individual species belonging to theabundant Fragilariopsis genera responded differently to theSAM indicating the importance of species-level observationin detecting SAM-induced changes in phytoplankton com-munities The day through the springndashsummer on which asample was collected explained a significant and larger pro-portion (154 ) of the variance in the phytoplankton com-munity composition than the SAM yet this covariate was aproxy for such environmental factors as ice cover and sea sur-face temperature factors that are regarded as drivers of theextreme seasonal variability in phytoplankton communitiesin Antarctic waters The impacts of SAM on phytoplanktonwhich are the pasture of the SO and principal energy sourcefor Antarctic life would have ramifications for both carbonexport and food availability for higher trophic levels in theSIZ of the SO
Published by Copernicus Publications on behalf of the European Geosciences Union
3816 B L Greaves et al SAM influences phytoplankton in SIZ
1 Introduction
Phytoplankton are the primary producers that feed almost alllife in the oceans In the Southern Ocean (SO) defined asthe southern portions of the Atlantic Ocean Indian Oceanand Pacific Ocean south of 60 S (Arndt et al 2013) springndashsummer phytoplankton blooms in the seasonal ice zone (SIZ)feed swarms of krill which in turn are key food for sea-birds fish whales and almost all Antarctic life (Smetacek2008 Cavicchioli et al 2019) Phytoplankton also play acritical role in ameliorating global climate change by captur-ing carbon through photosynthesis Around one-third of thecarbon fixed by phytoplankton in SIZ of the SO sinks out ofthe surface ocean (Henson et al 2015) more than the globalocean average of around 20 (Boyd and Trull 2007 Ciais etal 2013 Henson et al 2015) With total productivity withinthe SIZ of the SO estimated at 68ndash107 Tg C yrminus1 (Arrigo etal 2008) this equates to 23ndash36 Tg C yrminus1 around 02 ndash03 of the estimated annual global marine biota export of13 Pg (Ciais et al 2013) being sequestered to the deeperocean for climatically significant periods of time likely hun-dreds to thousands of years (Lampitt and Antia 1997) Evenso the SIZ of the SO shows a net release of CO2 fromthe ocean to the atmosphere due to off-gassing of carbon-rich deep-ocean water upwelling at the Antarctic Divergence(Takahashi et al 2009) Thus any changes in the composi-tion and abundance of phytoplankton in the SIZ are likely toinfluence both the trophodynamics of the SO and the contri-bution of the region to oceanicndashatmospheric carbon flux
Global standing stocks of phytoplankton are estimated tohave been declining by as much as 1 per year a declinelargely attributed to rising surface ocean temperature (Boyceet al 2010 2011 Mackas 2011) Furthermore global phy-toplankton productivity is predicted to drop by as much as9 from the years 1990 to 2090 (RCP85 ldquobusiness asusualrdquo) with a decline across most of the Earthrsquos ocean area(Bopp et al 2013) In contrast higher latitudes includingthe SIZ of the SO are predicted to experience an increasein phytoplankton productivity due to changes to seasonal iceextent and duration (Parkinson 2019 Turner et al 2013)andor increased upwelling of nutrient-rich deep ocean waterat the Antarctic Divergence (Steinacher et al 2010 Bopp etal 2013 Carranza and Gille 2015)
11 Importance of the SIZ phytoplankton bloom
The Antarctic SIZ is one of the most productive parts of theSO (Carranza and Gille 2015) It is also a significant com-ponent of the global carbon cycle by virtue of both carbonsequestration by phytoplankton (Henson et al 2015) as wellas upwelling and off-gassing of carbon-rich deep ocean wa-ter (Takahashi et al 2009) It is one of the largest and mostvariable biomes on Earth with sea ice extent varying fromaround 20 million km2 during winter to only 4 million km2
in summer (Turner et al 2015 Massom and Stammerjohn
2010 Parkinson 2019) The most macronutrient-rich surfacewaters of the SIZ occur over the Antarctic Divergence a cir-cumpolar region of the SO located at around 63 S wherecarbon- and nutrient-rich water upwells to the surface sup-plying the nutrients that drive much of the phytoplanktonproduction in the SO (Lovenduski and Gruber 2005 Car-ranza and Gille 2015)
In winter phytoplankton growth is limited by light avail-ability and temperature In spring and summer phytoplank-ton can proliferate in the high-light high-nutrient waters thattrail the southward retreat of sea ice (Fig 1a b) (Wilson etal 1986 Smetacek and Nicol 2005 Lannuzel et al 2007Saenz and Arrigo 2014 Rigual-Hernaacutendez et al 2015)The SIZ supports high phytoplankton standing stocks andproductivity and phytoplankton abundance in blooms candouble every few days (Wilson et al 1986 Sarthou et al2005) Wind speed is a primary determinant of phytoplank-ton bloom development in the SIZ with calmer conditionsfostering shallow mixed depths that maintain phytoplanktoncells in a high-light environment and maximise productivity(Savidge et al 1996 Fitch and Moore 2007) Phytoplank-ton populations are characterised by large-scale spatial andtemporal variability (Martin et al 2012) with only 17 ndash24 of ice edge waters experiencing phytoplankton bloomsin any springndashsummer period (Fitch and Moore 2007)
12 The Southern Annular Mode
The Southern Annular Mode (SAM) which is also variouslyalso called the High-Latitude Mode and the Antarctic Oscil-lation is well-represented by two alternative definitions (a)the normalised zonal mean sea-level pressure at 40 S mi-nus that at 65 S (Gong and Wang 1999 Marshall 2003)or (b) the principal mode of atmospheric circulation at highlatitudes of the Southern Hemisphere The SAM reflects theposition and intensity of a zonally symmetric structure of at-mospheric circulation in the Southern Hemisphere circlingthe Earth (annular) at around 50 S and it has been defined asthe alternating pattern of strengthening and weakening west-erly winds in conjunction with high- to low-pressure bands(Ho et al 2012) Variation in the SAM typically describesaround 35 of total Southern Hemisphere climate variabil-ity (Marshall 2007) and the SAM is currently the dominantlarge-scale mode through which climate change is expressedat SO latitudes (Thompson and Solomon 2002 Lenton andMatear 2007 Lovenduski et al 2007 Swart et al 2015)Between 1979 and 2017 the value of daily SAM averaged004 index points ranged fromminus513 to 464 and had a stan-dard deviation of 138 (after data by NOAA 2017) Averagemonthly SAM varied from minus27 to 25 index points over the11 years studied (Fig 1c)
There was a trend toward more positive SAM from 1979to 2017 of 0011 index points per year (NOAA 2017) at-tributed to both ozone depletion (Thompson and Solomon2002 Arblaster and Meehl 2006 Gillett and Fyfe 2013
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3817
Jones et al 2016) and increasing atmospheric greenhousegas concentrations (Thompson et al 2011) The long-termaverage SAM is now at its most positive level for at least thepast 1000 years (Abram et al 2014) Continuing increases inatmospheric greenhouse gases are expected to drive a furtherpositive increase in the SAM in all seasons (Arblaster andMeehl 2006 Swart and Fyfe 2012 Gillett and Fyfe 2013)despite the expected recovery in stratospheric ozone concen-trations to pre-ozone hole values by around 2065 (Son et al2009 Schiermeier 2009 Thompson et al 2011 Solomon etal 2016)
A more positive SAM indicates the occurrence of astrengthening circumpolar vortex (Marshall 2003 Ho etal 2012) leading to stronger westerly winds and increasedstorminess at high latitudes (Hall and Visbeck 2002 Kwokand Comiso 2002 Lovenduski and Gruber 2005 Arblasterand Meehl 2006) These changes are particularly markedsouth of 60 S in the atmospheric Southern CircumpolarTrough (Hines et al 2000 Mackintosh et al 2017) a re-gion characterised by strong winds with variable direction(Taljaard 1967) Stronger winds associated with more pos-itive SAM may result in increased transport of surface wa-ter northward from the Antarctic Divergence by Ekman drift(Lovenduski and Gruber 2005 DiFiore et al 2006) poten-tially driving increased upwelling of nutrient- and carbon-rich deep ocean water at the Antarctic Divergence (Hall andVisbeck 2002) More positive SAM is also associated withreduced near-surface air temperature over the SIZ due to anincreased frequency of strong southerly winds and increasedcloud cover (Lefebvre et al 2004 Sen Gupta and England2006 Marshall 2007) Sea ice extent around the Antarcticcontinent shows zonal relationships with the SAM with pos-itive relationships between the SAM and sea ice extent inthe western Pacific and Indian sectors of the SO and nega-tive or non-existent relationships in other sectors (Kohyamaand Hartmann 2016) Wind also affects the nature of the seaice breaking up floes via wave interactions increasing flood-ing and changing pack ice density (compressing or openingup the pack) and contributing to ice formation by generatingfrazil ice (Massom and Stammerjohn 2010 Squire 2020)Lower sea-surface temperatures have been observed to lagpositive SAM events by 1 to 4 months (Lefebvre et al 2004Meredith et al 2008) and changes in the SAM may takeweeks to months to be manifested in phytoplankton commu-nities (Sen Gupta and England 2006 Meredith et al 2008)Extreme SAM events might also impact phytoplankton com-munities for multiple years (Ottersen et al 2001)
By modulating upwelling ocean mixed depth air temper-ature and sea ice characteristics and duration it is likely thata more positive SAM will affect the composition and abun-dance of phytoplankton in the SIZ of the SO Lovenduski andGruber (2005) predicted that more positive SAM would sup-port higher phytoplankton productivity and subsequent anal-yses by Arrigo et al (2008) Boyce et al (2010) and Soppaet al (2016) have confirmed a positive relationship between
the SAM and phytoplankton standing stocks and productivitysouth of 60 S in the SIZ
13 The hypothesis
Based on the predicted and observed positive relationshipsbetween the SAM and phytoplankton standing stocks andproductivity in the SIZ of the SO we hypothesised thatchanges in the SAM could also elicit changes in the compo-sition of the phytoplankton community To test this hypothe-sis we conducted a scanning electron microscopic survey ofhard-shelled phytoplankton in surface waters of the AntarcticSIZ using samples collected between October and Februaryeach springndashsummer over 11 consecutive years (2002ndash2003to 2012ndash2013) We then related the composition of thesecommunities to environmental variables including the SAM
2 Methods
A total of 52 surface-water samples were collected from theseasonal ice zone (SIZ) of the Southern Ocean (SO) across11 consecutive austral springndashsummers from 2002ndash2003 to2012ndash2013 The samples were collected aboard the Frenchre-supply vessel MV LrsquoAstrolabe during resupply voyagesbetween Hobart Australia and Dumont drsquoUrville Antarc-tica between 20 October and 28 February Most sampleswere collected from ice-free water although some were col-lected south of the receding ice edge (Fig 1a)
The sampled area was in the Indian sector of the SO span-ning 270 km of latitude between 62 and 645 S and 625 kmof longitude between 136 and 148 E (Fig 2 inset) The arealies gt 100 km north of the Antarctic continental shelf breakin waters gt 3000 m depth
Samples were obtained from the clean seawater line ofthe re-supply vessel from around 3 m depth Each samplerepresented 250 mL of seawater filtered through a 25 mmdiameter polycarbonate-membrane filter with 08 microm pores(Poretics) The filter was then rinsed with two additions ofapproximately 2 mL of Milli-Q water to remove salt thenair dried and stored in a sealed container containing sil-ica gel desiccant Samples were prepared for scanning elec-tron microscope (SEM) survey by mounting each filter ontoa metal stub and sputter coating with 15 nm gold or plat-inum Only organisms possessing hard siliceous or calcare-ous shells were sufficiently well preserved through the sam-ple preparation technique that they could be identified bySEM and these included diatoms coccolithophores sili-coflagellates Pterosperma Parmales radiolarians and ar-moured dinoflagellates
21 Phytoplankton relative abundance
The composition of the phytoplankton community of eachsample was determined from times400 magnification imagescaptured using a JEOL JSM 840 Field Emission SEM Cell
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3818 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 1 (a) Latitude and timing of samples (black filled circles) and sea ice extent at 143 E (grey solid line) (b) monthly total chlorophyll(Acker and Leptoukh 2007 GMAO 2017) across the sampled area (longitude 1357ndash1478 E) northern extent (latitude minus62 N lightgreen solid circles) and southern extent (latitudeminus645 N olive-green open circles) and (c) monthly average of daily SAM (NOAA 2017)
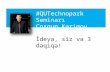
Figure 2 Example of phytoplankton identification on a single SEM image representing 00348 mL of seawater Overlying letters are taxacodes for individual phytoplankton taxa considered in the analysis (listed in Table 3) codes in parenthesis are rare taxa (see text) Insetsampling area in relation to southern Australia and the Antarctic coastline with sample locations indicated as open circles
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3819
numbers for each phytoplankton taxon were counted in arandom selection of captured images taken of each sam-ple Each captured image (Fig 2) represented an area of301 micromtimes 227 microm (area 0068 mm2) of each sample filterwhich was captured at a resolution of 85 pixels per microme-tre A minimum of three SEM images were assessed for eachsample with more images assessed when cell densities werelower ndash individual images were considered as incrementalincreases in the area of a sample covered and not samplingreplicates On average 387 cells were counted for each sam-ple Taxa were classified with the aid of Scott and Marchant(2005) Tomas (1997) and expert opinion Cell counts persample were converted to volume-specific abundances (cellsper mL) by dividing total counts by the number of imagesassessed multiplied by 00348 mL of seawater represented byeach captured image
A total of 48 phytoplankton taxa were identified many tospecies level Because the diatoms Fragilariopsis curta andF cylindrus could not be reliably discriminated at the micro-scope resolution employed they were pooled into a singletaxa group Other taxa were also grouped namely Nitzschiaacicularis with N decipiens to a single group and discoidcentric diatoms of the genera Thalassiosira Actinocyclusand Porosira to another Rare species with maximum rela-tive abundance lt 2 were removed from the data prior toanalysis as they were not considered to be sufficiently abun-dant to warrant further analysis (Webb and Bryson 1972Taylor and Sjunneskog 2002 Swiło et al 2016) After pool-ing taxa and deleting rare taxa 22 taxa and taxonomic-groups (species groups of species and families) remainedto describe the composition of the phytoplankton commu-nity A total of 19 499 phytoplankton organisms were identi-fied and counted 18 878 diatoms 322 Parmales 173 coccol-ithophores 81 silicoflagellates and 45 Petasaria
Phytoplankton abundance data were converted to relativeabundance by dividing each value by the total abundanceof the 22 taxa groups in the sample This was to alleviateany variation among samples resulting from dilution a phe-nomenon whereby the abundance of cells in surface waterscan be reduced in a matter of hours by an abrupt increase inwind speed and associated increase in the mixed layer depth(Carranza and Gille 2015) diluting near-surface cells into agreater water volume However relative abundance has thedisadvantage that blooming of one species will cause a re-duction in relative abundance of other present species whentheir absolute abundances may not have changed
22 Environmental covariates
Phytoplankton abundances were related to a range of envi-ronmental covariates available at the time of sampling Theseincluded the SAM sea surface temperature (SST) salinity(S) time since sea ice cover (DSSI defined below) mini-mum latitude of sea ice in the preceding winter latitude andlongitude of sample collection (LATS and LONGE respec-
tively) the days since 1st October that a sample was collected(D) the year of sampling (Y being the year that each springndashsummer sampling season began) the time of day that a sam-ple was collected and satellite-derived total chlorophyll con-tent Macronutrient concentrations phosphate (PO4) silicate(SiO4) and nitrate+ nitrite (hereafter nitrate NOx) were in-cluded as indicators of nutrient drawdown as a proxy for phy-toplankton productivity (Arrigo et al 1999)
We obtained daily estimates of the SAM from the USNWS Climate Prediction Center (NOAA 2017) This datasetuses the principal component method definition of the SAM(Mo 2000) rather than the simple zonal-mean normalisedpressure difference technique (Gong and Wang 1999) Weused these estimates principally because daily values werereadily available other available estimates were largely sea-sonal averages only (Ho et al 2012) Water samples for dis-solved macronutrients were collected frozen on the ship andlater analysed at the Commonwealth Scientific and IndustrialResearch Organisation in Hobart Australia using standardspectrophotometric methods (Hydes et al 2010) The vari-able DSSI was defined as the time since sea ice had meltedto 20 cover after Wright et al (2010) as determined fromdaily Special Sensor MicrowaveImager (SSMI) sea ice con-centration data distributed by the University of Hamburg(Spreen et al 2008) Total chlorophyll content was estimatedfor each sample location by estimating the total chlorophyllcontent over a 20 kmtimes 20 km area centred at each samplelocation for all available times from 31 August to 1 Mayin the year of sampling (monthly observations) (Acker andLeptoukh 2007 GMAO 2017) and interpolating betweenobservations to estimate total chlorophyll content on the datesampled (some examples are reproduced in Fig S3) By thismethod total chlorophyll was estimated for 49 of the 52 sam-ples the remainder of samples having a paucity of data whichprecluded estimation
23 Statistical analysis
Three statistical analyses were undertaken to explore the hy-pothesis (i) constrained analysis of principal coordinates(CAP Anderson and Willis 2003 also known as distance-based redundancy analysis Legendre and Anderson 1999)was used to estimate the influence of multiple environmentalcovariates in simultaneously explaining community compo-sition (ii) clustering techniques were used to explore similar-ities in phytoplankton community composition among sam-ples independently of environmental information to definesignificantly different groups of samples with similar phyto-plankton community composition and (iii) correlation anal-ysis was used to support observed relationships between phy-toplankton community composition and environmental co-variates
