AQUATIC MICROBIAL ECOLOGY Aquat Microb Ecol Vol. 60: 261–272, 2010 doi: 10.3354/ame01429 Published online August 3 INTRODUCTION Pelagic heterotrophic bacteria are extremely impor- tant in aquatic ecosystems, with abundance ranges from <10 5 to >10 8 cells ml –1 (e.g. Gasol & Vaqué 1993). Traditionally, the ecological role of heterotrophic bac- teria was assumed to be restricted to nutrient mineral- ization. However, with the acceptance of the micro- bial-loop concept (Pomeroy 1974, Azam et al. 1983), bacteria were conferred a new ecological role in food webs, representing an alternative route of organic- matter and nutrient transfer to metazoan trophic levels. The microbial loop is an important pathway of energy flow, especially in oligotrophic systems (Roland & Cole 1999, Biddanda et al. 2001). Bacterial metabolism is supported by both allochthonous and autochthonous dissolved organic matter (DOM). The aquatic food web can be supported by different allochthonous organic carbon sources, but a small fraction of this external dis- solved organic carbon (DOC) is transferred to zoo- © Inter-Research 2010 · www.int-res.com *Email: [email protected] Relationships between pelagic bacteria and phytoplankton abundances in contrasting tropical freshwaters Fábio Roland 1, *, Lúcia M. Lobão 1 , Luciana O. Vidal 1 , Erik Jeppesen 2 , Rodolfo Paranhos 3 , Vera L. M. Huszar 4 1 Federal University of Juiz de Fora, Laboratory of Aquatic Ecology, Minas Gerais, Brazil, 36036-900 2 Dept. of Freshwater Ecology, National Environmental Research Institute, Aarhus University, 8600 Silkeborg, Denmark 3 Federal University of Rio de Janeiro, Laboratory of Hydrobiology, Rio de Janeiro, Brazil, 21941-901 4 Federal University of Rio de Janeiro, Laboratory of Phycology, National Museum, Rio de Janeiro, Brazil, 20940-040 ABSTRACT: While microbial aquatic communities are dominated numerically by viruses, both bac- terioplankton and phytoplankton play a basal role in the carbon cycle, producing and mineralizing organic matter and driving CO 2 concentrations. Both weak and strong relationships between these 2 microbial groups have been reported for temperate ecosystems. However, data from the tropics and sub-tropics are still scarce, and no consistent pattern regarding the structural microbial connections in these aquatic environments is known so far. We examined bacteria-phytoplankton abundance relationships for tropical freshwaters in comparison to well-studied temperate aquatic ecosystems. We present data on bacterioplankton and phytoplankton abundances in a large data set (1644 sam- ples; lakes, rivers, and reservoirs) from sampling throughout an extensive gradient of latitude (3° N to 33° S) and longitude (35° to 70° W) in tropical waters. We found a generally weak, but significant, relationship between bacterioplankton and phytoplankton abundances and between bacterioplank- ton and chlorophyll. However, analyzing system by system, we observed an increase in the strength of the relationships (expressed by the determination coefficient, r 2 ), from 0.05 to 0.17 (bacterioplank- ton and phytoplankton abundances) and from 0.09 to 0.44 (bacterial abundance and chl a). Our data suggest that the in-system ecological drivers (e.g. water temperature, trophic state, and flushing characteristics, i.e. lentic or lotic) determine the bacterioplankton abundance patterns more than other factors such as latitude or system typology. In a global perspective, the comparison between non-tropical and tropical/sub-tropical freshwaters showed that a lower proportion of phytoplankton carbon is transformed into bacterial carbon in the tropics. KEY WORDS: Microbial dynamics · Bacterial–phytoplankton coupling · Tropical waters Resale or republication not permitted without written consent of the publisher

Roland_et_al._2010bacterioplankton e fito.pdf
Dec 17, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
-
AQUATIC MICROBIAL ECOLOGYAquat Microb Ecol
Vol. 60: 261272, 2010doi: 10.3354/ame01429
Published online August 3
INTRODUCTION
