Static Electricity Chapter 16 and 24

Static Electricity Chapter 16 and 24. Review: The 4 Fundamental Forces Strong Force – The force that is involved in holding the nucleus of an atom together.
Jan 03, 2016
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Static Electricity
Chapter 16 and 24

Review: The 4 Fundamental Forces
Strong Force – The force that is involved in holding the nucleus of an atom together
Electromagnetic Force – The force that exists between charged particles
Weak Force – The force involved in nuclear decay
Gravity – The force that exists between any two objects that have mass.

Static Electricity
Better known as Electrostatics comes from the roots electro and statics. – “Electro” is Greek for amber, a petrified tree resin
which when rubbed will attract other objects. When it was discovered that the movement of a sub-atomic particle was responsible for this attraction, the particles were called electrons; the force was called electric.
– “Stati” is Greek for standing or place.
Thus, electrostatics is the study of electrical forces at rest.

What do the forces do?
First – they are not like gravity
The electrical forces can either attract or repel one another.
Ben Franklin named the two types of forces positive and negative.

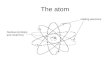
Structure of the AtomHelium Atom
Neutron
-Negative Charge-Charge = -1.6x10-19 C
Electron Proton-Positive Charge
-Charge = +1.6x10-19 C

- -
Charge Interaction: Like Charges
- -Like charges REPEL each other
+ +
+ +
- -- -- -
+ ++ ++ +

Charge Interaction: Unlike Charges
- +
+-
Opposite Charges Attract
- +- +- +

Coulomb’s Law
221
d
qqkF
The force between two electrically charged particles (q) is proportional to the product of their charges divided by the square of the distances between them.
K is the universal electrostatic constant. It is equal to 9.0 x 109 Nm2/C2.
Which is stronger, gravitational forces or electric forces?
Problem: Assume that you have two objects, one with a mass of 10 kg and the other with a mass of 15 kg, each with a charge of –3.0 x 10-2 C and separated by a distance of 2 meters. Compare the electrical and gravitational forces, which is greater?

IonsMost atoms have an equal amount of protons and electrons. Because of this they are neutral (they have no NET charge)
If an atom has too few or too many electrons, it will have either a net positive charge or a net negative charge. These are called IONS.
Ions are NEVER created by moving or trading protons. The only part of an atom that moves from place to place is an electron.

Charged AtomsNeutral Atom
Positive Ion
++
-
Lost an electron so it has a net positive charge

Charged AtomsNeutral Atom
Negative Ion
++
-
Gained an electron so it has a net negative charge
-
-

Insulators and ConductorsIn a conductor, electrons are NOT tightly bound to their atoms. These are called CONDUCTION (“free”) ELECTRONS.
- Therefore, it is easy to make charges move in and out of a conductor. This is the same reason we use conductors for wires. Charges (electrons) will flow easily through them.
- In a conductor, electrons will spread out so they are as far apart from each other as possible
- Metals, water with dissolved materials in it

Insulators and ConductorsIn an insulator, electrons ARE tightly bound to their atoms.
- Therefore, it is not easy to make charges move in and out of an insulator. In fact, even if an insulator is charged (maybe by friction) the charge will stay in one place and not spread out.
- Glass, rubber, plastic, wood, pure water

Increasing Conductivity AbilityIncreasing Conductivity Ability
•• RubberRubber–– GlassGlass
•• Wool Wool –– Dry AirDry Air
•• SiliconSilicon–– GermaniumGermanium
•• WaterWater–– CarbonCarbon
•• MercuryMercury–– IronIron
»» AluminumAluminum»» CopperCopper»» SilverSilver
Increasing Conducting A
bility

How to Charge and Object
Friction: Charging two objects by rubbing them together.
One takes electrons from the other so one becomes positive and one becomes negative
Conduction (Contact): Since charges like to spread out, touching a charged object to a neutral or differently charged object will transfer charge.
Induction: Bringing a charged object near a neutral object to induce a dipole in the neutral object.
NO TRANSFER OF CHARGE!!!!!

Induction
Before:

InductionInduce an “Electric Dipole”
Also called polarization
The negative charges move away from the negative sphere. REMEMER, the protons don’t move!

InductionInduced charge in both blocks when we separate them
Positive Block Negative Block

Law of Conservation of Electric Charge
In a closed system, the net amount of charge produced in any process is zeroThe strength of charged particles is measured in coulombs. An electron and a proton have the same magnitude of charge, just opposite signs. The magnitude of the charge of either of these two particles is: – 1.602x10–19 coulombs So, we can say that an electron has a charge
of –1.602x10–19Coulombs, and a proton has a charge of
+1.602x10–19Coulombs.

A little more on the conservation of charge
How much energy required to tear away electrons varies from substance to substance.– Rubber holds electrons more firmly than fur. When
rubber and fur are rubbed together, electrons transfer from the fur to the rubber rod.
• The rubber has excess electrons and is negatively charged.
– A glass or plastic rod rubbed with pure silk will transfer its electrons to the silk. Giving the silk a negative charge.
The electrons are not created or destroyed, they are simply transferred from one object to another.

Neutral ObjectsUsing your knowledge about induction and dipoles, how do you think Neutral Objects are affected by charged objects?
They Attract!
Neutral objects are attracted to charged objects

How does charge distribute itself on an object?
•Charges spread out as much as they can.
•In order to be in equilibrium, charges will bunch up at corners. (they gather so the net force in the center of a conductor is zero)
Charge Distribution

What should you do if a broken power lands on top of your car?
Charge distribution on a car

Shielding
Static charge occupies only the outer surface of a conductor; inside the conductor the electric field is zero.

Electric FieldsThe space around every electrical charge
Has both magnitude and direction, a vector quantity
Exploring Electrical Fields

Electrical Potential Energy
The work required to push a charged particle against the electric field of a charged object increases the particle’s electrical potential energy.
•Work done is equal to the energy gained.•Similar to how a mass’s PE depends on its location within the Earth’s gravitational force field

Electric Potential
Electrical potential is the electrical potential energy per charge.Electric potential = electrical potential energy/chargeOther terms for Electric Potential:– Voltage– EMF (electromotive force)
1 volt = 1 joule/coulomb

Lightning
Lightning on Science Joy Wagon
Related Documents