For CAP and cluster analysis relative abundance datawere square-root-transformed to reduce possible dominanceof the analysis by a few abundant taxa The BrayndashCurtis dis-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3820 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 3 Variance in phytoplankton community composition explained by the SAM versus timing and length of the averaged range ofdaily SAM values Response surfaces relate the fraction of total variance in phytoplankton community composition attributable to the SAMversus the number of days in the range of the averaged daily SAM (vertical axis) and the timing of the centre of the range of the averageddaily SAM (horizontal axis) The horizontal axis is expressed as (a) the time through the calendar year of the middle of the range and (b) thenumber of days before a sample was collected to the middle of the range Three obvious maxima are identified with crosses (SAMautumnSAMspring and SAMprior)
similarity index (Bray and Curtis 1957) was used to calcu-late the resemblance of samples based on their communitystructure The advantage of this index for the cell count datawas that similarity among samples was not strongly affectedby the absence of taxa
CAP was applied to the BrayndashCurtis resemblance matrixto partition total variance in community composition into un-constrained and constrained components with the latter rep-resenting the variation due to the environmental covariatesCAP is an example of a constrained ordination method inwhich the typical samplendashspecies matrix of abundances (asused in redundancy analysis) is replaced with a symmetricmatrix of pairwise sample similarities The advantage of thisdistance-based approach to redundancy analysis is that anyecologically relevant distance measure may be used herewe use the BrayndashCurtis metric because it discounts jointabsences between samples when determining similarity Aforward selection strategy was used to choose the optimummodel containing the minimum subset of constraints requiredto explain the most variation in phytoplankton communitystructure (Legendre et al 2011) Linear projections of sig-nificant covariates were plotted as arrows in the ordinationdiagram indicating the direction and magnitude of environ-mental gradients that were correlated with changes in thephytoplankton community (Davidson et al 2016) The vari-ance in phytoplankton community structure (as determinedfrom the ordination) explained by each environmental co-variate was calculated according to the procedure outlined inTer Braak and Verdonschot (1995) and attributed to Dargie(1984) Taxa were added to the CAP plots as weighted site
averages for each species thereby indicating the relative in-fluence of the fitted environmental constraints on each phy-toplankton taxa group
Hierarchical agglomerative clustering based on averagelinkage was performed on the BrayndashCurtis resemblance ma-trix Significant differences among sample clusters were de-termined according to the similarity profile (SIMPROF) per-mutation method of Clarke et al (2008) based on α = 005and 1000 permutations Clustering can identify the presenceof significant differences between the community composi-tion of the samples but clustering cannot identify an effect ofthe SAM at least not directly since environmental covariatesare not included in the cluster analysis
Pair-wise correlation analyses were performed using Pear-sonrsquos correlation coefficient r to explore the relationshipsamong environmental variables and between these environ-mental variables and the relative abundances of phytoplank-ton taxa (Rodgers and Nicewander 1988) Given the largenumber of pair-wise correlations considered we applied aBonferroni correction to give consideration to the family-wise error rate by setting alpha which is usually α = 005(Gibbons and Pratt 1975 Cohen 1990) to αm where mis the total number of correlations considered Recognisingthat αm may be conservative (Nakagawa 2004) we indi-cated when calculated correlations were significant at bothα lt 005 and at Bonferroni-corrected α lt 005m
Response surfaces were used to display the variance ex-plained from individual CAP analyses according to the num-ber of days averaged and the mid-point (or lagged mid-point) of the range of days averaged for each aggregated
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3816 B L Greaves et al SAM influences phytoplankton in SIZ
1 Introduction
Phytoplankton are the primary producers that feed almost alllife in the oceans In the Southern Ocean (SO) defined asthe southern portions of the Atlantic Ocean Indian Oceanand Pacific Ocean south of 60 S (Arndt et al 2013) springndashsummer phytoplankton blooms in the seasonal ice zone (SIZ)feed swarms of krill which in turn are key food for sea-birds fish whales and almost all Antarctic life (Smetacek2008 Cavicchioli et al 2019) Phytoplankton also play acritical role in ameliorating global climate change by captur-ing carbon through photosynthesis Around one-third of thecarbon fixed by phytoplankton in SIZ of the SO sinks out ofthe surface ocean (Henson et al 2015) more than the globalocean average of around 20 (Boyd and Trull 2007 Ciais etal 2013 Henson et al 2015) With total productivity withinthe SIZ of the SO estimated at 68ndash107 Tg C yrminus1 (Arrigo etal 2008) this equates to 23ndash36 Tg C yrminus1 around 02 ndash03 of the estimated annual global marine biota export of13 Pg (Ciais et al 2013) being sequestered to the deeperocean for climatically significant periods of time likely hun-dreds to thousands of years (Lampitt and Antia 1997) Evenso the SIZ of the SO shows a net release of CO2 fromthe ocean to the atmosphere due to off-gassing of carbon-rich deep-ocean water upwelling at the Antarctic Divergence(Takahashi et al 2009) Thus any changes in the composi-tion and abundance of phytoplankton in the SIZ are likely toinfluence both the trophodynamics of the SO and the contri-bution of the region to oceanicndashatmospheric carbon flux
Global standing stocks of phytoplankton are estimated tohave been declining by as much as 1 per year a declinelargely attributed to rising surface ocean temperature (Boyceet al 2010 2011 Mackas 2011) Furthermore global phy-toplankton productivity is predicted to drop by as much as9 from the years 1990 to 2090 (RCP85 ldquobusiness asusualrdquo) with a decline across most of the Earthrsquos ocean area(Bopp et al 2013) In contrast higher latitudes includingthe SIZ of the SO are predicted to experience an increasein phytoplankton productivity due to changes to seasonal iceextent and duration (Parkinson 2019 Turner et al 2013)andor increased upwelling of nutrient-rich deep ocean waterat the Antarctic Divergence (Steinacher et al 2010 Bopp etal 2013 Carranza and Gille 2015)
11 Importance of the SIZ phytoplankton bloom
The Antarctic SIZ is one of the most productive parts of theSO (Carranza and Gille 2015) It is also a significant com-ponent of the global carbon cycle by virtue of both carbonsequestration by phytoplankton (Henson et al 2015) as wellas upwelling and off-gassing of carbon-rich deep ocean wa-ter (Takahashi et al 2009) It is one of the largest and mostvariable biomes on Earth with sea ice extent varying fromaround 20 million km2 during winter to only 4 million km2
in summer (Turner et al 2015 Massom and Stammerjohn
2010 Parkinson 2019) The most macronutrient-rich surfacewaters of the SIZ occur over the Antarctic Divergence a cir-cumpolar region of the SO located at around 63 S wherecarbon- and nutrient-rich water upwells to the surface sup-plying the nutrients that drive much of the phytoplanktonproduction in the SO (Lovenduski and Gruber 2005 Car-ranza and Gille 2015)
In winter phytoplankton growth is limited by light avail-ability and temperature In spring and summer phytoplank-ton can proliferate in the high-light high-nutrient waters thattrail the southward retreat of sea ice (Fig 1a b) (Wilson etal 1986 Smetacek and Nicol 2005 Lannuzel et al 2007Saenz and Arrigo 2014 Rigual-Hernaacutendez et al 2015)The SIZ supports high phytoplankton standing stocks andproductivity and phytoplankton abundance in blooms candouble every few days (Wilson et al 1986 Sarthou et al2005) Wind speed is a primary determinant of phytoplank-ton bloom development in the SIZ with calmer conditionsfostering shallow mixed depths that maintain phytoplanktoncells in a high-light environment and maximise productivity(Savidge et al 1996 Fitch and Moore 2007) Phytoplank-ton populations are characterised by large-scale spatial andtemporal variability (Martin et al 2012) with only 17 ndash24 of ice edge waters experiencing phytoplankton bloomsin any springndashsummer period (Fitch and Moore 2007)
12 The Southern Annular Mode
The Southern Annular Mode (SAM) which is also variouslyalso called the High-Latitude Mode and the Antarctic Oscil-lation is well-represented by two alternative definitions (a)the normalised zonal mean sea-level pressure at 40 S mi-nus that at 65 S (Gong and Wang 1999 Marshall 2003)or (b) the principal mode of atmospheric circulation at highlatitudes of the Southern Hemisphere The SAM reflects theposition and intensity of a zonally symmetric structure of at-mospheric circulation in the Southern Hemisphere circlingthe Earth (annular) at around 50 S and it has been defined asthe alternating pattern of strengthening and weakening west-erly winds in conjunction with high- to low-pressure bands(Ho et al 2012) Variation in the SAM typically describesaround 35 of total Southern Hemisphere climate variabil-ity (Marshall 2007) and the SAM is currently the dominantlarge-scale mode through which climate change is expressedat SO latitudes (Thompson and Solomon 2002 Lenton andMatear 2007 Lovenduski et al 2007 Swart et al 2015)Between 1979 and 2017 the value of daily SAM averaged004 index points ranged fromminus513 to 464 and had a stan-dard deviation of 138 (after data by NOAA 2017) Averagemonthly SAM varied from minus27 to 25 index points over the11 years studied (Fig 1c)
There was a trend toward more positive SAM from 1979to 2017 of 0011 index points per year (NOAA 2017) at-tributed to both ozone depletion (Thompson and Solomon2002 Arblaster and Meehl 2006 Gillett and Fyfe 2013
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3817
Jones et al 2016) and increasing atmospheric greenhousegas concentrations (Thompson et al 2011) The long-termaverage SAM is now at its most positive level for at least thepast 1000 years (Abram et al 2014) Continuing increases inatmospheric greenhouse gases are expected to drive a furtherpositive increase in the SAM in all seasons (Arblaster andMeehl 2006 Swart and Fyfe 2012 Gillett and Fyfe 2013)despite the expected recovery in stratospheric ozone concen-trations to pre-ozone hole values by around 2065 (Son et al2009 Schiermeier 2009 Thompson et al 2011 Solomon etal 2016)
A more positive SAM indicates the occurrence of astrengthening circumpolar vortex (Marshall 2003 Ho etal 2012) leading to stronger westerly winds and increasedstorminess at high latitudes (Hall and Visbeck 2002 Kwokand Comiso 2002 Lovenduski and Gruber 2005 Arblasterand Meehl 2006) These changes are particularly markedsouth of 60 S in the atmospheric Southern CircumpolarTrough (Hines et al 2000 Mackintosh et al 2017) a re-gion characterised by strong winds with variable direction(Taljaard 1967) Stronger winds associated with more pos-itive SAM may result in increased transport of surface wa-ter northward from the Antarctic Divergence by Ekman drift(Lovenduski and Gruber 2005 DiFiore et al 2006) poten-tially driving increased upwelling of nutrient- and carbon-rich deep ocean water at the Antarctic Divergence (Hall andVisbeck 2002) More positive SAM is also associated withreduced near-surface air temperature over the SIZ due to anincreased frequency of strong southerly winds and increasedcloud cover (Lefebvre et al 2004 Sen Gupta and England2006 Marshall 2007) Sea ice extent around the Antarcticcontinent shows zonal relationships with the SAM with pos-itive relationships between the SAM and sea ice extent inthe western Pacific and Indian sectors of the SO and nega-tive or non-existent relationships in other sectors (Kohyamaand Hartmann 2016) Wind also affects the nature of the seaice breaking up floes via wave interactions increasing flood-ing and changing pack ice density (compressing or openingup the pack) and contributing to ice formation by generatingfrazil ice (Massom and Stammerjohn 2010 Squire 2020)Lower sea-surface temperatures have been observed to lagpositive SAM events by 1 to 4 months (Lefebvre et al 2004Meredith et al 2008) and changes in the SAM may takeweeks to months to be manifested in phytoplankton commu-nities (Sen Gupta and England 2006 Meredith et al 2008)Extreme SAM events might also impact phytoplankton com-munities for multiple years (Ottersen et al 2001)
By modulating upwelling ocean mixed depth air temper-ature and sea ice characteristics and duration it is likely thata more positive SAM will affect the composition and abun-dance of phytoplankton in the SIZ of the SO Lovenduski andGruber (2005) predicted that more positive SAM would sup-port higher phytoplankton productivity and subsequent anal-yses by Arrigo et al (2008) Boyce et al (2010) and Soppaet al (2016) have confirmed a positive relationship between
the SAM and phytoplankton standing stocks and productivitysouth of 60 S in the SIZ
13 The hypothesis
Based on the predicted and observed positive relationshipsbetween the SAM and phytoplankton standing stocks andproductivity in the SIZ of the SO we hypothesised thatchanges in the SAM could also elicit changes in the compo-sition of the phytoplankton community To test this hypothe-sis we conducted a scanning electron microscopic survey ofhard-shelled phytoplankton in surface waters of the AntarcticSIZ using samples collected between October and Februaryeach springndashsummer over 11 consecutive years (2002ndash2003to 2012ndash2013) We then related the composition of thesecommunities to environmental variables including the SAM
2 Methods
A total of 52 surface-water samples were collected from theseasonal ice zone (SIZ) of the Southern Ocean (SO) across11 consecutive austral springndashsummers from 2002ndash2003 to2012ndash2013 The samples were collected aboard the Frenchre-supply vessel MV LrsquoAstrolabe during resupply voyagesbetween Hobart Australia and Dumont drsquoUrville Antarc-tica between 20 October and 28 February Most sampleswere collected from ice-free water although some were col-lected south of the receding ice edge (Fig 1a)
The sampled area was in the Indian sector of the SO span-ning 270 km of latitude between 62 and 645 S and 625 kmof longitude between 136 and 148 E (Fig 2 inset) The arealies gt 100 km north of the Antarctic continental shelf breakin waters gt 3000 m depth
Samples were obtained from the clean seawater line ofthe re-supply vessel from around 3 m depth Each samplerepresented 250 mL of seawater filtered through a 25 mmdiameter polycarbonate-membrane filter with 08 microm pores(Poretics) The filter was then rinsed with two additions ofapproximately 2 mL of Milli-Q water to remove salt thenair dried and stored in a sealed container containing sil-ica gel desiccant Samples were prepared for scanning elec-tron microscope (SEM) survey by mounting each filter ontoa metal stub and sputter coating with 15 nm gold or plat-inum Only organisms possessing hard siliceous or calcare-ous shells were sufficiently well preserved through the sam-ple preparation technique that they could be identified bySEM and these included diatoms coccolithophores sili-coflagellates Pterosperma Parmales radiolarians and ar-moured dinoflagellates
21 Phytoplankton relative abundance
The composition of the phytoplankton community of eachsample was determined from times400 magnification imagescaptured using a JEOL JSM 840 Field Emission SEM Cell
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3818 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 1 (a) Latitude and timing of samples (black filled circles) and sea ice extent at 143 E (grey solid line) (b) monthly total chlorophyll(Acker and Leptoukh 2007 GMAO 2017) across the sampled area (longitude 1357ndash1478 E) northern extent (latitude minus62 N lightgreen solid circles) and southern extent (latitudeminus645 N olive-green open circles) and (c) monthly average of daily SAM (NOAA 2017)
Figure 2 Example of phytoplankton identification on a single SEM image representing 00348 mL of seawater Overlying letters are taxacodes for individual phytoplankton taxa considered in the analysis (listed in Table 3) codes in parenthesis are rare taxa (see text) Insetsampling area in relation to southern Australia and the Antarctic coastline with sample locations indicated as open circles
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3819
numbers for each phytoplankton taxon were counted in arandom selection of captured images taken of each sam-ple Each captured image (Fig 2) represented an area of301 micromtimes 227 microm (area 0068 mm2) of each sample filterwhich was captured at a resolution of 85 pixels per microme-tre A minimum of three SEM images were assessed for eachsample with more images assessed when cell densities werelower ndash individual images were considered as incrementalincreases in the area of a sample covered and not samplingreplicates On average 387 cells were counted for each sam-ple Taxa were classified with the aid of Scott and Marchant(2005) Tomas (1997) and expert opinion Cell counts persample were converted to volume-specific abundances (cellsper mL) by dividing total counts by the number of imagesassessed multiplied by 00348 mL of seawater represented byeach captured image
A total of 48 phytoplankton taxa were identified many tospecies level Because the diatoms Fragilariopsis curta andF cylindrus could not be reliably discriminated at the micro-scope resolution employed they were pooled into a singletaxa group Other taxa were also grouped namely Nitzschiaacicularis with N decipiens to a single group and discoidcentric diatoms of the genera Thalassiosira Actinocyclusand Porosira to another Rare species with maximum rela-tive abundance lt 2 were removed from the data prior toanalysis as they were not considered to be sufficiently abun-dant to warrant further analysis (Webb and Bryson 1972Taylor and Sjunneskog 2002 Swiło et al 2016) After pool-ing taxa and deleting rare taxa 22 taxa and taxonomic-groups (species groups of species and families) remainedto describe the composition of the phytoplankton commu-nity A total of 19 499 phytoplankton organisms were identi-fied and counted 18 878 diatoms 322 Parmales 173 coccol-ithophores 81 silicoflagellates and 45 Petasaria
Phytoplankton abundance data were converted to relativeabundance by dividing each value by the total abundanceof the 22 taxa groups in the sample This was to alleviateany variation among samples resulting from dilution a phe-nomenon whereby the abundance of cells in surface waterscan be reduced in a matter of hours by an abrupt increase inwind speed and associated increase in the mixed layer depth(Carranza and Gille 2015) diluting near-surface cells into agreater water volume However relative abundance has thedisadvantage that blooming of one species will cause a re-duction in relative abundance of other present species whentheir absolute abundances may not have changed
22 Environmental covariates
Phytoplankton abundances were related to a range of envi-ronmental covariates available at the time of sampling Theseincluded the SAM sea surface temperature (SST) salinity(S) time since sea ice cover (DSSI defined below) mini-mum latitude of sea ice in the preceding winter latitude andlongitude of sample collection (LATS and LONGE respec-