Pelagic heterotrophic bacteria are extremely impor-tant in aquatic ecosystems, with abundance rangesfrom 108 cells ml1 (e.g. Gasol & Vaqu 1993).Traditionally, the ecological role of heterotrophic bac-teria was assumed to be restricted to nutrient mineral-ization. However, with the acceptance of the micro-bial-loop concept (Pomeroy 1974, Azam et al. 1983),bacteria were conferred a new ecological role in food
webs, representing an alternative route of organic-matter and nutrient transfer to metazoan trophic levels.The microbial loop is an important pathway of energyflow, especially in oligotrophic systems (Roland & Cole1999, Biddanda et al. 2001). Bacterial metabolism issupported by both allochthonous and autochthonousdissolved organic matter (DOM). The aquatic food webcan be supported by different allochthonous organiccarbon sources, but a small fraction of this external dis-solved organic carbon (DOC) is transferred to zoo-
Inter-Research 2010 www.int-res.com*Email: [email protected]
Relationships between pelagic bacteria and phytoplankton abundances in contrasting
tropical freshwaters
Fbio Roland1,*, Lcia M. Lobo1, Luciana O. Vidal1, Erik Jeppesen2,Rodolfo Paranhos3, Vera L. M. Huszar4
1Federal University of Juiz de Fora, Laboratory of Aquatic Ecology, Minas Gerais, Brazil, 36036-9002Dept. of Freshwater Ecology, National Environmental Research Institute, Aarhus University, 8600 Silkeborg, Denmark
3Federal University of Rio de Janeiro, Laboratory of Hydrobiology, Rio de Janeiro, Brazil, 21941-9014Federal University of Rio de Janeiro, Laboratory of Phycology, National Museum, Rio de Janeiro, Brazil, 20940-040
ABSTRACT: While microbial aquatic communities are dominated numerically by viruses, both bac-terioplankton and phytoplankton play a basal role in the carbon cycle, producing and mineralizingorganic matter and driving CO2 concentrations. Both weak and strong relationships between these 2microbial groups have been reported for temperate ecosystems. However, data from the tropics andsub-tropics are still scarce, and no consistent pattern regarding the structural microbial connectionsin these aquatic environments is known so far. We examined bacteria-phytoplankton abundancerelationships for tropical freshwaters in comparison to well-studied temperate aquatic ecosystems.We present data on bacterioplankton and phytoplankton abundances in a large data set (1644 sam-ples; lakes, rivers, and reservoirs) from sampling throughout an extensive gradient of latitude (3 N to33 S) and longitude (35 to 70 W) in tropical waters. We found a generally weak, but significant,relationship between bacterioplankton and phytoplankton abundances and between bacterioplank-ton and chlorophyll. However, analyzing system by system, we observed an increase in the strengthof the relationships (expressed by the determination coefficient, r2), from 0.05 to 0.17 (bacterioplank-ton and phytoplankton abundances) and from 0.09 to 0.44 (bacterial abundance and chl a). Our datasuggest that the in-system ecological drivers (e.g. water temperature, trophic state, and flushingcharacteristics, i.e. lentic or lotic) determine the bacterioplankton abundance patterns more thanother factors such as latitude or system typology. In a global perspective, the comparison betweennon-tropical and tropical/sub-tropical freshwaters showed that a lower proportion of phytoplanktoncarbon is transformed into bacterial carbon in the tropics.
KEY WORDS: Microbial dynamics Bacterialphytoplankton coupling Tropical waters
Resale or republication not permitted without written consent of the publisher
-
Aquat Microb Ecol 60: 261272, 2010
plankton and fish through bacterial biomass (Cole etal. 2006). It is generally believed that autochthonousDOM from phytoplankton is more available for bacter-ial consumption than allochthonous terrestrial DOC(Kritzberg et al. 2005). Bacteria rapidly assimilate phy-toplanktonic carbon compared to terrestrial DOC(Chen & Wangersky 1996). In tropical freshwaters, forinstance, humic substances are an important energysource for aquatic bacteria (Amado et al. 2006), but thissource is probably not very relevant as a carbon sourcefor bacterial production, since consumption of humicsubstances appears to be mostly channeled throughmicrobial respiration (Farjalla et al. 2009).
