Review Chapter-2

Review Chapter-2. Atoms An atom is made up of protons, electrons and neutrons.protonselectronsneutrons The mass of an atom is found in the nucleus,
Dec 20, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
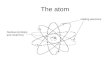
Atoms
An atom is made up of protons, electrons and neutrons.
The mass of an atom is found in the nucleus, since the neutrons and protons each weigh considerably more than the electrons orbiting it.
The electrons can be removed or shared with other atoms without having agreat impact on the mass of a chemical, and therefore it is through movement of electrons that chemical reactions take place.
Of course, electrons could just happily spin off and completely leave the atom - it's not like they're part of the nucleus.
However, the positive charge of the protons attracts the negatively charged electrons to it (since positive and negative charges attract each other). This keeps them spinning around the nucleus
Molecule
• A molecule consists of two or more atoms of the same element, or different elements, that are chemically bound together
Compounds
• A substance formed by bonding two or more elements in definite proportions.
• Can be broken down into a simpler elements
Mixtures
• Consists of two or more different elements and/or compounds physically intermingled
• Can be separated into its components
Water
• Neutral – 10 protons/10 electrons• Polar
– Oxygen (-) charge– Hydrogen (+) charge
Covalent Bond
Covalent Bond
Water
• A water molecule can form (H+) hydrogen ion and a (OH-) hydroxide ion.
• If H+ = OH-, water
• If H+ > OH-, acid
• If OH- > H+, base
Examples: Acids
• citric acid (from certain fruits and veggies, notably citrus fruits)
• ascorbic acid (vitamin C, as from certain fruits)
• vinegar
• carbonic acid (for carbonation of soft drinks)
• lactic acid (in buttermilk)
Properties of bases
• taste bitter (don't taste them!)
• feel slippery or soapy
• react with acids to form salts and water
Cohesion/Adhesion
• Cohesion: (attraction btw molecules of same substances)– Water is attracted to other water. This is called
cohesion.
• Adhesion: (attraction btw molecules of different substances)– Water can also be attracted to other materials.
This is called adhesion.
Related Documents