tively) the days since 1st October that a sample was collected(D) the year of sampling (Y being the year that each springndashsummer sampling season began) the time of day that a sam-ple was collected and satellite-derived total chlorophyll con-tent Macronutrient concentrations phosphate (PO4) silicate(SiO4) and nitrate+ nitrite (hereafter nitrate NOx) were in-cluded as indicators of nutrient drawdown as a proxy for phy-toplankton productivity (Arrigo et al 1999)
We obtained daily estimates of the SAM from the USNWS Climate Prediction Center (NOAA 2017) This datasetuses the principal component method definition of the SAM(Mo 2000) rather than the simple zonal-mean normalisedpressure difference technique (Gong and Wang 1999) Weused these estimates principally because daily values werereadily available other available estimates were largely sea-sonal averages only (Ho et al 2012) Water samples for dis-solved macronutrients were collected frozen on the ship andlater analysed at the Commonwealth Scientific and IndustrialResearch Organisation in Hobart Australia using standardspectrophotometric methods (Hydes et al 2010) The vari-able DSSI was defined as the time since sea ice had meltedto 20 cover after Wright et al (2010) as determined fromdaily Special Sensor MicrowaveImager (SSMI) sea ice con-centration data distributed by the University of Hamburg(Spreen et al 2008) Total chlorophyll content was estimatedfor each sample location by estimating the total chlorophyllcontent over a 20 kmtimes 20 km area centred at each samplelocation for all available times from 31 August to 1 Mayin the year of sampling (monthly observations) (Acker andLeptoukh 2007 GMAO 2017) and interpolating betweenobservations to estimate total chlorophyll content on the datesampled (some examples are reproduced in Fig S3) By thismethod total chlorophyll was estimated for 49 of the 52 sam-ples the remainder of samples having a paucity of data whichprecluded estimation
23 Statistical analysis
Three statistical analyses were undertaken to explore the hy-pothesis (i) constrained analysis of principal coordinates(CAP Anderson and Willis 2003 also known as distance-based redundancy analysis Legendre and Anderson 1999)was used to estimate the influence of multiple environmentalcovariates in simultaneously explaining community compo-sition (ii) clustering techniques were used to explore similar-ities in phytoplankton community composition among sam-ples independently of environmental information to definesignificantly different groups of samples with similar phyto-plankton community composition and (iii) correlation anal-ysis was used to support observed relationships between phy-toplankton community composition and environmental co-variates
For CAP and cluster analysis relative abundance datawere square-root-transformed to reduce possible dominanceof the analysis by a few abundant taxa The BrayndashCurtis dis-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3820 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 3 Variance in phytoplankton community composition explained by the SAM versus timing and length of the averaged range ofdaily SAM values Response surfaces relate the fraction of total variance in phytoplankton community composition attributable to the SAMversus the number of days in the range of the averaged daily SAM (vertical axis) and the timing of the centre of the range of the averageddaily SAM (horizontal axis) The horizontal axis is expressed as (a) the time through the calendar year of the middle of the range and (b) thenumber of days before a sample was collected to the middle of the range Three obvious maxima are identified with crosses (SAMautumnSAMspring and SAMprior)
similarity index (Bray and Curtis 1957) was used to calcu-late the resemblance of samples based on their communitystructure The advantage of this index for the cell count datawas that similarity among samples was not strongly affectedby the absence of taxa
CAP was applied to the BrayndashCurtis resemblance matrixto partition total variance in community composition into un-constrained and constrained components with the latter rep-resenting the variation due to the environmental covariatesCAP is an example of a constrained ordination method inwhich the typical samplendashspecies matrix of abundances (asused in redundancy analysis) is replaced with a symmetricmatrix of pairwise sample similarities The advantage of thisdistance-based approach to redundancy analysis is that anyecologically relevant distance measure may be used herewe use the BrayndashCurtis metric because it discounts jointabsences between samples when determining similarity Aforward selection strategy was used to choose the optimummodel containing the minimum subset of constraints requiredto explain the most variation in phytoplankton communitystructure (Legendre et al 2011) Linear projections of sig-nificant covariates were plotted as arrows in the ordinationdiagram indicating the direction and magnitude of environ-mental gradients that were correlated with changes in thephytoplankton community (Davidson et al 2016) The vari-ance in phytoplankton community structure (as determinedfrom the ordination) explained by each environmental co-variate was calculated according to the procedure outlined inTer Braak and Verdonschot (1995) and attributed to Dargie(1984) Taxa were added to the CAP plots as weighted site
averages for each species thereby indicating the relative in-fluence of the fitted environmental constraints on each phy-toplankton taxa group
Hierarchical agglomerative clustering based on averagelinkage was performed on the BrayndashCurtis resemblance ma-trix Significant differences among sample clusters were de-termined according to the similarity profile (SIMPROF) per-mutation method of Clarke et al (2008) based on α = 005and 1000 permutations Clustering can identify the presenceof significant differences between the community composi-tion of the samples but clustering cannot identify an effect ofthe SAM at least not directly since environmental covariatesare not included in the cluster analysis
Pair-wise correlation analyses were performed using Pear-sonrsquos correlation coefficient r to explore the relationshipsamong environmental variables and between these environ-mental variables and the relative abundances of phytoplank-ton taxa (Rodgers and Nicewander 1988) Given the largenumber of pair-wise correlations considered we applied aBonferroni correction to give consideration to the family-wise error rate by setting alpha which is usually α = 005(Gibbons and Pratt 1975 Cohen 1990) to αm where mis the total number of correlations considered Recognisingthat αm may be conservative (Nakagawa 2004) we indi-cated when calculated correlations were significant at bothα lt 005 and at Bonferroni-corrected α lt 005m
Response surfaces were used to display the variance ex-plained from individual CAP analyses according to the num-ber of days averaged and the mid-point (or lagged mid-point) of the range of days averaged for each aggregated
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3817
Jones et al 2016) and increasing atmospheric greenhousegas concentrations (Thompson et al 2011) The long-termaverage SAM is now at its most positive level for at least thepast 1000 years (Abram et al 2014) Continuing increases inatmospheric greenhouse gases are expected to drive a furtherpositive increase in the SAM in all seasons (Arblaster andMeehl 2006 Swart and Fyfe 2012 Gillett and Fyfe 2013)despite the expected recovery in stratospheric ozone concen-trations to pre-ozone hole values by around 2065 (Son et al2009 Schiermeier 2009 Thompson et al 2011 Solomon etal 2016)
A more positive SAM indicates the occurrence of astrengthening circumpolar vortex (Marshall 2003 Ho etal 2012) leading to stronger westerly winds and increasedstorminess at high latitudes (Hall and Visbeck 2002 Kwokand Comiso 2002 Lovenduski and Gruber 2005 Arblasterand Meehl 2006) These changes are particularly markedsouth of 60 S in the atmospheric Southern CircumpolarTrough (Hines et al 2000 Mackintosh et al 2017) a re-gion characterised by strong winds with variable direction(Taljaard 1967) Stronger winds associated with more pos-itive SAM may result in increased transport of surface wa-ter northward from the Antarctic Divergence by Ekman drift(Lovenduski and Gruber 2005 DiFiore et al 2006) poten-tially driving increased upwelling of nutrient- and carbon-rich deep ocean water at the Antarctic Divergence (Hall andVisbeck 2002) More positive SAM is also associated withreduced near-surface air temperature over the SIZ due to anincreased frequency of strong southerly winds and increasedcloud cover (Lefebvre et al 2004 Sen Gupta and England2006 Marshall 2007) Sea ice extent around the Antarcticcontinent shows zonal relationships with the SAM with pos-itive relationships between the SAM and sea ice extent inthe western Pacific and Indian sectors of the SO and nega-tive or non-existent relationships in other sectors (Kohyamaand Hartmann 2016) Wind also affects the nature of the seaice breaking up floes via wave interactions increasing flood-ing and changing pack ice density (compressing or openingup the pack) and contributing to ice formation by generatingfrazil ice (Massom and Stammerjohn 2010 Squire 2020)Lower sea-surface temperatures have been observed to lagpositive SAM events by 1 to 4 months (Lefebvre et al 2004Meredith et al 2008) and changes in the SAM may takeweeks to months to be manifested in phytoplankton commu-nities (Sen Gupta and England 2006 Meredith et al 2008)Extreme SAM events might also impact phytoplankton com-munities for multiple years (Ottersen et al 2001)
By modulating upwelling ocean mixed depth air temper-ature and sea ice characteristics and duration it is likely thata more positive SAM will affect the composition and abun-dance of phytoplankton in the SIZ of the SO Lovenduski andGruber (2005) predicted that more positive SAM would sup-port higher phytoplankton productivity and subsequent anal-yses by Arrigo et al (2008) Boyce et al (2010) and Soppaet al (2016) have confirmed a positive relationship between
the SAM and phytoplankton standing stocks and productivitysouth of 60 S in the SIZ
13 The hypothesis
Based on the predicted and observed positive relationshipsbetween the SAM and phytoplankton standing stocks andproductivity in the SIZ of the SO we hypothesised thatchanges in the SAM could also elicit changes in the compo-sition of the phytoplankton community To test this hypothe-sis we conducted a scanning electron microscopic survey ofhard-shelled phytoplankton in surface waters of the AntarcticSIZ using samples collected between October and Februaryeach springndashsummer over 11 consecutive years (2002ndash2003to 2012ndash2013) We then related the composition of thesecommunities to environmental variables including the SAM
2 Methods
A total of 52 surface-water samples were collected from theseasonal ice zone (SIZ) of the Southern Ocean (SO) across11 consecutive austral springndashsummers from 2002ndash2003 to2012ndash2013 The samples were collected aboard the Frenchre-supply vessel MV LrsquoAstrolabe during resupply voyagesbetween Hobart Australia and Dumont drsquoUrville Antarc-tica between 20 October and 28 February Most sampleswere collected from ice-free water although some were col-lected south of the receding ice edge (Fig 1a)
The sampled area was in the Indian sector of the SO span-ning 270 km of latitude between 62 and 645 S and 625 kmof longitude between 136 and 148 E (Fig 2 inset) The arealies gt 100 km north of the Antarctic continental shelf breakin waters gt 3000 m depth
Samples were obtained from the clean seawater line ofthe re-supply vessel from around 3 m depth Each samplerepresented 250 mL of seawater filtered through a 25 mmdiameter polycarbonate-membrane filter with 08 microm pores(Poretics) The filter was then rinsed with two additions ofapproximately 2 mL of Milli-Q water to remove salt thenair dried and stored in a sealed container containing sil-ica gel desiccant Samples were prepared for scanning elec-tron microscope (SEM) survey by mounting each filter ontoa metal stub and sputter coating with 15 nm gold or plat-inum Only organisms possessing hard siliceous or calcare-ous shells were sufficiently well preserved through the sam-ple preparation technique that they could be identified bySEM and these included diatoms coccolithophores sili-coflagellates Pterosperma Parmales radiolarians and ar-moured dinoflagellates
21 Phytoplankton relative abundance
The composition of the phytoplankton community of eachsample was determined from times400 magnification imagescaptured using a JEOL JSM 840 Field Emission SEM Cell
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3818 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 1 (a) Latitude and timing of samples (black filled circles) and sea ice extent at 143 E (grey solid line) (b) monthly total chlorophyll(Acker and Leptoukh 2007 GMAO 2017) across the sampled area (longitude 1357ndash1478 E) northern extent (latitude minus62 N lightgreen solid circles) and southern extent (latitudeminus645 N olive-green open circles) and (c) monthly average of daily SAM (NOAA 2017)
Figure 2 Example of phytoplankton identification on a single SEM image representing 00348 mL of seawater Overlying letters are taxacodes for individual phytoplankton taxa considered in the analysis (listed in Table 3) codes in parenthesis are rare taxa (see text) Insetsampling area in relation to southern Australia and the Antarctic coastline with sample locations indicated as open circles
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3819
numbers for each phytoplankton taxon were counted in arandom selection of captured images taken of each sam-ple Each captured image (Fig 2) represented an area of301 micromtimes 227 microm (area 0068 mm2) of each sample filterwhich was captured at a resolution of 85 pixels per microme-tre A minimum of three SEM images were assessed for eachsample with more images assessed when cell densities werelower ndash individual images were considered as incrementalincreases in the area of a sample covered and not samplingreplicates On average 387 cells were counted for each sam-ple Taxa were classified with the aid of Scott and Marchant(2005) Tomas (1997) and expert opinion Cell counts persample were converted to volume-specific abundances (cellsper mL) by dividing total counts by the number of imagesassessed multiplied by 00348 mL of seawater represented byeach captured image
A total of 48 phytoplankton taxa were identified many tospecies level Because the diatoms Fragilariopsis curta andF cylindrus could not be reliably discriminated at the micro-scope resolution employed they were pooled into a singletaxa group Other taxa were also grouped namely Nitzschiaacicularis with N decipiens to a single group and discoidcentric diatoms of the genera Thalassiosira Actinocyclusand Porosira to another Rare species with maximum rela-tive abundance lt 2 were removed from the data prior toanalysis as they were not considered to be sufficiently abun-dant to warrant further analysis (Webb and Bryson 1972Taylor and Sjunneskog 2002 Swiło et al 2016) After pool-ing taxa and deleting rare taxa 22 taxa and taxonomic-groups (species groups of species and families) remainedto describe the composition of the phytoplankton commu-nity A total of 19 499 phytoplankton organisms were identi-fied and counted 18 878 diatoms 322 Parmales 173 coccol-ithophores 81 silicoflagellates and 45 Petasaria
Phytoplankton abundance data were converted to relativeabundance by dividing each value by the total abundanceof the 22 taxa groups in the sample This was to alleviateany variation among samples resulting from dilution a phe-nomenon whereby the abundance of cells in surface waterscan be reduced in a matter of hours by an abrupt increase inwind speed and associated increase in the mixed layer depth(Carranza and Gille 2015) diluting near-surface cells into agreater water volume However relative abundance has thedisadvantage that blooming of one species will cause a re-duction in relative abundance of other present species whentheir absolute abundances may not have changed
22 Environmental covariates
Phytoplankton abundances were related to a range of envi-ronmental covariates available at the time of sampling Theseincluded the SAM sea surface temperature (SST) salinity(S) time since sea ice cover (DSSI defined below) mini-mum latitude of sea ice in the preceding winter latitude andlongitude of sample collection (LATS and LONGE respec-
tively) the days since 1st October that a sample was collected(D) the year of sampling (Y being the year that each springndashsummer sampling season began) the time of day that a sam-ple was collected and satellite-derived total chlorophyll con-tent Macronutrient concentrations phosphate (PO4) silicate(SiO4) and nitrate+ nitrite (hereafter nitrate NOx) were in-cluded as indicators of nutrient drawdown as a proxy for phy-toplankton productivity (Arrigo et al 1999)
We obtained daily estimates of the SAM from the USNWS Climate Prediction Center (NOAA 2017) This datasetuses the principal component method definition of the SAM(Mo 2000) rather than the simple zonal-mean normalisedpressure difference technique (Gong and Wang 1999) Weused these estimates principally because daily values werereadily available other available estimates were largely sea-sonal averages only (Ho et al 2012) Water samples for dis-solved macronutrients were collected frozen on the ship andlater analysed at the Commonwealth Scientific and IndustrialResearch Organisation in Hobart Australia using standardspectrophotometric methods (Hydes et al 2010) The vari-able DSSI was defined as the time since sea ice had meltedto 20 cover after Wright et al (2010) as determined fromdaily Special Sensor MicrowaveImager (SSMI) sea ice con-centration data distributed by the University of Hamburg(Spreen et al 2008) Total chlorophyll content was estimatedfor each sample location by estimating the total chlorophyllcontent over a 20 kmtimes 20 km area centred at each samplelocation for all available times from 31 August to 1 Mayin the year of sampling (monthly observations) (Acker andLeptoukh 2007 GMAO 2017) and interpolating betweenobservations to estimate total chlorophyll content on the datesampled (some examples are reproduced in Fig S3) By thismethod total chlorophyll was estimated for 49 of the 52 sam-ples the remainder of samples having a paucity of data whichprecluded estimation
23 Statistical analysis
Three statistical analyses were undertaken to explore the hy-pothesis (i) constrained analysis of principal coordinates(CAP Anderson and Willis 2003 also known as distance-based redundancy analysis Legendre and Anderson 1999)was used to estimate the influence of multiple environmentalcovariates in simultaneously explaining community compo-sition (ii) clustering techniques were used to explore similar-ities in phytoplankton community composition among sam-ples independently of environmental information to definesignificantly different groups of samples with similar phyto-plankton community composition and (iii) correlation anal-ysis was used to support observed relationships between phy-toplankton community composition and environmental co-variates