The dependence of bacterioplankton on autochtho-nous carbon has been supported by positive relation-ships between phytoplankton (expressed as chloro-phyll a [chl a], cell numbers, or biovolume) andheterotrophic bacteria (expressed as numbers or bio-mass, Bird & Kalff 1984, Stewart & Fritsen 2004; or pro-duction, White et al. 1991). A strong relationship istaken as an indication that the growth of bacterio-plankton is directly dependent on phytoplankton (Coleet al. 1988, Jeppesen et al. 1997, Gasol & Duarte 2000).However, this bacterial dependence on phytoplanktonhas been the focus of recent debate (Lee & Bong 2008,Sarmento et al. 2008, Stenuite et al. 2009). Recent liter-ature provides support for both strong (Sarmento et al.2008, Stenuite et al. 2009) and weak dependence(Canosa & Pinilla 2007, Lee & Bong 2008) of bacterialgrowth on phytoplankton activity.
When expressed mathematically as a regressionequation between bacteria and algae, the parametersthat define the relationships (the slope and the y-inter-cept) can describe important ecological parameters.The slope of the equation between the bacterial andphytoplankton attributes (either abundance or bio-mass) indicates the proportion of phytoplankton car-bon that is transformed into bacterial carbon, while they-intercept estimates the fraction of the bacterialstanding stock that appears to be independent ofphytoplankton (Currie 1990, Simon et al. 1992, delGiorgio & Peters 1993).
The strength of the bacterioplanktonphytoplank-ton relationship varies with the relative importance ofautochthonous and allochthonous carbon sources andthe nutrient status of the ecosystem. Strong positiverelationships are often found in highly productivesystems, where the carbon available to bacteria ismainly autochthonous (del Giorgio et al. 1997). How-ever, the proportion of bacterioplankton to phyto-plankton biomass tends to be higher in oligotrophicrather than in eutrophic systems, because bacterialbiomass increases somewhat more slowly than phyto-plankton biomass along a trophic gradient (Cole et al.1988). In contrast, weak or no relationships have
been found in unproductive systems and in systemswith high inputs of allochthonous material (Findlay etal. 1991, del Giorgio & Peters 1994) as allochthonousorganic matter can be an alternative energy sourcefor bacteria, decoupling the bacterioplanktonphyto-plankton relationship. In this case, bacterial respira-tion can often exceed phytoplankton production(Karlsson et al. 2002).
It is important to point out that a positive bacterio-planktonphytoplankton relationship does not neces-sarily indicate bacterial dependence on phytoplanktoncarbon. In some systems, high inputs of inorganicnutrients (nitrogen and phosphorus) may stimulate thegrowth of both microbial groups (Currie 1990, Brett etal. 1999). Since, in nutrient-limited conditions, phyto-plankton and bacterioplankton compete for nutrients,these relationships may therefore even be negative(Carr et al. 2005).
Predation is another factor that might affect the bac-terioplanktonphytoplankton relationship, generallydecoupling their dependence (Jeppesen et al. 1997).Bacterial abundance is affected by predation by proto-zoans and metazoans (Pace et al. 1990) and by viralinfection (Fuhrman 1999), with different intensitiesdepending on system type. Grazers control the fate ofbacterial communities, and heterotrophic flagellatestend to be the major bacterivores in freshwaters, fol-lowed by ciliates, rotifers, and cladocerans (Jrgens &Jeppesen 2000, Zllner et al. 2003). Warm lakes arecharacterized by the dominance of small-bodied zoo-plankton and higher abundances of rotifers, ciliates,and nanoflagellates, with an elevated grazing impacton bacterioplankton (Crisman & Beaver 1990, Jeppe-sen et al. 2007).
