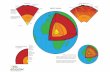
The Rock Cycle

The Rock Cycle. Elements in the Earth’s ATMOSPHERE Element Amount Nitrogen78.1 % Oxygen20.9 % Argon0.96 %
Dec 26, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Elements in the
Earth’s OCEANS
Element Amount
Oxygen 85.84 %
Hydrogen 10.82 %
Chlorine 1.94 %
Sodium 1.08 %
Magnesium 0.1292 %
Elements in the
Earth’s CRUSTElement
Oxygen
Silicon
Aluminum
Iron
Calcium
Amount47 %
28.2 %
8.23 %
5.63 %
3.5 %
IGNEOUS ROCKS• Forms from cooled lava.
• Most have crystals… the size of the crystal depends on how fast the lava cooled.
- Slow cooling = large crystals - Fast cooling = small to microscopic • Some rocks have pores (like a sponge) from
the gases in the lava escaping during the cooling process.
Uses of Igneous Rocks
• Road building materials• Gravestones• Creating lasting monuments• Used as an abrasive in polish things.• For trim and decoration in buildings. • Decorative landscape stone. • Jewelry
Igneous rock forms in two ways!
Intrusive
- Forms from magma cooling and solidifying inside the earth’s crust.
Extrusive
- Forms from lava cooling and solidifying outside of the earth’s crust.
SEDIMENTARY ROCKS
• Formed from weathered igneous and metamorphic rocks that are compacted and cemented together!
• Usually layers can be seen. • Some are conglomerates… i.e. you
can see smaller rocks or shells embedded.
Uses of Sedimentary Rocks
• Gypsum is used to make plaster of Paris and in drywall.
• Sandstone and limestone are used as building stones.
• Limestone is also used for hard core cement roads.
METAMORPHIC ROCKS
Formed within the Earth… rocks that undergo an extreme amount of heat and pressure.
These rocks are usually very dense rocks.
Uses of Metamorphic Rocks
• Used for pool table tops • Carved into statues • Buildings materials (floor tiles roofing
tiles and counter tops)• Jewelry = Diamonds
The Importance of Rocks and Minerals
IgneousObsidian Used in making arrowheads and knives Basalt Used in road building materials PumiceUsed in scouring, scrubbing, and polishing
materials Granite Used for buildings, monuments, and
tombstones
The Importance of Rocks and Minerals
SedimentarySandstone Used in the building industry for houses Gypsum (mineral) is used to make plaster of Paris and in
drywall
Limestone is also used for hard core cement roads.
The Importance of Rocks and Minerals
MetamorphicMarble Used in building, floors, tile in
bathrooms Slate Used forroofs, chalkboards, patio walks
and pool tables
The Importance of Rocks and Minerals
MineralsCalcite Used in cements and mortars and
the production of lime QuartzUsed in making glass, electrical
components, and optical lenses
MineralsA mineral is…
• Naturally occurring
• Inorganic- (made up of none living things)
• Definite chemical composition & crystal structure
• Solid
All physical properties of minerals come from the “internal arrangement of atoms”
Mineral Identification Tests
• The Streak Test
• The Color Test
• The Luster Test
• Hardness (Moh’s Scale)
• Cleavage
• Crystal Shape
• Reactions with Acids
• Specific gravity
The Streak Test• The color of the powdered mineral
performed by rubbing the unknown mineral on an unglazed tile.
The Luster Test• The way a mineral shines or doesn't shine
• The only way to really learn the different luster's is to see them for yourself.
• 2 Types of Luster
• Metallic and Non-Metallic
• Metallic Luster
Rocks look
like shiny metal
• Ex. Hematite
Non-Metallic Luster• Non-metallic- all the other ways that a mineral can
shine – Glassy- shines like a piece of broken glass (most
common non-metallic) – Dull/earthy- no shine at all – Resinous/waxy- looks like a piece of plastic or dried
glue – Pearly- looks oily it may have a slight rainbow like an
oil slick on water. Also looks like the inside of some clam shells
– Adamantine- brilliant, sparkling shine like a diamond
Color Test• Color is used to determine the type
of rock but it is not always reliable. • Sulfur is (almost) always yellow,
and there are a few others, but not many minerals have a fixed color. Small amounts of impurities can drastically change a mineral's color.
Hardness Test• Hardness- a minerals resistance
to scratching. • This should not be confused with
brittleness. A diamond is very hard and will scratch a hammer but a hammer will smash a diamond.
Moh’s Scale of Hardness• Talc (Softest)
• Gypsum • Calcite • Fluorite • Apatite
• Feldspar (AKA Albite) • Quartz • Topaz
• Corundum • Diamond (Hardest)
Cleavage Test• Cleavage –The rocks
will break along flat surfaces or…
• Cubic- Rocks will break into cubes.
Crystal Formation• Minerals will grow
crystal formations when given time & space to grow.
• Can be recognized by their beautiful regular shapes once you have seen a few examples.
Quartz Crystal Formation
Miscellaneous Tests• Acid- Calcite and powdered dolomite will
Effervescence (fizz) in diluted hydrochloric acid (HCl)
• Smell- Sphalerite will give off a rotten-egg smell when streaked on a streak plate. Magnetism- Magnetite (AKA Lodestone) will pick up paper clips
Miscellaneous Tests Cont.
• Taste- Halite is rock salt and will taste salty.
• Fluorescence- some minerals (mostly forms of calcite) will glow in fluorescent colors under a black (UV) light.
• Double refraction- some clear forms of calcite (Iceland Spar) will make a double image of words.
Specific gravity• Specific gravity indicates how many times more
the mineral weighs compared to an equal amount of water .
• Since water has a density of 1 gram/cm3, and since all of the units cancel, specific gravity is the same number as density but without any units.
• Remember… Density = Mass / Volume
• Density is how much matter packed in a space.
Related Documents