For CAP and cluster analysis relative abundance datawere square-root-transformed to reduce possible dominanceof the analysis by a few abundant taxa The BrayndashCurtis dis-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3820 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 3 Variance in phytoplankton community composition explained by the SAM versus timing and length of the averaged range ofdaily SAM values Response surfaces relate the fraction of total variance in phytoplankton community composition attributable to the SAMversus the number of days in the range of the averaged daily SAM (vertical axis) and the timing of the centre of the range of the averageddaily SAM (horizontal axis) The horizontal axis is expressed as (a) the time through the calendar year of the middle of the range and (b) thenumber of days before a sample was collected to the middle of the range Three obvious maxima are identified with crosses (SAMautumnSAMspring and SAMprior)
similarity index (Bray and Curtis 1957) was used to calcu-late the resemblance of samples based on their communitystructure The advantage of this index for the cell count datawas that similarity among samples was not strongly affectedby the absence of taxa
CAP was applied to the BrayndashCurtis resemblance matrixto partition total variance in community composition into un-constrained and constrained components with the latter rep-resenting the variation due to the environmental covariatesCAP is an example of a constrained ordination method inwhich the typical samplendashspecies matrix of abundances (asused in redundancy analysis) is replaced with a symmetricmatrix of pairwise sample similarities The advantage of thisdistance-based approach to redundancy analysis is that anyecologically relevant distance measure may be used herewe use the BrayndashCurtis metric because it discounts jointabsences between samples when determining similarity Aforward selection strategy was used to choose the optimummodel containing the minimum subset of constraints requiredto explain the most variation in phytoplankton communitystructure (Legendre et al 2011) Linear projections of sig-nificant covariates were plotted as arrows in the ordinationdiagram indicating the direction and magnitude of environ-mental gradients that were correlated with changes in thephytoplankton community (Davidson et al 2016) The vari-ance in phytoplankton community structure (as determinedfrom the ordination) explained by each environmental co-variate was calculated according to the procedure outlined inTer Braak and Verdonschot (1995) and attributed to Dargie(1984) Taxa were added to the CAP plots as weighted site
averages for each species thereby indicating the relative in-fluence of the fitted environmental constraints on each phy-toplankton taxa group
Hierarchical agglomerative clustering based on averagelinkage was performed on the BrayndashCurtis resemblance ma-trix Significant differences among sample clusters were de-termined according to the similarity profile (SIMPROF) per-mutation method of Clarke et al (2008) based on α = 005and 1000 permutations Clustering can identify the presenceof significant differences between the community composi-tion of the samples but clustering cannot identify an effect ofthe SAM at least not directly since environmental covariatesare not included in the cluster analysis
Pair-wise correlation analyses were performed using Pear-sonrsquos correlation coefficient r to explore the relationshipsamong environmental variables and between these environ-mental variables and the relative abundances of phytoplank-ton taxa (Rodgers and Nicewander 1988) Given the largenumber of pair-wise correlations considered we applied aBonferroni correction to give consideration to the family-wise error rate by setting alpha which is usually α = 005(Gibbons and Pratt 1975 Cohen 1990) to αm where mis the total number of correlations considered Recognisingthat αm may be conservative (Nakagawa 2004) we indi-cated when calculated correlations were significant at bothα lt 005 and at Bonferroni-corrected α lt 005m
Response surfaces were used to display the variance ex-plained from individual CAP analyses according to the num-ber of days averaged and the mid-point (or lagged mid-point) of the range of days averaged for each aggregated
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3818 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 1 (a) Latitude and timing of samples (black filled circles) and sea ice extent at 143 E (grey solid line) (b) monthly total chlorophyll(Acker and Leptoukh 2007 GMAO 2017) across the sampled area (longitude 1357ndash1478 E) northern extent (latitude minus62 N lightgreen solid circles) and southern extent (latitudeminus645 N olive-green open circles) and (c) monthly average of daily SAM (NOAA 2017)
Figure 2 Example of phytoplankton identification on a single SEM image representing 00348 mL of seawater Overlying letters are taxacodes for individual phytoplankton taxa considered in the analysis (listed in Table 3) codes in parenthesis are rare taxa (see text) Insetsampling area in relation to southern Australia and the Antarctic coastline with sample locations indicated as open circles
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3819
numbers for each phytoplankton taxon were counted in arandom selection of captured images taken of each sam-ple Each captured image (Fig 2) represented an area of301 micromtimes 227 microm (area 0068 mm2) of each sample filterwhich was captured at a resolution of 85 pixels per microme-tre A minimum of three SEM images were assessed for eachsample with more images assessed when cell densities werelower ndash individual images were considered as incrementalincreases in the area of a sample covered and not samplingreplicates On average 387 cells were counted for each sam-ple Taxa were classified with the aid of Scott and Marchant(2005) Tomas (1997) and expert opinion Cell counts persample were converted to volume-specific abundances (cellsper mL) by dividing total counts by the number of imagesassessed multiplied by 00348 mL of seawater represented byeach captured image
A total of 48 phytoplankton taxa were identified many tospecies level Because the diatoms Fragilariopsis curta andF cylindrus could not be reliably discriminated at the micro-scope resolution employed they were pooled into a singletaxa group Other taxa were also grouped namely Nitzschiaacicularis with N decipiens to a single group and discoidcentric diatoms of the genera Thalassiosira Actinocyclusand Porosira to another Rare species with maximum rela-tive abundance lt 2 were removed from the data prior toanalysis as they were not considered to be sufficiently abun-dant to warrant further analysis (Webb and Bryson 1972Taylor and Sjunneskog 2002 Swiło et al 2016) After pool-ing taxa and deleting rare taxa 22 taxa and taxonomic-groups (species groups of species and families) remainedto describe the composition of the phytoplankton commu-nity A total of 19 499 phytoplankton organisms were identi-fied and counted 18 878 diatoms 322 Parmales 173 coccol-ithophores 81 silicoflagellates and 45 Petasaria
Phytoplankton abundance data were converted to relativeabundance by dividing each value by the total abundanceof the 22 taxa groups in the sample This was to alleviateany variation among samples resulting from dilution a phe-nomenon whereby the abundance of cells in surface waterscan be reduced in a matter of hours by an abrupt increase inwind speed and associated increase in the mixed layer depth(Carranza and Gille 2015) diluting near-surface cells into agreater water volume However relative abundance has thedisadvantage that blooming of one species will cause a re-duction in relative abundance of other present species whentheir absolute abundances may not have changed
22 Environmental covariates
Phytoplankton abundances were related to a range of envi-ronmental covariates available at the time of sampling Theseincluded the SAM sea surface temperature (SST) salinity(S) time since sea ice cover (DSSI defined below) mini-mum latitude of sea ice in the preceding winter latitude andlongitude of sample collection (LATS and LONGE respec-
tively) the days since 1st October that a sample was collected(D) the year of sampling (Y being the year that each springndashsummer sampling season began) the time of day that a sam-ple was collected and satellite-derived total chlorophyll con-tent Macronutrient concentrations phosphate (PO4) silicate(SiO4) and nitrate+ nitrite (hereafter nitrate NOx) were in-cluded as indicators of nutrient drawdown as a proxy for phy-toplankton productivity (Arrigo et al 1999)
We obtained daily estimates of the SAM from the USNWS Climate Prediction Center (NOAA 2017) This datasetuses the principal component method definition of the SAM(Mo 2000) rather than the simple zonal-mean normalisedpressure difference technique (Gong and Wang 1999) Weused these estimates principally because daily values werereadily available other available estimates were largely sea-sonal averages only (Ho et al 2012) Water samples for dis-solved macronutrients were collected frozen on the ship andlater analysed at the Commonwealth Scientific and IndustrialResearch Organisation in Hobart Australia using standardspectrophotometric methods (Hydes et al 2010) The vari-able DSSI was defined as the time since sea ice had meltedto 20 cover after Wright et al (2010) as determined fromdaily Special Sensor MicrowaveImager (SSMI) sea ice con-centration data distributed by the University of Hamburg(Spreen et al 2008) Total chlorophyll content was estimatedfor each sample location by estimating the total chlorophyllcontent over a 20 kmtimes 20 km area centred at each samplelocation for all available times from 31 August to 1 Mayin the year of sampling (monthly observations) (Acker andLeptoukh 2007 GMAO 2017) and interpolating betweenobservations to estimate total chlorophyll content on the datesampled (some examples are reproduced in Fig S3) By thismethod total chlorophyll was estimated for 49 of the 52 sam-ples the remainder of samples having a paucity of data whichprecluded estimation
23 Statistical analysis
Three statistical analyses were undertaken to explore the hy-pothesis (i) constrained analysis of principal coordinates(CAP Anderson and Willis 2003 also known as distance-based redundancy analysis Legendre and Anderson 1999)was used to estimate the influence of multiple environmentalcovariates in simultaneously explaining community compo-sition (ii) clustering techniques were used to explore similar-ities in phytoplankton community composition among sam-ples independently of environmental information to definesignificantly different groups of samples with similar phyto-plankton community composition and (iii) correlation anal-ysis was used to support observed relationships between phy-toplankton community composition and environmental co-variates
For CAP and cluster analysis relative abundance datawere square-root-transformed to reduce possible dominanceof the analysis by a few abundant taxa The BrayndashCurtis dis-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3820 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 3 Variance in phytoplankton community composition explained by the SAM versus timing and length of the averaged range ofdaily SAM values Response surfaces relate the fraction of total variance in phytoplankton community composition attributable to the SAMversus the number of days in the range of the averaged daily SAM (vertical axis) and the timing of the centre of the range of the averageddaily SAM (horizontal axis) The horizontal axis is expressed as (a) the time through the calendar year of the middle of the range and (b) thenumber of days before a sample was collected to the middle of the range Three obvious maxima are identified with crosses (SAMautumnSAMspring and SAMprior)
similarity index (Bray and Curtis 1957) was used to calcu-late the resemblance of samples based on their communitystructure The advantage of this index for the cell count datawas that similarity among samples was not strongly affectedby the absence of taxa
CAP was applied to the BrayndashCurtis resemblance matrixto partition total variance in community composition into un-constrained and constrained components with the latter rep-resenting the variation due to the environmental covariatesCAP is an example of a constrained ordination method inwhich the typical samplendashspecies matrix of abundances (asused in redundancy analysis) is replaced with a symmetricmatrix of pairwise sample similarities The advantage of thisdistance-based approach to redundancy analysis is that anyecologically relevant distance measure may be used herewe use the BrayndashCurtis metric because it discounts jointabsences between samples when determining similarity Aforward selection strategy was used to choose the optimummodel containing the minimum subset of constraints requiredto explain the most variation in phytoplankton communitystructure (Legendre et al 2011) Linear projections of sig-nificant covariates were plotted as arrows in the ordinationdiagram indicating the direction and magnitude of environ-mental gradients that were correlated with changes in thephytoplankton community (Davidson et al 2016) The vari-ance in phytoplankton community structure (as determinedfrom the ordination) explained by each environmental co-variate was calculated according to the procedure outlined inTer Braak and Verdonschot (1995) and attributed to Dargie(1984) Taxa were added to the CAP plots as weighted site
averages for each species thereby indicating the relative in-fluence of the fitted environmental constraints on each phy-toplankton taxa group
Hierarchical agglomerative clustering based on averagelinkage was performed on the BrayndashCurtis resemblance ma-trix Significant differences among sample clusters were de-termined according to the similarity profile (SIMPROF) per-mutation method of Clarke et al (2008) based on α = 005and 1000 permutations Clustering can identify the presenceof significant differences between the community composi-tion of the samples but clustering cannot identify an effect ofthe SAM at least not directly since environmental covariatesare not included in the cluster analysis
Pair-wise correlation analyses were performed using Pear-sonrsquos correlation coefficient r to explore the relationshipsamong environmental variables and between these environ-mental variables and the relative abundances of phytoplank-ton taxa (Rodgers and Nicewander 1988) Given the largenumber of pair-wise correlations considered we applied aBonferroni correction to give consideration to the family-wise error rate by setting alpha which is usually α = 005(Gibbons and Pratt 1975 Cohen 1990) to αm where mis the total number of correlations considered Recognisingthat αm may be conservative (Nakagawa 2004) we indi-cated when calculated correlations were significant at bothα lt 005 and at Bonferroni-corrected α lt 005m
Response surfaces were used to display the variance ex-plained from individual CAP analyses according to the num-ber of days averaged and the mid-point (or lagged mid-point) of the range of days averaged for each aggregated
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3819
numbers for each phytoplankton taxon were counted in arandom selection of captured images taken of each sam-ple Each captured image (Fig 2) represented an area of301 micromtimes 227 microm (area 0068 mm2) of each sample filterwhich was captured at a resolution of 85 pixels per microme-tre A minimum of three SEM images were assessed for eachsample with more images assessed when cell densities werelower ndash individual images were considered as incrementalincreases in the area of a sample covered and not samplingreplicates On average 387 cells were counted for each sam-ple Taxa were classified with the aid of Scott and Marchant(2005) Tomas (1997) and expert opinion Cell counts persample were converted to volume-specific abundances (cellsper mL) by dividing total counts by the number of imagesassessed multiplied by 00348 mL of seawater represented byeach captured image
A total of 48 phytoplankton taxa were identified many tospecies level Because the diatoms Fragilariopsis curta andF cylindrus could not be reliably discriminated at the micro-scope resolution employed they were pooled into a singletaxa group Other taxa were also grouped namely Nitzschiaacicularis with N decipiens to a single group and discoidcentric diatoms of the genera Thalassiosira Actinocyclusand Porosira to another Rare species with maximum rela-tive abundance lt 2 were removed from the data prior toanalysis as they were not considered to be sufficiently abun-dant to warrant further analysis (Webb and Bryson 1972Taylor and Sjunneskog 2002 Swiło et al 2016) After pool-ing taxa and deleting rare taxa 22 taxa and taxonomic-groups (species groups of species and families) remainedto describe the composition of the phytoplankton commu-nity A total of 19 499 phytoplankton organisms were identi-fied and counted 18 878 diatoms 322 Parmales 173 coccol-ithophores 81 silicoflagellates and 45 Petasaria
Phytoplankton abundance data were converted to relativeabundance by dividing each value by the total abundanceof the 22 taxa groups in the sample This was to alleviateany variation among samples resulting from dilution a phe-nomenon whereby the abundance of cells in surface waterscan be reduced in a matter of hours by an abrupt increase inwind speed and associated increase in the mixed layer depth(Carranza and Gille 2015) diluting near-surface cells into agreater water volume However relative abundance has thedisadvantage that blooming of one species will cause a re-duction in relative abundance of other present species whentheir absolute abundances may not have changed
22 Environmental covariates
Phytoplankton abundances were related to a range of envi-ronmental covariates available at the time of sampling Theseincluded the SAM sea surface temperature (SST) salinity(S) time since sea ice cover (DSSI defined below) mini-mum latitude of sea ice in the preceding winter latitude andlongitude of sample collection (LATS and LONGE respec-
tively) the days since 1st October that a sample was collected(D) the year of sampling (Y being the year that each springndashsummer sampling season began) the time of day that a sam-ple was collected and satellite-derived total chlorophyll con-tent Macronutrient concentrations phosphate (PO4) silicate(SiO4) and nitrate+ nitrite (hereafter nitrate NOx) were in-cluded as indicators of nutrient drawdown as a proxy for phy-toplankton productivity (Arrigo et al 1999)
We obtained daily estimates of the SAM from the USNWS Climate Prediction Center (NOAA 2017) This datasetuses the principal component method definition of the SAM(Mo 2000) rather than the simple zonal-mean normalisedpressure difference technique (Gong and Wang 1999) Weused these estimates principally because daily values werereadily available other available estimates were largely sea-sonal averages only (Ho et al 2012) Water samples for dis-solved macronutrients were collected frozen on the ship andlater analysed at the Commonwealth Scientific and IndustrialResearch Organisation in Hobart Australia using standardspectrophotometric methods (Hydes et al 2010) The vari-able DSSI was defined as the time since sea ice had meltedto 20 cover after Wright et al (2010) as determined fromdaily Special Sensor MicrowaveImager (SSMI) sea ice con-centration data distributed by the University of Hamburg(Spreen et al 2008) Total chlorophyll content was estimatedfor each sample location by estimating the total chlorophyllcontent over a 20 kmtimes 20 km area centred at each samplelocation for all available times from 31 August to 1 Mayin the year of sampling (monthly observations) (Acker andLeptoukh 2007 GMAO 2017) and interpolating betweenobservations to estimate total chlorophyll content on the datesampled (some examples are reproduced in Fig S3) By thismethod total chlorophyll was estimated for 49 of the 52 sam-ples the remainder of samples having a paucity of data whichprecluded estimation