The bacterioplanktonphytoplankton relationshiphas been the focus of a great deal of work (Bird & Kalff1984, Cole et al. 1988, Gasol & Duarte 2000), but themajority of data are from non-tropical aquatic ecosys-tems. Recent studies have been carried out in tropicalregions (Bouvy et al. 1998, Canosa & Pinilla 2007, Pir-lot et al. 2007, Sarmento et al. 2008, Stenuite et al.2009), but a comprehensive understanding of struc-tural dependency between these aquatic communitiesis still required. Potentially, the tropics exhibit a largespectrum of types of aquatic ecosystems, especiallyfreshwaters, i.e. running waters and lakes (includingshallow, floodplain, and man-made). This heterogene-ity among systems is also mixed with a large range ofturbidity, DOC, nutrients, and temperature. Here, weinvestigated bacterial abundance in relation to phyto-plankton in tropical waters and evaluated similaritiesand differences to known patterns derived mainly fromtemperate systems. A broad survey was conducted inBrazilian lakes, rivers, and reservoirs that vary introphic state and DOC content. In addition, we com-
262
-
Roland et al.: Bacterioplankton and phytoplankton relationships in tropical freshwaters
pared our data set to most of the reported availabledata. We hypothesized that the large impact of the sur-rounding environment in a tropical climate will forcetropical freshwaters to have bacterial abundancesweakly correlated with phytoplankton abundancesand more related to the geological and hydrodynamicsetting of each particular environment.
MATERIALS AND METHODS
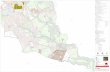
Data sources. Samples were taken between 1999and 2007 from freshwaters located in Brazil between04 21 46 N and 33 29 53 S (Fig. 1). Our data setconsisted of a total of 1644 samples, from rivers (890samples), reservoirs (529), floodplain lakes (131), andcoastal lakes (94). All samples were taken in the lim-netic zone, below the surface. A single sample wastaken from rivers and coastal lakes. The samples, 1 persystem from reservoirs, were taken during the pre-rainy, post-rainy, and dry seasons. One sample for eachfloodplain lake was taken in different seasons (filling,high water, drawdown, and low water). This procedureallowed us to evaluate the complete hydrologic cycleof this diverse group of environments.
One set of samples (564) was fixed with formalin at a2% final concentration for counting the bacteria withan epifluorescence microscope. The remaining set ofsamples (1080) was fixed with sterile paraformalde-hyde at a final concentration of 2% (Andrade et al.2003), placed in liquid nitrogen, and stored at 80Cuntil laboratory analysis by flow cytometry. Phyto-
plankton samples were fixed with Lugols solution;samples for nutrient analyses and filtered samples forchl a estimates were frozen.
Bacterial abundance (106 cells ml1) was estimated inall samples. Phytoplankton abundance (ind. ml1) wasestimated in 1103 samples, and chl a concentrations(g l1) in 385 samples. DOC concentrations wereobtained from 1487 samples, dissolved inorganic nitro-gen (DIN, g N l1) from 1384 samples, and solublereactive phosphorus (SRP) from 1420 samples.
Sample analysis. Bacterioplankton abundance wasestimated by direct counts at 1000 magnification,with an epifluorescence microscope (Olympus BX-60)or by cell counts performed in a CyAn ADP flowcytometer (Dako) equipped with a solid-state laser(488 nm, 24 mW) and filter modifications (green fluo-rescence [FL1] at 510 15 nm, red fluorescence [FL4]at 650 10 nm). For direct counts, samples (fromreservoirs, floodplain lakes, and most coastal lakes)were filtered throughout black polycarbonate filters(0.2 m, Nucleopore) and stained with acridineorange (final concentration, 0.05%; Hobbie et al.1977), and at least 200 cells were counted. For sam-ples counted by flow cytometry (all river samples andsome coastal lakes), abundance was determined afternucleic-acid staining with Syto13 (Molecular Probes)at 2.5 M (del Giorgio et al. 1996). For calibration ofside scatter and green fluorescence signals, and as aninternal standard for cytometric counts and measure-ments, fluorescent latex beads (Polysciences, 1.5 mdiameter) were systematically added. To validate ourmixed bacterial abundance data set, we performed acomparison between the 2 methods. We carried outadditional sampling on 20 different systems, includ-ing rivers, reservoirs, coastal lakes and floodplainlakes. Bacterial abundances were estimated in thesesamples using both the epifluorescence (stained withacridine orange) and flow-cytometer techniques. Wefound a significant relationship between the epifluo-rescence and flow-cytometer methods (r2 = 0.75, p
Related Documents