23 Statistical analysis
Three statistical analyses were undertaken to explore the hy-pothesis (i) constrained analysis of principal coordinates(CAP Anderson and Willis 2003 also known as distance-based redundancy analysis Legendre and Anderson 1999)was used to estimate the influence of multiple environmentalcovariates in simultaneously explaining community compo-sition (ii) clustering techniques were used to explore similar-ities in phytoplankton community composition among sam-ples independently of environmental information to definesignificantly different groups of samples with similar phyto-plankton community composition and (iii) correlation anal-ysis was used to support observed relationships between phy-toplankton community composition and environmental co-variates
For CAP and cluster analysis relative abundance datawere square-root-transformed to reduce possible dominanceof the analysis by a few abundant taxa The BrayndashCurtis dis-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3820 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 3 Variance in phytoplankton community composition explained by the SAM versus timing and length of the averaged range ofdaily SAM values Response surfaces relate the fraction of total variance in phytoplankton community composition attributable to the SAMversus the number of days in the range of the averaged daily SAM (vertical axis) and the timing of the centre of the range of the averageddaily SAM (horizontal axis) The horizontal axis is expressed as (a) the time through the calendar year of the middle of the range and (b) thenumber of days before a sample was collected to the middle of the range Three obvious maxima are identified with crosses (SAMautumnSAMspring and SAMprior)
similarity index (Bray and Curtis 1957) was used to calcu-late the resemblance of samples based on their communitystructure The advantage of this index for the cell count datawas that similarity among samples was not strongly affectedby the absence of taxa
CAP was applied to the BrayndashCurtis resemblance matrixto partition total variance in community composition into un-constrained and constrained components with the latter rep-resenting the variation due to the environmental covariatesCAP is an example of a constrained ordination method inwhich the typical samplendashspecies matrix of abundances (asused in redundancy analysis) is replaced with a symmetricmatrix of pairwise sample similarities The advantage of thisdistance-based approach to redundancy analysis is that anyecologically relevant distance measure may be used herewe use the BrayndashCurtis metric because it discounts jointabsences between samples when determining similarity Aforward selection strategy was used to choose the optimummodel containing the minimum subset of constraints requiredto explain the most variation in phytoplankton communitystructure (Legendre et al 2011) Linear projections of sig-nificant covariates were plotted as arrows in the ordinationdiagram indicating the direction and magnitude of environ-mental gradients that were correlated with changes in thephytoplankton community (Davidson et al 2016) The vari-ance in phytoplankton community structure (as determinedfrom the ordination) explained by each environmental co-variate was calculated according to the procedure outlined inTer Braak and Verdonschot (1995) and attributed to Dargie(1984) Taxa were added to the CAP plots as weighted site
averages for each species thereby indicating the relative in-fluence of the fitted environmental constraints on each phy-toplankton taxa group
Hierarchical agglomerative clustering based on averagelinkage was performed on the BrayndashCurtis resemblance ma-trix Significant differences among sample clusters were de-termined according to the similarity profile (SIMPROF) per-mutation method of Clarke et al (2008) based on α = 005and 1000 permutations Clustering can identify the presenceof significant differences between the community composi-tion of the samples but clustering cannot identify an effect ofthe SAM at least not directly since environmental covariatesare not included in the cluster analysis
Pair-wise correlation analyses were performed using Pear-sonrsquos correlation coefficient r to explore the relationshipsamong environmental variables and between these environ-mental variables and the relative abundances of phytoplank-ton taxa (Rodgers and Nicewander 1988) Given the largenumber of pair-wise correlations considered we applied aBonferroni correction to give consideration to the family-wise error rate by setting alpha which is usually α = 005(Gibbons and Pratt 1975 Cohen 1990) to αm where mis the total number of correlations considered Recognisingthat αm may be conservative (Nakagawa 2004) we indi-cated when calculated correlations were significant at bothα lt 005 and at Bonferroni-corrected α lt 005m
Response surfaces were used to display the variance ex-plained from individual CAP analyses according to the num-ber of days averaged and the mid-point (or lagged mid-point) of the range of days averaged for each aggregated
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3820 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 3 Variance in phytoplankton community composition explained by the SAM versus timing and length of the averaged range ofdaily SAM values Response surfaces relate the fraction of total variance in phytoplankton community composition attributable to the SAMversus the number of days in the range of the averaged daily SAM (vertical axis) and the timing of the centre of the range of the averageddaily SAM (horizontal axis) The horizontal axis is expressed as (a) the time through the calendar year of the middle of the range and (b) thenumber of days before a sample was collected to the middle of the range Three obvious maxima are identified with crosses (SAMautumnSAMspring and SAMprior)
similarity index (Bray and Curtis 1957) was used to calcu-late the resemblance of samples based on their communitystructure The advantage of this index for the cell count datawas that similarity among samples was not strongly affectedby the absence of taxa
CAP was applied to the BrayndashCurtis resemblance matrixto partition total variance in community composition into un-constrained and constrained components with the latter rep-resenting the variation due to the environmental covariatesCAP is an example of a constrained ordination method inwhich the typical samplendashspecies matrix of abundances (asused in redundancy analysis) is replaced with a symmetricmatrix of pairwise sample similarities The advantage of thisdistance-based approach to redundancy analysis is that anyecologically relevant distance measure may be used herewe use the BrayndashCurtis metric because it discounts jointabsences between samples when determining similarity Aforward selection strategy was used to choose the optimummodel containing the minimum subset of constraints requiredto explain the most variation in phytoplankton communitystructure (Legendre et al 2011) Linear projections of sig-nificant covariates were plotted as arrows in the ordinationdiagram indicating the direction and magnitude of environ-mental gradients that were correlated with changes in thephytoplankton community (Davidson et al 2016) The vari-ance in phytoplankton community structure (as determinedfrom the ordination) explained by each environmental co-variate was calculated according to the procedure outlined inTer Braak and Verdonschot (1995) and attributed to Dargie(1984) Taxa were added to the CAP plots as weighted site
averages for each species thereby indicating the relative in-fluence of the fitted environmental constraints on each phy-toplankton taxa group
Hierarchical agglomerative clustering based on averagelinkage was performed on the BrayndashCurtis resemblance ma-trix Significant differences among sample clusters were de-termined according to the similarity profile (SIMPROF) per-mutation method of Clarke et al (2008) based on α = 005and 1000 permutations Clustering can identify the presenceof significant differences between the community composi-tion of the samples but clustering cannot identify an effect ofthe SAM at least not directly since environmental covariatesare not included in the cluster analysis
Pair-wise correlation analyses were performed using Pear-sonrsquos correlation coefficient r to explore the relationshipsamong environmental variables and between these environ-mental variables and the relative abundances of phytoplank-ton taxa (Rodgers and Nicewander 1988) Given the largenumber of pair-wise correlations considered we applied aBonferroni correction to give consideration to the family-wise error rate by setting alpha which is usually α = 005(Gibbons and Pratt 1975 Cohen 1990) to αm where mis the total number of correlations considered Recognisingthat αm may be conservative (Nakagawa 2004) we indi-cated when calculated correlations were significant at bothα lt 005 and at Bonferroni-corrected α lt 005m
Response surfaces were used to display the variance ex-plained from individual CAP analyses according to the num-ber of days averaged and the mid-point (or lagged mid-point) of the range of days averaged for each aggregated
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3821
Table 1 Variance in the community composition of 22 phytoplankton taxa groups attributable to constraining environmental covariables inthe CAP analysis
CAP analysis Variance Covariate Variance Fraction p
category of totalvariance
D 061 154 lt 0001SST 057 146 lt 0001SAMautumn 052 133 lt 0001LONGE 047 119 lt 0001
(a) Variables fit individually as SAMspring 041 103 lt 0001the only constraining covariate SAMprior 039 99 lt 0001
DSSI 023 59 0004S 018 47 0018Y 013 34 0086LATS 010 25 0228Minimum latitude of sea ice the previous winter 006 16 0537
Variance explained by all constraining covariables 148 375 lt 0001
(b) Optimum Individual D 061 154 lt 0001multi-covariate constraining SAMautumn 050 126 lt 0001model covariables LONGE 021 52 lt 0001
SAMprior 017 43 0006
Unexplained residual 246 625 Total variance in taxa composition between samples 394 100
SAM index These allowed identification of maxima in cor-relation between the SAM and phytoplankton communitystructure Response surfaces were derived by evaluating sep-arate CAP analyses for each combination of (i) the tempo-ral positioning of the daily-SAM averaging range and (ii) thelength of the daily-SAM averaging range In constructing theresponse surfaces the range of the averaged daily SAM wascentred on (i) each calendar day individually (1 Januaryndash31 December) through the year associated with each sam-ple and alternatively (ii) relative to the time of sampling andlagged from 1 to 365 d prior to each sample collection datein 1 d increments The length of the SAM averaging rangewas varied in 2 d increments from zero to plus and minus182 d from the centre of the range Similar response surfaceswere constructed relating the correlation between the aver-aged daily SAM and (i) total chlorophyll and (ii) [PO4]
Data management and manipulation summary statisticscorrelation analyses and scatter plots were undertaken in Mi-crosoft Excel (2016) and R (R Core Team 2016) Clusteranalysis and SIMPROF were undertaken using the R pack-age clustsig (Whitaker and Christman 2014) CAP analyseswere conducted using the capscale function in the R packagevegan (Dixon 2003)
3 Results
31 The influence of the SAM on phytoplanktoncommunity composition
CAP analysis and pairwise correlation analysis both indi-cated the presence of a relationship between the SAM andphytoplankton community composition Clustering analysisshowed there to be sufficient and systematic variation in phy-toplankton community composition between samples thatsamples could be grouped
Empirical identification of the time between variation inthe SAM and the manifestation of this variation in the phyto-plankton community structure revealed three maxima in phy-toplankton community composition explained by the SAMThe first of the maxima was an autumn seasonal SAM in-dex (SAMautumn) which was determined to be the average of57 daily SAM estimates centred on the preceding 11 March(11 Februaryndash8 April) SAMautumn explained up to 133 of the variance in phytoplankton community composition es-timated through CAP analysis (Fig 3a Table 1a) The sec-ond of the maxima was a spring seasonal index (SAMspring)which was determined to be the average of 75 daily SAMestimates centred on 25 October (20 Septemberndash3 Decem-ber) SAMspring explained up to 103 of variance in phyto-plankton community composition (Fig 3a Table 1a) Unlikethe other maxima that were related to the time of year thethird of the maxima was timed relative to the date of sample
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3822 B L Greaves et al SAM influences phytoplankton in SIZ
Table 2 (a) Summary statistics for environmental variables (b) correlations between taxa group relative abundances and environmental vari-ables (c) correlations among environmental variables (d) correlations between macronutrient concentrations and environmental variables(e) as in (f) but involving only the 50 of samples collected latest in the springndashsummer Correlations significant at α le 005 are in italicand correlations significant after Bonferroni adjustment are also underlined (α lt 00519 for correlations among environmental variablesα lt 00520 for correlations with taxa group relative abundance)
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(a) Statistics for environmental covariables
Unit days index index index E days C PSU year mg mminus3
Average 96 minus02 01 04 142 65 06 337 ndash 029Min 20 minus08 minus13 minus15 136 minus26 minus18 332 2002 007Max 151 06 20 100 148 gt 365 30 341 2012 070n 52 11 52 11 52 52 5 52 11 49Average standard error of estimate ndash 014 013 014 ndash ndash ndash ndash ndash ndash
(b) Correlations with taxa group relative abundance
Chaetoceros atlanticus minus015 055 057 063 020 minus001 minus020 022 013 037Chaetoceros concavicorniscurvatus 037 036 027 035 minus007 027 025 minus014 011 025Chaetoceros castracanei minus036 minus002 026 020 041 minus012 minus036 minus007 minus007 020Chaetoceros dichaeta 048 038 031 029 minus013 037 035 minus017 020 036Chaetoceros neglectus minus070 minus006 042 024 048 minus040 minus069 056 minus004 033Cylindrotheca closterium 013 009 minus010 minus003 002 032 012 002 minus011 003Dactyliosolen antarcticus 018 037 034 027 minus006 018 013 minus008 006 037Dactyliosolen tenuijunctus minus018 minus044 minus008 minus016 016 minus019 minus017 023 minus002 minus010Dictyocha speculum (silicoflagellate) minus078 minus017 030 014 068 minus041 minus075 036 minus014 017discoid centric diatoms minus057 015 006 024 052 minus011 minus057 021 minus015 021Emiliania huxleyi (haptophyte) minus028 minus038 minus042 minus038 021 012 minus025 minus001 minus037 minus024Fragilariopsis cylindruscurta 026 minus006 minus008 minus009 minus058 minus008 035 minus012 024 minus015Fragilariopsis kerguelensis 023 052 016 025 minus007 019 022 minus046 minus005 007Fragilariopsis pseudonana minus013 022 minus002 022 minus010 minus005 minus003 012 022 002Fragilariopsis rhombica 016 minus039 minus058 minus057 minus013 013 022 minus012 minus024 minus059Fragilariopsis ritscheri 011 minus010 000 minus003 minus002 002 010 minus003 003 minus001Guinardia cylindrus 009 012 minus006 minus006 005 017 010 minus003 minus002 012Nitzschia acicularisdecipiens minus047 minus045 minus029 minus031 042 minus032 minus046 009 minus022 minus019Parmales spp (chrysophyte) minus060 minus029 015 minus009 042 minus042 minus065 036 minus028 016Petasaria heterolepis minus025 minus013 minus027 minus008 015 minus017 minus025 002 minus002 minus004Pseudo-nitzschia lineola minus035 039 019 037 036 minus009 minus035 018 001 026Thalassiothrix antarctica minus016 032 012 016 015 minus011 minus011 minus019 minus015 000
(c) Correlations among environmental variables
SAMautumn 032SAMprior minus006 051SAMspring 004 056 083LONGE minus063 minus017 010 005DSSI 056 018 minus003 007 minus027SST 092 027 minus014 minus003 minus068 060S minus043 minus014 031 021 023 minus013 minus041Y 018 027 035 032 minus024 002 027 minus006total chlorophyll minus002 050 072 069 011 minus008 minus015 014 043
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3823
Table 2 Continued
Environmental variables
D SAM
autu
mn
SAM
prio
r
SAM
spri
ng
LO
NGE
DSS
I
SST
S Y tota
lchl
orop
hyll
(d) Correlations with macronutrients (n= 51)
[NOx ] minus 077 -039 023 004 053 minus 043 minus 072 054 minus014 012[PO4] minus 073 minus 056 minus007 minus026 062 minus 052 minus 070 039 minus013 minus010[SiO4] minus 056 minus 042 026 minus005 040 minus 049 minus 063 039 009 022
(e) Correlations with macronutrients (n= 26 later-in-season 50 of samples)
[NOx ] minus018 minus 058 minus005 minus025 minus023 minus019 002 027 minus017 ndash[PO4] minus013 minus 074 minus051 minus 068 009 minus031 minus001 003 minus002 ndash[SiO4] minus010 minus051 minus004 minus031 minus016 minus035 minus044 minus005 034 ndash
Figure 4 Maxima of SAM influence on phytoplankton community composition SAMprior was determined relative to sample collection thedepicted solid line represents the average temporal location of the 97 d period and the broken lines represent the earliest and latest extent ofthe range associated with the earliest and latest samples
collection for each sample and comprised the average of the97 daily SAM estimates centred 102 d prior to each samplecollection date It explained 99 of the variance in phy-toplankton composition (SAMprior Fig 3b Table 1a) Notethat SAMprior and SAMspring temporally overlapped to vary-ing extents across the 52 samples (Fig 4) and so were notentirely independent covariates for example a sample col-lected in the summer had previous days contributing to bothSAMprior and SAMspring
The optimum CAP model contained four covariates thatexplained the variance in phytoplankton community com-position among samples (Table 1b) While four CAP axeswere statistically significant (p lt 005) the first two axes to-gether explained a total of 311 of the variance in phyto-plankton community composition and the third and fourthaxes together only explained a further 64 (not tabu-lated) Thus Fig 6a illustrates most of the variance explainedby the CAP analysis SAMautumn explained the most vari-ance in community composition (126 ) and SAMprior ex-plained a further 43 of variance (Table 1b) These twoSAM indices were moderately and significantly positively
correlated (r = 051 Table 2c p lt 0001) Both showedsimilar negative correlations (Table 2b) with the relativeabundances of the small diatoms Fragilariopsis rhombica(Fig 5a) and Nitzschia acicularisdecipiens and the coc-colithophorid Emiliana huxleyi and similar positive cor-relations with the abundances of larger diatoms Chaeto-ceros atlanticus Chaetoceros dichaeta and Dactyliosolenantarcticus A further six taxa showed a correlation withSAMautumn but not SAMprior namely positive correla-tions with Chaetoceros concavicorniscurvatus Fragilari-opsis kerguelensis (Fig 5b) Pseudo-nitzschia lineola andThalassiothrix antarctica and negative correlations withDactyliosolen tenuijunctus and the Parmales Three taxashowed correlations with SAMprior but not SAMautumnnamely positive correlations with Chaetoceros neglectus andthe silicoflagellate Dictyocha speculum and a negative cor-relation with Petasaria heterolepis
In total 15 of the 22 taxa groups showed significantpairwise correlations (p lt 005) with one or more of theSAM indices with SAMautumn being the most influential (Ta-ble 2b) showing significant correlation with 12 of the 22 taxa
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3824 B L Greaves et al SAM influences phytoplankton in SIZ
Table3Identifiedtaxa
groupstaxataxacodecellscountedcellsm
easuredaverageindividualcellvolum
eabundance(averagem
inimum
andm
aximum
)averagerelative
abundanceaverage
totalvolumeaverage
relativevolum
eandpercentage
ofsamples
inw
hicheach
taxagroup
was
identified
TaxonTaxa
codeC
ellsC
ellsA
verageA
bundanceR
elativeA
verageA
veragevolum
eSam
plescounted
measured
individualabundance
totalfraction
ofw
ithtaxon
cellvolume
averagevolum
etotalcellvolum
e
Average
Min
Max
Num
berN
umber
microm3
cellsmLminus
1cellsm
Lminus
1cellsm
Lminus
1microm
3m
Lminus
1
Chaetoceros
atlanticusca
356479
131651
0364
22
81382
14
90
Chaetoceros
castracaneicca
4834
9406
038
03
18616
04
48
Chaetoceros
concavicorniscurvatuscc
120200
344320
0135
07
78443
14
77
Chaetoceros
dichaetacd
25631943
491423
02503
13
145999
29
94
Chaetoceros
neglectuscn
634488
17683
0697
35
11906
02
81
Cylindrotheca
closteriumcyc
12250
12117
079
07
410601
77
D
actyliosolenantarcticus
da277
472(61
899)44
0195
16
1860
68027
98
D
actyliosolentenuijunctus
dt1981
13503828
2967
131599
895
36716
100
D
ictyochaspeculum
(silicoflagellate)ds
8184
492010
069
05
99301
15
48
discoidcentric
diatoms
dcx965
12808572
13312
69652
437
55673
100
E
miliania
huxleyi(haptophyte)ehu
17370
6524
0192
08
355201
58
Fragilariopsis
cylindruscurtafcx
39873013
70632
08796
17
44167
09
98
Fragilariopsiskerguelensis
fk1031
40553748
1670
105458
369
49265
98
Fragilariopsis
pseudonanafps
170115
35526
0201
09
1899904
69
Fragilariopsis
rhombica
fr4542
346936
65829
207022
23359
06
100
Fragilariopsisritscheri
fri46
19572
70
8602
11
02002
35
G
uinardiacylindrus
guc110
8110
40515
079
06
225921
41
67
Nitzschia
acicularisdecipiensnix
1133509
251162
0977
57
46705
10
98
Parmales
spp(chrysophyte)parm
3222
838
0668
17
33400
27
Petasaria
heterolepis(other)
pet45
ndash(65)
70
18703
2667
01
6
Pseudonitzschia
lineolapsl
681403
109391
4376
41
8446015
100
Thalassiothrix
antarcticata
112269
(63000)
130
17206
314
42448
85
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3825
Figure 5 Scatter-plots (a b) examples of phytoplankton taxon relative abundance versus SAMautumn (c) LONGE of sample collectionversus D and (d) [PO4] versus SAMautumn Each figure shows r2 and p associated with the relationship A line of least-squares best fit isprovided to give an indication of trend
groups When applying the conservative Bonferroni-adjustedα = 00025 seven taxa groups showed significant correlation(p lt 00025) with any SAM index and four with SAMautumn
SAMprior and SAMspring represented a similar time span inthe spring immediately prior to sampling (Fig 4) and werestrongly and significantly correlated (r = 083 Table 2cp lt 0001) Samples were collected over a calendar rangeof 140 d (20 Octoberndash28 February Table 2a) and thus the97 d period represented by SAMprior varied in its positionin the calendar across the 140 d spread of the 52 samples(Fig 4) SAMprior and SAMspring also showed similar corre-lation signs with taxa group relative abundances (Table 2b)It was not possible however to determine whether the pre-season SAM influence was a spring effect or a prior-to-sampling effect and whilst both appear to be important ex-planatory terms only SAMprior was retained in the optimumCAP model (Table 1b)
In the optimum multi-covariate CAP model D explainedthe greatest proportion of the observed variance in phyto-plankton community composition (Table 1b) D was signif-icantly correlated (p lt 00025) with SST S and DSSI andthe variable singly captured the most variation in phytoplank-ton community composition associated with seasonal suc-cession Alone it explained 154 of the total variance (Ta-
ble 1b) with its effect on the phytoplankton community be-ing approximately orthogonal to that of the SAM (Fig 6a) Aweak positive relationship detected between SAMautumn andD indicated a weak trend of sampling later in the springndashsummer period in years with a higher autumn SAM (r =032 Table 2c p = 002) but otherwise the SAM indicesand D were un-related
A total of 10 taxa groups showed significant correlation(p lt 005) between their relative abundance and D (Ta-ble 2b) Chaetoceros castracanei C neglectus D specu-lum E huxleyi N acicularisdecipiens Parmales P line-ola and the discoid centric diatoms showed negative relative-abundance correlations with D indicating greatest relativeabundance early in the springndashsummer while C concavicor-niscurvatus and C dichaeta showed greater relative abun-dance later in the springndashsummer A negative correlation(minus063 p lt 0001) was detected between the longitude ofindividual sample collection (LONGE) and D indicatingthat samples collected later in the springndashsummer were morelikely to have been collected towards the west in the sampledregion (Table 2c Fig 5c)
Following cluster analysis similarity profile (SIMPROF)permutation analysis identified seven significantly differentgroups (p lt 005) with samples loosely grouped on the ba-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3826 B L Greaves et al SAM influences phytoplankton in SIZ
Figure 6 (a) CAP analysis of phytoplankton community composition Dots represent individual samples with colours corresponding tosignificant clusters (Fig 6b) The 22 phytoplankton taxagroups are overlain as weighted averages of their sample scores (red abbreviationsafter Fig 2) with positions plotted with a 3-times-larger distance from the origin to more easily visualise their relationships with constrainingenvironmental variables Linear projections of the significant constraining environmental covariates appear as blue arrows the length andangle of which represent the magnitude and direction of influence of each variable on community composition The inset shows the taxalocated close to the origin diatoms fri and cyc collocating (b) Cluster analysis dendrogram of the 52 samples based on similarities inphytoplankton community structure using colour to show seven significantly different groups (numbered 1ndash7 solid lines α = 005) Samplelabels contain season and voyage (eg 0809v2b= austral springndashsummer over 2008ndash2009 voyage designation 2 sample b is the secondsample obtained from the SIZ during that voyage) SAMautumn value SAMprior value and the D value
sis of their within-season successional maturity (D) and theSAM (Fig 6b) and demonstrated that there were signifi-cant differences between the community composition of thesamples The group structure determined by cluster analy-sis was displayed in the CAP ordination (using colour) todemonstrate that samples that clustered together were indeedclose to one another in the two-dimensional (2D) ordina-tion (Fig 6a) with their positioning further indicating theinfluences of D and the SAM on cluster groupings This lentconfidence that the 2D ordination was a reasonable approx-imation to the full high-dimensional structure As we knewthe values for the environmental covariates for each sam-ple it was possible to determine the correlation between the2D CAP solution and each environmental covariate We dis-played these correlations as a projected vector (arrow) wheredirection indicates the sign and length indicates strengthThis showed samples in clusters 3 and 4 (Fig 6b) were com-monly associated with a more positive SAM while those inclusters 5 6 and 7 were commonly associated with morenegative SAM values Samples in clusters 2 and 5 were com-
monly collected earlier in the springndashsummer period (lowerD) while those in clusters 1 4 6 and 7 were commonly col-lected later (Fig 6)
Other considered environmental covariates that did notsignificantly influence community composition were thetime of the day that a sample was collected and the mini-mum latitude reached by sea ice cover in the previous winter(Supplement Table S1)
These analyses were also undertaken using phytoplanktonabsolute abundances rather than with relative abundances asreported above The analysis of absolute abundance showedsimilar temporal peaks in variance explained (SupplementFig S4) although it explained less variance (SAMautumn ex-plaining 109 SAMspring 91 and SAMprior 92 ) (Ta-ble S3) Individual taxa correlations with SAM indices (Ta-ble S4) showed a similar pattern to those estimated using rel-ative abundances (Table 2b)
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3827
32 Influence of the SAM on phytoplanktonproductivity
Two indicators of the influence of the SAM on phytoplank-ton productivity were obtained (i) the influence of the SAMon satellite-derived total chlorophyll and (ii) the influence ofthe SAM on macronutrient concentrations indicating nutri-ent drawdown associated with productivity Using the timesand locations of the 52 samples over the 11 years of ourstudy satellite-derived total chlorophyll showed positive cor-relation with all SAM indices r = 050 (p lt 0001) withSAMautumn r = 072 (p lt 0001) with SAMprior and r =069 (p lt 0001) with SAMspring (Table 2c) Peaks in thecorrelation of total chlorophyll with the SAM were evidentin the preceding autumn and spring and prior to sampling inresponse surfaces for NASA satellite total chlorophyll alongwith a peak in early winter (Fig S1) While further data arerequired to confirm this correlation the results obtained inthis study supported the presence of a positive relationshipbetween productivity and the SAM
The observed concentrations of the macronutrients NOx PO4 and SiO4 showed significant negative correlationswith SAMautumn (r =minus039 minus056 minus042 respectively Ta-ble 2d p 0005 lt 0001 0002 respectively) The concen-trations of these nutrients showed stronger negative correla-tions with SAMautumn when the 50 of samples collectedlatest in the springndashsummer season was considered (r =minus058 minus074 minus051 Table 2e p 0002 lt 0001 0008respectively) Macronutrient concentrations were unrelatedto either SAMprior or SAMspring (Table 2d) Peaks in neg-ative correlation of the SAM on [PO4] were evident in thepreceding autumn and spring prior to sampling in responsesurfaces with the peaks being more negative when only the50 of samples collected later in the springndashsummer wereconsidered (Fig S2) The concentrations of macronutrientsalso showed expected decline through the springndashsummercorrelations between [NOx] [PO4] and [SiO4] withD wereminus077minus073 andminus056 respectively (Table 2d p lt 0001lt 0001 lt 0001 respectively)
33 Observed occurrence and abundance
Abundance of individual taxa groups averaged 133 cells permillilitre and ranged to a maximum of 8796 cells per mL (Ta-ble 3) Individual cell volume ranged from 8 microm3 for the Par-males spp to gt 60 000 microm3 for the diatoms Dactyliosolenantarcticus and Thalassiothrix antarctica Average relativeabundance ranged from 02 for the diatom Fragilariopsisritscheri to 17 for the combined taxa group Fragilariop-sis cylindruscurta Of the 22 taxa groups resolved in thisstudy four taxa groups were identified in all 52 samples and11 taxa groups were identified in more than 90 of samples(Table 3)
4 Discussion
41 The SAM and phytoplankton communitycomposition
Our results show that the SAM shows a relationship withthe community composition of phytoplankton in the sea-sonal ice zone (SIZ) of the Southern Ocean (SO) This con-clusion was supported by a combination of three analyses(i) Permutation-based analyses of cluster structure demon-strated that the 52 samples were separable into seven statisti-cally different groups on the basis of community abundancecomposition of the 22 taxa groups (Fig 6b) and thus thatthere was variation between samples that might be explain-able with known environmental variables if clustering hadrevealed few or no clusters it would have been indicative oflevels of community variance (either high or low) unlikelyto be systematically explainable with the environmental vari-ables (ii) CAP analysis identified the SAM as a significantexplanatory variable on the structure of the phytoplanktoncommunity (Table 1b) and showed that groups identified incluster analysis were generally distinguished by the SAMand the D that a sample was collected (Fig 6) (iii) 15 ofthe 22 taxa groups resolved showed significant pairwise cor-relations (p lt 005) between relative abundance and at leastone of the three derived SAM indices (Table 2b)
The derived SAM index with greatest influence on phy-toplankton community composition SAMautumn (Figs 3 4)explained 126 of the variance of phytoplankton commu-nity composition in the optimum multi-variable CAP model(Table 1b) SAMautumn represented the average SAM aroundthe time that sea ice was extending northward through theSIZ (Fig 1a) At this time phytoplankton productivity inthe SIZ would have declined to around 30 of its mid-summer maximum (Moore and Abbott 2000 Arrigo et al2008 Constable et al 2014) and phytoplankton would bepreparing for winter by variously producing energy stor-age products producing resting spores or cysts reducingmetabolic rate and engaging in heterotrophic consumptionfor energy (Fryxell 1989 McMinn and Martin 2013) Theformation of sea ice reduces available light by as much as999 (McMinn et al 1999) severely limiting light forphytoplankton for around half of each year at the rangeof longitude sampled latitude 64 S was covered in seaice for half the time across the sampled years (Fig 1a)Windier conditions associated with a more positive SAM inautumn may delay the consolidation of sea ice into largerfloes (Roach et al 2018) extending the phytoplankton grow-ing season and possibly increasing the relative abundanceof taxa that occur later in the springndashsummer season Thequantity of phytoplankton that survive the Antarctic winteris extremely low (McMinn and Martin 2013) and the abun-dance of taxa present and their metabolic condition whenthe autumn sea ice forms may strongly influence their vi-ability relative vigour and availability to seed the subse-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3828 B L Greaves et al SAM influences phytoplankton in SIZ
quent post-winter bloom This possibility was supported bythe observation that the only two taxa groups observed tohave significantly (p lt 005) higher relative abundance laterin the springndashsummer the Chaetoceros species C dichaetaand C concavicorniscurvatus were both observed to alsoshow significantly higher relative abundances when the pre-ceding SAMautumn was more positive (Table 2b) Thus SAM-induced effects on phytoplankton in the autumn could wellinfluence the phytoplankton community structure in the fol-lowing post-winter productive season
Extending the springndashsummer productive season by de-laying the autumn consolidation of sea ice may result inmore prolonged declines in relative abundance of taxa thatare more prolific earlier in the springndashsummer and may thusreduce the population from which the following post-winterbloom is initiated Of the eight taxa groups showing sta-tistically higher relative abundance earlier in the springndashsummer (p lt 005) three showed corresponding statisticallylower relative abundances with higher preceding SAMautumn(Emiliana huxleyi Nitzschia acicularisdecipiens and Par-males spp p lt 005 Table 2b) supporting this conjec-ture Of the remaining five taxa groups of the eight fourshowed no detectable relationship with SAMautumn and one(Pseudonitzschia lineola) showed a positive relationship
Two other derived SAM indices were found to influencephytoplankton SAMspring and SAMprior These indices weredifficult to distinguish due to their largely overlapping timeperiods (Fig 4) and they were strongly correlated (r = 083p lt 005 Table 2c) with similar influence on taxonomicabundances (Table 2b) SAMprior was the preferred parame-ter for the multiparameter CAP model in which it explained43 of total variance Windier and stormier conditions as-sociated with a higher SAM in the months prior to sam-pling would increase nutrient input to the euphotic zone fromdeeper waters (Lovenduski and Gruber 2005) promotingproductivity whilst at the same time episodically dilutingsurface phytoplankton through deeper mixing More stormyconditions may also have brought about a faster break-upof winter sea ice promoting earlier spring phytoplanktongrowth Conversely windier conditions would also restrictstratification of the surface ocean precluding phytoplanktonbloom formation lessening productivity (Fitch and Moore2007) and reducing the abundance of early blooming taxaThis may explain the responses of Emiliania huxleyi and thecombined Nitzschia acicularisdecipiens group which bothshowed early maximum abundances (r =minus028 and minus047respectively with D p lt 005 Table 2b) and also nega-tive correlations with SAMspring and SAMprior (r =minus029to minus042 p lt 005 Table 2b) Five other taxa groups withearly maximum abundance (negative correlation with Dp lt 005) showed no detectable correlation with SAMspringand one (Pseudonitzschia lineola) showed a positive rela-tionship indicating that their abundances were determinedby environmental factors that prevail early in the season butnot those factors altered by variations in the SAM Histori-
cally the variance in the SAM is lower in the spring quar-ter than in other quarters (NOAA 2005) perhaps explainingwhy SAMspring and SAMprior explained less variance in com-munity composition than SAMautumn
We expected the SAM prior to sampling (SAMprior andSAMspring) would show a relationship with phytoplanktoncomposition and a lesser relationship of the SAM in thewinter is plausible because the surface of the ocean is in-sulated from atmospheric conditions by sea ice The relation-ship with the SAM the previous autumn was not expected butis also plausible as it coincides with the time when sea ice isforming and thus a critical time for phytoplankton preparingto hibernate the half-year of sea ice cover We also observeda similar relationship between SAMautumn and (i) NASAsatellite total chlorophyll and (ii) macronutrient concentra-tions across all samples as well as (iii) a stronger correla-tion with macronutrient concentrations when only the sam-ples collected in the latter half of the season were considered(Table 2c d and e respectively) We also observed maximain the autumn SAM relationship in response-surface analy-ses of the correlation between the SAM and (i) NASA satel-lite total chlorophyll and (ii) [PO4] in all samples as well as(iii) a stronger maxima with [PO4] when only the samplescollected later in the season were considered (Figs S1 andS2) Both total chlorophyll and [PO4] were observationallyindependent of the taxonomic cell counts and whilst [PO4]was estimated from parallel samples as the taxonomic analy-sis NASA satellite total chlorophyll had no material connec-tion with collected samples being linked only geographicallyand temporally and thus offers independent support for theunexpected observation that phytoplankton community com-position in the springndashsummer is related to the SAM in theprevious autumn The empirically defined SAMautumn alsoshowed significant (p lt 005) pairwise correlations with 12of the 22 taxa groups resolved (Table 2b)
42 Effect of the SAM on phytoplankton taxa
Nothing has been previously reported with respect to the cli-matic preferences of the majority of taxa identified in thisstudy and only 10 of the 22 taxa groups considered in ourresearch had data records in the Ocean Biogeographic In-formation System (OBIS 2020) Some of the observed taxahave been reported to show various relationships with en-vironmental factors including sea-surface temperature timethrough the season and latitude but often at the taxonomiclevel of genera rather than at a species level (Burckle et al1987 Chiba et al 2000 Waters et al 2000 Green and Sam-brotto 2006 Gomi et al 2007) We however observed dif-fering responses to environmental variables among closelyrelated taxa This was exemplified by the opposite correla-tions of Chaetoceros species C dicheata and C neglectuswith D (048 and minus070 respectively p lt 00025 Table 2b)and the opposite correlations of Fragilariopsis species Frhombica and F kerguelensis with SAMautumn (minus039 and
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3829
052 respectively p lt 005 Table 2b Fig 5a b) The strongand opposite response to these variables by species belong-ing to the same genus indicates the importance of species-level observation in detecting subtle changes in pelagic phy-toplankton communities
A third of analysed taxa comprising 7 taxa and 23 of all counted cells showed no detectable relationship withthe SAM This could be due to large errors associated withlow counts of rarer taxa because unaccounted variation wasmasking any relationship or because the taxa were insensi-tive to the SAM There is less chance of detecting relation-ships between taxa and environment variables when fewerindividuals are counted however some less represented taxadid show relationships with SAM indices (eg Emilianiahuxleyi |r|gt 038 Table 2b) Of the 22 taxa resolved 5showed no significant relationships with either the SAM orD All were comparatively scarce and together representedonly 2 of all cells counted Assessing species composi-tions across a greater fraction of each sample and thus count-ing more of the scarcer taxa may have revealed relationshipsbetween these rarer taxa and environmental variables (Nak-agawa and Cuthill 2007) Yet it remains possible that thesetaxa are actually unaffected by seasonal succession and theSAM instead responding to other environmental variablesthat were not measured as part of this study or that they re-main as persistent but relatively rare background taxa withrespect to the overall phytoplankton assemblage
This is the first study to show a link between variationin the SAM and the composition of phytoplankton commu-nities in the SO although similar findings have been re-ported for other major climatic phenomena in other partsof the globe The climatically similar Northern HemisphereAnnular Mode (NAM) causes increased westerly winds anddeeper mixed layers at middle to high northern latitudes inits positive phase (Nehring 1998 Thompson et al 2003Kahru et al 2011) The NAM has been related to the tim-ing abundance and biomass of phytoplankton taxa at highnorthern latitudes (Nehring 1998 Belgrano et al 1999 Ot-tersen et al 2001 Blenckner and Hillebrand 2002) andto the delayed occurrence of maximum chlorophyll in theNorth Atlantic Summer (Kahru et al 2011) Similarly theEl NintildeondashSouthern Oscillation (ENSO) equatorial mode hasbeen shown to influence the distribution and abundance ofphytoplankton in the tropical oceans (Blanchot et al 1992)
Phytoplankton are the pastures of the oceans and it is plau-sible that the climate in both autumn and spring influencethe phytoplankton community composition of phytoplank-ton and their ecological progression through the productivespringndashsummer period in the SIZ Climate change impactshave now been documented across every type of ecosystemon Earth (Scheffers et al 2016 Harris et al 2018) and thedistribution abundance phenology and productivity of phy-toplankton communities throughout the world are changingin response to warming acidifying and stratifying oceans(Hoegh-Guldberg and Bruno 2010) We have detected an
association between variation in phytoplankton communitycomposition and variation in the SAM over a relatively brief11-year monitoring period despite all the other environmen-tal factors that elicit variability in phytoplankton communi-ties in the SIZ of the SO
43 The effects of the SAM on productivity andbiomass
A positive SAM has previously been shown to be associ-ated with increased standing stocks and productivity of phy-toplankton in the SIZ of the SO (Arrigo et al 2008 Boyce etal 2010 Soppa et al 2016) In the SIZ above the AntarcticDivergence nutrients are replenished from the deeper oceanthrough the unproductive winter and the levels of nutritionremaining at the end of summer integrate the total draw-down of nutrients by phytoplankton production over the en-tire springndashsummer growing season (Arrigo et al 1999) Weobserved this nutrient drawdown through the springndashsummeras the negative correlation between all macronutrient con-centrations and D (Table 2d) We also observed a nega-tive relationship between all macronutrient concentrations inthe springndashsummer and the previous SAMautumn (Table 2dFig 5d) suggesting that an elevated SAM in autumn leadsto greater productivity and thus greater nutrient drawdownduring the following springndashsummer The nutrient concen-trations at the end of the springndashsummer productive seasonwould be expected to best represent the total productivityover the season we observed that the correlation between nu-trient concentrations and SAMautumn were higher when onlythe 50 of samples collected later in the springndashsummerwere considered (Table 2e) further supporting the conjec-ture that a higher SAM in the autumn is linked with greaterproductivity through the following springndashsummer
The observed positive relationship between total chloro-phyll and all the SAM indices (r = 05 to 072 p lt 00025Table 2c) and the presence of apparent spring and autumnmaxima in the response surfaces of the variance in totalchlorophyll explained by the SAM (Fig S1) further sup-port the conjecture that a more positive SAM is linked withgreater total chlorophyll and thus greater total productivityin the SIZ The total chlorophyll data considered were limitedto the 52 samples collected that is estimated for the timesand locations of each sample collection Estimates werecoarsely determined as interpolations of available monthlypredictions (Fig S3) and estimates could be thus obtainedfor only 49 of the 52 samples Yet there are indicators of re-liability in the sparse information the diatom Fragilariopsisrhombica is always relatively small (Table 3) and when therelative abundance of this taxon was high total chlorophyllwas lower (r =minus059 p lt 00025 Table 2b) and when therelative abundance of larger diatoms were high total chloro-phyll was also often high (eg Dactyliosolen antarcticusr = 037 p lt 005 Table 2b)
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3830 B L Greaves et al SAM influences phytoplankton in SIZ
44 Implications
The SIZ is a productive region of the SO (Moore and Abbott2000) and changes to the SIZ phytoplankton communityhave potentially far-reaching implications for the ecosystemservices these organisms provide including carbon exportto the deep ocean and supporting the productivity of almostall Antarctic life Increases in the relative abundance of thelarger Chaetoceros spp diatoms would favour grazing bylarge metazooplankton especially krill (Boyd et al 1984Kawaguchi et al 1999 Moline et al 2004) which linkphytoplankton to whales seabirds seals and most higherAntarctic life forms (Smetacek 2008) Such changes wouldalso increase the efficiency of the biological pump as thelarger phytoplankton sink more rapidly than small phyto-plankton (Alldredge and Gotschalk 1989) and increasedgrazing by krill would reparcel some phytoplankton biomassinto faeces that would also sink more rapidly (Cadeacutee etal 1992) Such changes in carbon flux and trophodynam-ics would act as a negative feedback on climate change byspeeding the sequestration of carbon to the deep ocean
The SAM is predicted to become increasingly positivein the future (Arblaster and Meehl 2006 Swart and Fyfe2012 Gillett and Fyfe 2013 Abram et al 2014 Solomonet al 2016) Our results cannot necessarily be extrapolatedto infer changes that will likely occur as the SAM contin-ues to increase as evolutionary responses can partly miti-gate adverse effects on phytoplankton of longer-term climatechange and future changes in climate are likely to imposeother co-stressors on phytoplankton inhabiting these waters(Lohbeck et al 2014 Schluumlter et al 2014 Deppeler andDavidson 2017) Our study showed that some of the vari-ation in the phytoplankton composition in the seasonal icezone was significantly related to variation in the SAM andthat the sign and magnitude of the correlation with the SAMdiffered among species
5 Conclusions
Statistical analyses indicated that together the autumn andspring SAM explained a higher percentage (179 ) of thevariation in phytoplankton community composition than anyvariable mostly due to the autumn SAM (up to 133 ) Intotal this exceeded the variance explained by any other vari-able even that attributable to the time of the season thatthe sample was collected (154 ) or other critical phys-ical variables such as temperature salinity and latitudeFurthermore 15 of the 22 phytoplankton taxa identified inthis study showed significant correlation with the SAM andthere were indications that a more positive SAM was relatedto increased phytoplankton productivity in the SIZ Whilethis study was limited in both timespan (11 austral springndashsummers) and the overall variance in phytoplankton compo-sition explained by all the constraining variables (375 ) it
suggests that the phytoplankton of the SIZ are indeed sensi-tive to changes in the SAM and thus possibly responsive toclimate change
Data availability The dataset used in this paper is available athttpsdoiorg10261795d9181f7308bd (Greaves et al 2019)
Supplement The supplement related to this article is available on-line at httpsdoiorg105194bg-17-3815-2020-supplement
Author contributions Author contributions BLG contributed toconceptualisation data curation formal analysis investigationmethodology software and supervision validation visualisationwriting of the original draft writing and review and editing ATDcontributed to conceptualisation funding acquisition formal anal-ysis methodology project administration resources supervisionwriting and review and editing ADF contributed to formal analy-sis methodology resources writing and review and editing JPMcontributed to formal analysis methodology software writing andreview and editing AM contributed to project administration su-pervision writing and review and editing AMcM contributed tofunding acquisition project administration resources writing andreview and editing SWM contributed to conceptualisation fund-ing acquisition formal analysis writing and review and editing
Competing interests The authors declare that they have no conflictof interest
Acknowledgements Sampling on Astrolabe was supported bya FrenchndashAustralian research collaboration The Institut PolaireFranccedilais Paul-Eacutemile-Victor supported access to the ship and fieldoperations The biogeochemical data collection was coordinatedby Alain Poisson and Nicolas Metzl Sorbonne Universiteacute andBronte Tilbrook CSIRO Oceans and Atmosphere Steve Rintoul(CSIRO) and Rose Morrow (LEGOS) coordinated the collection ofsalinity and temperature data The Antarctic Climate and Ecosys-tems CRC and the Integrated Marine Observing System are thankedfor supporting the operation of sensors the collection of water sam-ples and nutrient analyses reported in this study Alan Poole MattSherlock John Akl Kate Berry Lesley Clementson Brian Grif-fiths (CSIRO) Rick van den Enden Rob Johnson (AAD) and themany dedicated volunteers and shipsrsquo officers and crew are thankedfor their important contributions to the field efforts and data man-agement We thank the University of Tasmania and the AustralianAntarctic Division for the space and resources needed to undertakethis work Thanks to Nathaniel Bindoff and Simon Wotherspoon fortheir consideration of parts of the paper Thanks are due to the re-viewer Damiano Righetti for the valuable input he provided in par-ticular for pointing out ambiguities and small errors and improvingthe clarity of the paper and an anonymous reviewer for the struc-tural and theoretical considerations Total chlorophyll data used inthis paper were produced with the Giovanni online data system de-veloped and maintained by the NASA GES DISC
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3831
Financial support This research has been supported by the Uni-versity of Tasmania (Institute of Marine and Antarctic Studies) bythe Australian Governmentrsquos Cooperative Research Centre programthrough the Antarctic Climate and Ecosystems CRC the AustralianAntarctic Division (projects 40 and 4107) and by the Australian Re-search Councilrsquos Special Research Initiative for Antarctic GatewayPartnership (project no SR140300001)
Review statement This paper was edited by Julia Uitz and re-viewed by Damiano Righetti and one anonymous referee
References
Abram N J Mulvaney R Vimeux F Phipps S J Turner Jand England M H Evolution of the Southern Annular Modeduring the past millennium Nat Clim Change 4 564ndash569httpsdoiorg101038nclimate2235 2014
Acker J G and Leptoukh G Online analysis enhances use ofNASA earth science data Eos Transactions American Geophys-ical Union 88 14ndash17 https1010292007EO020003 2007
Alldredge A L and Gotschalk C C Direct observations of themass flocculation of diatom blooms characteristics settling ve-locities and formation of diatom aggregates Deep-Sea Res PtI 36 159ndash171 httpsdoiorg1010160198-0149(89)90131-31989
Anderson M J and Willis T J Canonical analysis of prin-cipal coordinates a useful method of constrained ordinationfor ecology Ecology 84 511ndash525 httpsdoiorg1018900012-9658(2003)084[0511CAOPCA]20CO2 2003
Arblaster J M and Meehl G A Contributions of external forc-ings to southern annular mode trends J Clim 19 2896ndash2905httpsdoiorg101175JCLI37741 2006
Arndt JE Schenke HW Jakobsson M Nitsche FO Buys GGoleby B Rebesco M Bohoyo F Hong J Black J andGreku R The International Bathymetric Chart of the South-ern Ocean (IBCSO) Version 10 ndash A new bathymetric compila-tion covering circum-Antarctic waters Geophys Res Lett 403111ndash3117 httpsdoiorg101002grl50413 2013
Arrigo K R Robinson D H Worthen D Dunbar R BDiTullio G R VanWoert M and Lizotte M P Phyto-plankton Community Structure and the Drawdown of Nutri-ents and CO2 in the Southern Ocean Science 283 365ndash367httpsdoiorg101126science2835400365 1999
Arrigo K R van Dijken G L and Bushinsky SPrimary production in the Southern Ocean 1997ndash2006 J Geophys Res-Ocean 113 1997ndash2006httpsdoiorg1010292007JC004551 2008
Belgrano A Lindahl O and Hernroth B North Atlantic Oscil-lation primary productivity and toxic phytoplankton in the Gull-mar Fjord Sweden (1985ndash1996) P Roy Soc B 266 425ndash430httpsdoiorg101098rspb19990655 1999
Blanchot J Rodier M and Le Bouteiller A Effect ofel nintildeo southern oscillation events on the distribution andabundance of phytoplankton in the Western Pacific Trop-ical Ocean along 165 E J Plankton Res 14 137ndash156httpsdoiorg101093plankt141137 1992
Blenckner T and Hillebrand H North Atlantic Oscillation sig-natures in aquatic and terrestrial ecosystems ndash A meta-analysisGlob Change Biol 8 203ndash212 httpsdoiorg101046j1365-2486200200469x 2002
Bopp L Resplandy L Orr J C Doney S C Dunne J PGehlen M Halloran P Heinze C Ilyina T Seacutefeacuterian RTjiputra J and Vichi M Multiple stressors of ocean ecosys-tems in the 21st century projections with CMIP5 modelsBiogeosciences 10 6225ndash6245 httpsdoiorg105194bg-10-6225-2013 2013
Boyce D G Lewis M R and Worm B Global phyto-plankton decline over the past century Nature 466 591ndash596httpsdoiorg101038nature09268 2010
Boyce D Lewis M and Worm B Boyce et al reply Nature472 E8ndashE9 httpsdoiorg101038nature09953 2011
Boyd C M Heyraud M and Boyd C N Feeding of the Antarc-tic krill Euphausia superba J Crustacean Biol 4 123ndash141httpsdoiorg1011631937240X84X00543 1984
Boyd P W and Trull T W Understanding the export of biogenicparticles in oceanic waters Is there consensus Prog Oceanogr72 276ndash312 httpsdoiorg101016jpocean2006100072007
Bray J R and Curtis J T An Ordination of the Upland ForestCommunities of Southern Wisconsin Ecol Monogr 27 325ndash349 httpsdoiorg1023071942268 1957
Burckle L H Jacobs S S and McLaughlin R B Late australspring diatom distribution between New Zealand and the RossIce Shelf Antarctica Hydrographic and sediment correlationsMicropaleontology 74ndash81 httpsdoiorg10230714855281987
Cadeacutee G C Gonzaacutelez H and Schnack-Schiel S B Krill dietaffects faecal string settling in Weddell Sea Ecology SpringerBerlin Germany 1992
Carranza M M and Gille S T Southern Ocean wind-driven entrainment enhances satellite chlorophyll-a throughthe summer J Geophys Res-Ocean 120 304ndash323httpsdoiorg1010022014JC010203 2015
Cavicchioli R Ripple W J Timmis K N Azam F BakkenL R Baylis M Behrenfeld M J Boetius A Boyd PW Classen A T and Crowther T W Scientistsrsquo warning tohumanity microorganisms and climate change Nat Rev Mi-crobiol 1 569ndash586 httpsdoiorg101038s41579-019-0222-5 2019
Chiba S Hirawake T Ushio S Horimoto N Satoh R Naka-jima Y Ishimaru T and Yamaguchi Y An overview of thebiologicaloceanographic survey by the RTV Umitaka-Maru IIIoff Adelie Land Antarctica in JanuaryndashFebruary 1996 Deep-Sea Res Pt II 47 2589ndash2613 httpsdoiorg101016S0967-0645(00)00037-0 2000
Ciais P Sabine C Bala G Bopp L Brovkin V CanadellJ Chhabra A DeFries R Galloway J Heimann M andJones C Carbon and other biogeochemical cycles Climatechange 2013 the physical science basis Contribution of Work-ing Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate Change Cambridge University PressCambridge United Kingdom and New York NY USA 465ndash570httpsdoiorg101017CBO9781107415324015 2013
Clarke K R Somerfield P J and Gorley R N Testing of nullhypotheses in exploratory community analyses similarity pro-
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3832 B L Greaves et al SAM influences phytoplankton in SIZ
files and biota-environment linkage J Exp Mar Biol Ecol366 56ndash69 httpsdoiorg101016jjembe200807009 2008
Cohen J Things I have learned (so far) some things youlearn arenrsquot so less is more Am Psychol 45 1304ndash1312httpsdoiorg1010370003-066X45121304 1990
Constable A J Melbourne-Thomas J Corney S P Arrigo KR Barbraud C Barnes D K A Bindoff N L Boyd P WBrandt A Costa D P and Davidson A T Climate change andSouthern Ocean ecosystems I How changes in physical habitatsdirectly affect marine biota Glob Change Biol 20 3004ndash3025httpsdoiorg101111gcb12623 2014
Dargie T C D On the integrated interpretation of in-direct site ordinations a case study using semi-aridvegetation in southeastern Spain Vegetatio 55 37ndash55httpsdoiorg101007BF00039980 1984
Davidson A T McKinlay J Westwood K Thomson P Gvan den Enden R de Salas M Wright S Johnson R andBerry K Enhanced CO2 concentrations change the structure ofAntarctic marine microbial communities Mar Ecol Prog Ser552 93ndash113 httpsdoiorg103354meps11742 2016
Deppeler S L and Davidson A T Southern Ocean phyto-plankton in a changing climate Front Mar Sci 4 1ndash28httpsdoiorg103389fmars201700040 2017
DiFiore P J Sigman D M Trull T W Lourey M J KarshK Cane G and Ho R Nitrogen isotope constraints on sub-antarctic biogeochemistry J Geophys Res-Ocean 111 1ndash19httpsdoiorg1010292005JC003216 2006
Dixon P VEGAN a package of R functions for community ecol-ogy J Veg Sci 14 631ndash637 httpsdoiorg101111j1654-11032003tb02228x 2003
Fitch D T and Moore J K Wind speed influence onphytoplankton bloom dynamics in the Southern OceanMarginal Ice Zone J Geophys Res-Ocean 112 1ndash13httpsdoiorg1010292006JC004061 2007
Fryxell G A Marine phytoplankton at the Weddell Sea ice edgeSeasonal changes at the specific level Polar Biol 10 6ndash7httpsdoiorg101007BF00238285 1989
Gibbons J D and Pratt J W P-values interpre-tation and methodology Am Stat 29 20ndash25httpsdoiorg10108000031305197510479106 1975
Gillett N P and Fyfe J C Annular mode changes in theCMIP5 simulations Geophys Res Lett 40 1189ndash1193httpsdoiorg101002grl50249 2013
GMAO NASA Ocean Biogeochemical Model assimilating ESRIDdata global monthly 067x125 degrees VR2014 Greenbelt MDUSA Goddard Earth Sciences Data and Information ServicesCenter (GES DISC) Goddard Modeling and Assimilation Of-fice 2017
Gomi Y Taniguchi A and Fukuchi M Temporal and spatialvariation of the phytoplankton assemblage in the eastern Indiansector of the Southern Ocean in summer 20012002 Polar Biol30 817ndash827 httpsdoiorg101007s00300-006-0242-2 2007
Gong D and Wang S Definition of Antarctic os-cillation index Geophys Res Lett 26 459ndash462httpsdoiorg1010291999GL900003 1999
Greaves B L Davidson A T and Fraser A D The SouthernAnnular Mode (SAM) influences phytoplankton communities inthe seasonal ice zone of the Southern Ocean Ver 1 Australian
Antarctic Data Centre httpsdoiorg10261795d9181f7308bd2019
Green S E and Sambrotto R N Plankton communitystructure and export of C N P and Si in the Antarc-tic Circumpolar Current Deep-Sea Res Pt II 53 620ndash643httpsdoiorg101016jdsr2200601022 2006
Hall A and Visbeck M Synchronous variabil-ity in the Southern Hemisphere atmosphere seaice and ocean resulting from the annular mode JClim 15 3043ndash3057 httpsdoiorg1011751520-0442(2002)015lt3043SVITSHgt20CO2 2002
Harris R M B Beaumont L J Vance T R Tozer C R Re-menyi T A Perkins-Kirkpatrick S E Mitchell PJ NicotraAB McGregor S Andrew NR Letnic M Kearney M RWernberg T Hutley L B Chambers L E Fletcher M-SKeatley M R Woodward C A Williamson G Duke N Cand Bowman D M J S Biological responses to the press andpulse of climate trends and extreme events Nat Clim Change8 579ndash587 httpsdoiorg101038s41558-018-0187-9 2018
Henson S A Yool A and Sanders R Variabilityin efficiency of particulate organic carbon export Amodel study Global Biogeochem Cy 29 33ndash45httpsdoiorg1010022014GB004965 2015
Hines K M Bromwich D H and Marshall G J Ar-tificial surface pressure trends in the NCEP-NCAR re-analysis over the Southern Ocean and Antartica JClim 13 3940ndash3952 httpsdoiorg1011751520-0442(2000)013lt3940ASPTITgt20CO2 2000
Ho M Kiem A S and Verdon-Kidd D C The Southern An-nular Mode a comparison of indices Hydrol Earth Syst Sci16 967ndash982 httpshttpsdoiorg105194hess-16-967-20122012
Hoegh-Guldberg O and Bruno J F The impact of climate changeon the worldrsquos marine ecosystems Science 328 1523ndash1528httpsdoiorg101126science1189930 2010
Hydes D J Aoyama M Aminot A Bakker K Becker S Cov-erly S Daniel A Dickson A G Grosso O Kerouel Rvan Ooijen J Sato K Tanhua T Woodward E M S andZhang J Z Determination of Dissolved Nutrients (N P SI)in Seawater With High Precision and Inter-Comparability Us-ing Gas-Segmented Continuous Flow Analysers in The GO-SHIP repeat hydrography manual a collection of expert re-ports and guidelines edited by Hood E M Sabine C Land Sloyan B M IOCCP report number 14 ICPO publicationseries number 134 UNESCO-IOC Paris France available athttpwwwgo-shiporgHydroManhtml (last access 15 January2020) 2010
Clem K R Crosta X de Lavergne C Eisenman I Eng-land M H Fogt R L Frankcombe L M MarshallG J Masson-Delmotte V Morrison A K Orsi A JRaphael M N Renwick J A Schneider D P Simp-kins G R Steig E J Stenni B Swingedouw D andVance T R Assessing recent trends in high-latitude SouthernHemisphere surface climate Nat Clim Change 6 917ndash926httpsdoiorg101038nclimate3103 2016
Kahru M Brotas V Manzano-Sarabia M and Mitchell B GAre phytoplankton blooms occurring earlier in the Arctic GlobChange Biol 17 1733ndash1739 httpsdoiorg101111j1365-2486201002312x 2011
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3833
Kawaguchi S Ichii T and Naganobu M Green krill the indi-cator of micro-and nano-size phytoplankton availability to krillPolar Biol 22 133ndash136 1999
Kohyama T and Hartmann D L Antarctic sea ice response toweather and climate modes of variability J Clime 29 721ndash741httpsdoiorg101175JCLI-D-15-03011 2016
Kwok R and Comiso J C Southern Ocean climate andsea ice anomalies associated with the Southern Oscilla-tion J Clim 15 487ndash501 httpsdoiorg1011751520-0442(2002)015lt0487SOCASIgt20CO2 2002
Lampitt R S and Antia A N Particle flux in deep seas Regionalcharacteristics and temporal variability Deep-Sea Res Pt I44 1377ndash1403 httpsdoiorg101016S0967-0637(97)00020-4 1997
Lannuzel D Schoemann V de Jong J Tison J L andChou L Distribution and biogeochemical behaviour of ironin the East Antarctic sea ice Mar Chem 106 18ndash32httpsdoiorg101016jmarchem200606010 2007
Lefebvre W Goosse H Timmermann R and FichefetT Influence of the Southern Annular Mode on the seaice-ocean system J Geophys Res-Ocean 109 1ndash12httpsdoiorg1010292004JC002403 2004
Legendre P and Anderson M J Distance-based re-dundancy analysis testing multispecies responsesin multifactorial ecological experiments EcolMonogr 69 1ndash24 httpsdoiorg1018900012-9615(1999)069[0001DBRATM]20CO2 1999
Legendre P Oksanen J and ter Braak C J Testing thesignificance of canonical axes in redundancy analysis Meth-ods Ecol Evol 2 269ndash277 httpsdoiorg101111j2041-210X201000078x 2011
Lenton A and Matear R J Role of the Southern Annular Mode(SAM) in Southern Ocean CO2 uptake Global Biogeochem Cy21 1-17 httpsdoiorg1010292006GB002714 2007
Lohbeck K T Riebesell U and Reusch T B H Gene expres-sion changes in the coccolithophore Emiliania huxleyi after 500generations of selection to ocean acidification P Roy Soc B281 1ndash7 httpsdoiorg101098rspb20140003 2014
Lovenduski N S Gruber N Doney S C and Lima I D En-hanced CO2 outgassing in the Southern Ocean from a positivephase of the Southern Annular Mode Global Biogeochem Cy21 1ndash14 httpsdoiorg1010292006GB002900 2007
Lovenduski N S and Gruber N Impact of the Southern AnnularMode on Southern Ocean circulation and biology Geophys ResLett 32 1ndash4 httpsdoiorg1010292005GL022727 2005
Mackas D L Does blending of chlorophylldata bias temporal trend Nature 472 E4ndashE5httpsdoiorg101038nature09951 2011
Mackintosh A N Anderson B M Lorrey A M Renwick JA Frei P and Dean S M Regional cooling caused recentNew Zealand glacier advances in a period of global warmingNat Commun 8 1ndash13 httpsdoiorg101038ncomms142022017
Marshall G J Trends in the Southern Annu-lar Mode from Observations and Reanalyses JClim 16 4134ndash4143 httpsdoiorg1011751520-0442(2003)016lt4134TITSAMgt20CO2 2003
Marshall G J Half-century seasonal relationships between theSouthern Annular mode and Antarctic temperatures Int J Cli-matol 27 373ndash383 httpsdoiorg101002joc1407 2007
Martin A McMinn A Heath M Hegseth E N and Ryan KG The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo SoundAntarctica and Tromsoslash Sound Norway J Exp Mar Biol Ecol428 57ndash66 httpsdoiorg101016jjembe201206006 2012
Massom R A and Stammerjohn S E Antarctic sea ice changeand variability ndash Physical and ecological implications Polar Sci4 149ndash186 httpsdoiorg101016jpolar201005001 2010
McMinn A Ashworth C and Ryan K Growth and Productivityof Antarctic Sea Ice Algae under PAR and UV Irradiances BotMar 42 401ndash407 httpsdoiorg101515BOT1999046 1999
McMinn A and Martin A Dark survival in awarming world P Roy Soc B 280 20122909httpsdoiorg101098rspb20122909 2013
Meredith M P Murphy E J Hawker E J King JC and Wallace M I On the interannual variability ofocean temperatures around South Georgia Southern OceanForcing by El NintildeoSouthern Oscillation and the South-ern Annular Mode Deep-Sea Res Pt II 55 2007ndash2022httpsdoiorg101016jdsr2200805020 2008
Mo K C Relationships between low-frequency variability inthe Southern Hemisphere and sea surface temperature anoma-lies J Clim 13 3599ndash3610 httpsdoiorg1011751520-0442(2000)013lt3599rblfvigt20co2 2000
Moline M A Claustre H Frazer T K Schofield O andVernet M Alteration of the food web along the Antarc-tic Peninsula in response to a regional warming trend GlobChange Biol 10 1973ndash1980 httpsdoiorg101111j1365-2486200400825x 2004
Moore J K and Abbott M R Phytoplankton chloro-phyll distributions and primary production in the South-ern Ocean J Geophys Res-Ocean 105 28709ndash28722httpsdoiorg1010291999JC000043 2000
Nakagawa S A farewell to Bonferroni the problems of low sta-tistical power and publication bias Behav Ecol 15 1044ndash1045httpsdoiorg101093behecoarh107 2004
Nakagawa S and Cuthill I C Effect size confidence inter-val and statistical significance a practical guide for biolo-gists Biol Rev 82 591ndash605 httpsdoiorg101111j1469-185X200700027x 2007
Nehring S Establishment of thermophilic phytoplankton speciesin the North Sea biological indicators of climatic changesShort communication ICES J Mar Sci 55 818ndash823httpsdoiorg101006jmsc19980389 1998
NOAA Teleconnection Pattern Calculation ProceduresClimate Prediction Center Internet Team available athttpswwwcpcncepnoaagovproductsprecipCWlinkdaily_ao_indexhistorymethodshtmlvar (last access 15 June 2017)2005
NOAA NCEP-DOE Reanalysis 2 data provided by theNOAAOARESRL PSD Boulder Colorado USA available athttpswwwcpcncepnoaagovproductsprecipCWlinkENSOverfnewaaoshtml last access 25 June 2017
OBIS Ocean Biogeographic Information System Intergovernmen-tal Oceanographic Commission of UNESCO available at httpwwwiobisorg last access 18 February 2020
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

3834 B L Greaves et al SAM influences phytoplankton in SIZ
Ottersen G Planque B Belgrano A Post E ReidP C and Stenseth N C Ecological effects of theNorth Atlantic Oscillation Oecologia 128 1ndash14httpsdoiorg101007s004420100655 2001
Parkinson C L A 40-y record reveals gradual Antarctic sea iceincreases followed by decreases at rates far exceeding the ratesseen in the Arctic P Natl Acad Sci USA 116 14414ndash14423httpsdoiorg101073pnas1906556116 2019
R Core Team R A Language and Environment for Statistical Com-puting R Foundation for Statistical Computing Vienna Austria2016
Rigual-Hernaacutendez A S Trull T W Bray S G Closset Iand Armand L K Seasonal dynamics in diatom and par-ticulate export fluxes to the deep sea in the Australian sec-tor of the southern Antarctic Zone J Mar Syst 142 62ndash74httpsdoiorg101016jjmarsys201410002 2015
Roach L A Smith M M and Dean S M Quantify-ing growth of pancake sea ice floes using images fromdrifting buoys J Geophys Res-Ocean 123 2851ndash2866httpsdoiorg1010022017JC013693 2018
Rodgers J L and Nicewander W A Thirteen Ways toLook at the Correlation Coefficient Am Stat 42 59ndash66httpsdoiorg10108000031305198810475524 1988
Saenz B T and Arrigo K R Annual primary produc-tion in Antarctic sea ice during 2005-2006 from a sea icestate estimate J Geophys Res-Ocean 119 3645ndash3678httpsdoiorg1010022013JC009677 2014
Sarthou G Timmermans K R Blain S and Treacuteguer P Growthphysiology and fate of diatoms in the ocean a review J Sea Res53 25ndash42 httpsdoiorg101016jseares200401007 2005
Savidge G Priddle J Gilpin L C Bathmann U Murphy EJ Owens N J P Pollard R T Turner D R Veth C andBoyd P An assessment of the role of the marginal ice zone inthe carbon cycle of the Southern Ocean Antarct Sci 8 349ndash358 httpsdoiorg101017S0954102096000521 1996
Scheffers B R De Meester L Bridge T C L HoffmannA A Pandolfi J M Corlett R T Butchart S H MPearce-Kelly P Kovacs K M Dudgeon D Pacifici MRondinini C Foden W B Martin T G Mora C Bick-ford D and Watson J E M The broad footprint of climatechange from genes to biomes to people Science 354 aaf7671httpsdoiorg101126scienceaaf7671 2016
Schiermeier Q Atmospheric science fixing the sky Nature 460792ndash795 httpsdoiorg101038460792a 2009
Schluumlter L Lohbeck K T Gutowska M A Groumlger J P Riebe-sell U and Reusch T B H Adaptation of a globally importantcoccolithophore to ocean warming and acidification Nat ClimChange 4 1024ndash1030 httpsdoiorg101038nclimate23792014
Scott F J and Marchant H J (Eds) Antarctic marine protistsAustralian Biological Resources Study Canberra and HobartAustralia 541 pp httpsdoiorg101017s00322474052448192005
Sen Gupta A and England M H Coupled oceanndashatmospherendashiceresponse to variations in the Southern Annular Mode J Clim19 4457ndash4486 httpsdoiorg101175JCLI38431 2006
Smetacek V and Nicol S Polar ocean ecosys-tems in a changing world Nature 437 362ndash368httpsdoiorg101038nature04161 2005
Smetacek V Are declining krill stocks a result of global warmingor of the decimation of the whales in Impacts of global warmingon polar systems Fundacioacuten BBVA edited by Duarte C MBilbao 47ndash83 2008
Solomon S Ivy D J Kinnison D Mills M J Neely R R andSchmidt A Emergence of healing in the Antarctic ozone layerScience 353 269ndash274 httpsdoiorg101126scienceaae00612016
Son S W Tandon N F Polvani L M and Waugh D W Ozonehole and Southern Hemisphere climate change Geophys ResLett 36 1ndash5 httpsdoiorg1010292009GL038671 2009
Soppa M Voumllker C and Bracher A Diatom Phenol-ogy in the Southern Ocean Mean Patterns Trends andthe Role of Climate Oscillations Remote Sens 8 1ndash7httpsdoiorg103390rs8050420 2016
Spreen G Kaleschke L and Heygster G Sea ice remote sensingusing AMSR-E 89-GHz channels J Geophys Res-Ocean 113C02S03 httpsdoiorg1010292005JC003384 2008
Squire V A Ocean wave interactions with sea icea reappraisal Annu Rev Fluid Mech 52 37ndash60httpsdoiorg101146annurev-fluid-010719-060301 2020
Steinacher M Joos F Froumllicher T L Bopp L Cadule PCocco V Doney S C Gehlen M Lindsay K Moore J KSchneider B and Segschneider J Projected 21st century de-crease in marine productivity a multi-model analysis Biogeo-sciences 7 979ndash1005 httpsdoiorg105194bg-7-979-20102010
Swart N C and Fyfe J C Observed and simulated changes inthe Southern Hemisphere surface westerly wind-stress GeophysRes Lett 39 1ndash6 httpsdoiorg1010292012GL0528102012
Swart N C Fyfe J C Gillett N and Marshall G J Compar-ing Trends in the Southern Annular Mode and Surface WesterlyJet J Clim 28 8840ndash8859 httpsdoiorg101175JCLI-D-15-03341 2015
Swiło M Majewski W Minzoni R T and Ander-son J B Diatom assemblages from coastal settingsof West Antarctica Mar Micropaleontol 125 95ndash109httpsdoiorg101016jmarmicro201604001 2016
Takahashi T Sutherland S C Wanninkhof R Sweeney CFeely R A Chipman D W Hales B Friederich G ChavezF Sabine C Watson A Bakker D C E Schuster U MetzlN Yoshikawa-Inoue H Ishii M Midorikawa T Nojiri YKoumlrtzinger A Steinhoff T Hoppema M Olafsson J Arnar-son T S Tilbrook B Johannessen T Olsen A Bellerby RWong C S Delille B Bates N R and de Baar H J W Cli-matological mean and decadal change in surface ocean pCO2and net seandashair CO2 flux over the global oceans Deep-Sea ResPt II 56 554ndash577 httpsdoiorg101016jdsr22008120092009
Taljaard J J Development Distribution and Move-ment of Cyclones and Anticyclones in the South-ern Hemisphere During the IGY J Appl Me-teorol 6 973ndash987 httpsdoiorg1011751520-0450(1967)006lt0973DDAMOCgt20CO2 1967
Taylor F and Sjunneskog C Postglacial marine diatom recordof the Palmer Deep Antarctic Peninsula (ODP Leg 178 Site1098) 2 Diatom assemblages Paleoceanography 17 1ndash12httpsdoiorg1010292000PA000564 2002
Biogeosciences 17 3815ndash3835 2020 httpsdoiorg105194bg-17-3815-2020
B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-

B L Greaves et al SAM influences phytoplankton in SIZ 3835
Ter Braak C J and Verdonschot P F Canonical correspondenceanalysis and related multivariate methods in aquatic ecologyAquat Sci 57 255ndash289 httpsdoiorg101007BF008774301995
Thompson D W Lee S and Baldwin M P Atmospheric pro-cesses governing the northern hemisphere annular modeNorthAtlantic oscillation Geoph Monog Series 134 81ndash112 2003
Thompson D W Solomon S Kushner P J England M HGrise K M and Karoly D J Signatures of the Antarcticozone hole in Southern Hemisphere surface climate change NatGeosci 4 741ndash749 2011
Thompson D W J and Solomon S Interpretation of RecentSouthern Hemisphere Climate Change Science 296 895ndash899httpsdoiorg101126science1069270 2002
Tomas C R (Ed) Identifying marine phytoplankton Academicpress San Diego California 858 pp 1997
Turner J Bracegirdle T J Phillips T Marshall G J and Hosk-ing J S An initial assessment of Antarctic sea ice extent in theCMIP5 models J Clim 26 1473ndash1484 2013
Turner J Summerhayes C Sparrow M Mayewski P ConveyP di Prisco G Gutt J Hodgson D Speich S Worby TBo S and Klepikov A Antarctic climate change and the envi-ronment ndash 2015 update Antarctic Treaty Consultative MeetingSofia Bulgaria June 2015 IP 92 2015
Waters R L Van Den Enden R and Marchant H J Summer mi-crobial ecology off East Antarctica (80ndash150 E) protistan com-munity structure and bacterial abundance Deep-Sea Res Pt II47 2401ndash2435 httpsdoiorg101016S0967-0645(00)00030-8 2000
Webb T and Bryson R A Late-and postglacial climatic changein the northern Midwest USA quantitative estimates derivedfrom fossil pollen spectra by multivariate statistical analy-sis Quaternary Res 2 70ndash115 httpsdoiorg1010160033-5894(72)90005-1 1972
Whitaker D and Christman M clustsig Significant Cluster Anal-ysis R package version 11 2014
Wilson D L Smith Jr W O and Nelson D M Phytoplanktonbloom dynamics of the western Ross Sea ice edge ndash I Primaryproductivity and species-specific production Deep-Sea Res PtI 33 1375ndash1387 httpsdoiorg1010160198-0149(86)90041-5 1986
Wright S W van den Enden R L Pearce I David-son A T Scott F J and Westwood K J Phyto-plankton community structure and stocks in the SouthernOcean (30ndash80 E) determined by CHEMTAX analysis ofHPLC pigment signatures Deep-Sea Res Pt II 57 758ndash778httpsdoiorg101016jdsr2200906015 2010
httpsdoiorg105194bg-17-3815-2020 Biogeosciences 17 3815ndash3835 2020
- Abstract
- Introduction
-
- Importance of the SIZ phytoplankton bloom
- The Southern Annular Mode
- The hypothesis
-
- Methods
-
- Phytoplankton relative abundance
- Environmental covariates
- Statistical analysis
-
- Results
-
- The influence of the SAM on phytoplankton community composition
- Influence of the SAM on phytoplankton productivity
- Observed occurrence and abundance
-
- Discussion
-
- The SAM and phytoplankton community composition
- Effect of the SAM on phytoplankton taxa
- The effects of the SAM on productivity and biomass
- Implications
-
- Conclusions
- Data availability
- Supplement
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
-
Related Documents