RATE OF REACTION CHAPTER 1

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

RATE OF REACTIONCHAPTER 1

In this chapter, you will learn to:
Analyse the rate of reaction.Synthesise factors affecting the rate of reaction.
Synthesise ideas on the collison theoryLearn about practising scientific knowledge to enhance quality
of life.

1.2 SYNTHESISE FACTORS AFFECTING THE RATE OF
REACTION

LEARNING OUTCOMES
Give examples of reactions that are affected by size of reactant, concentration, temperature, and catalyst.
Explain how each factor affects the rate of reaction.
Describe how factors affecting the rate of reaction are applied in daily life and in industrial processes.
Solve problems involving factors affecting rate of reaction.

INTRODUCTION
The rate of reaction is affected by the following factors:
The size of reactants (or surface area) Concentration of reactants Temperature Catalyst Pressure

Size of ReactantsFor a given quantity of the same reactants, the size of the
particles affects the reaction.The smaller the sizes of the particles, the larger is the surface
area exposed for reaction.Example:
If 2g of marble chips (large particles) and 2 g of calcium carbonate powder ( small particles) are reacted separately with 50 cm3 of 1 mole dm-3 hydrochloric acid, the following results will be obtained:
Conditions Time taken to obtain 50 cm3 CO2 (min)
2 g marble chips with 50 cm3 HCl, 1 mole dm-3
4
2 g CaCO3 with 50 cm3 HCl, 1 mole dm-3
½

Concentration of ReactantsIf 2 g of marble chips are reacted separately with dilute
hydrochloric acid of two different strengths, the following results will be obtained:
The higher the concentration of a reactant, the higher is the rate of that reaction.
Conditions Time taken to obtain 50 cm3 CO2 (s)
2 g marble chips with 50 cm3 HCl, 1 mole dm-3
90
2 g marble chips with 50 cm3 HCl, 2 mole dm-3
45

TemperatureThe rate of reaction increases when the temperature at which
the reaction is taking place is increased.Example:
Conditions Time taken to obtain 50 cm3 CO2 (min)
2 g marble chips with 50 cm3 HCl, 1 mole dm-3 at 27 oC
3
2 g marble chips with 50 cm3 HCl, 1 mole dm-3 at 35 oC
2

CatalystCatalyst is a substance which causes a change in the rate of
reaction without itself undergoing a chemical change at the end of the reaction.
Example: Hydrogen peroxide, H2O2, does not decompose at room conditions,
but in the presence of a catalyst, it decomposes into water and oxygen. The catalyst used is manganese (IV) oxide.
A catalyst can either hasten or slow down a reaction. A catalyst which quickens (Hastens) a reaction is called a positive catalyst or promoter, while a catalyst which slow down a reaction is called a negative catalyst of inhibitor.

Continue…Characteristics of a catalyst include the following:
A catalyst does not undergo any chemical change at the end of the reaction. The quantity and chemical composition of the catalyst remain the same at the end of the reaction.
However, the catalyst can undergo a physical change during the reaction. For example, coarse manganese(IV) oxide change to the powdery form at end of the decomposition of potassium chlorate(V).
Only a small quantity of catalyst is needed to change a rate of reaction but a larger quantity of catalyst can speed up the reaction and therefore increase the rate of reaction.
A catalyst only changes the rate of reaction but not the quantity of products. The product produced in the reaction will not increase by the addition of a catalyst.
The efficiency of a catalyst is reduced if impurities are present in it.

Continue…Some catalysts used in industrial processes are as follows:
Process Reaction Catalyst
Contact process To produce sulphur trioxide in the manufacture of sulphuric acid.
Vanadium pentoxide
V2O5
Haber process To manufacture ammonia IronFe
Ostwald process To produce nitrogen monoxide in the manufacture of nitric acid
PlatinumPt
Hydrogenation of margarine
Vegetable oils are converted to margarine by reaction with
hydrogen
NickelNi

PressurePressure can affect the rate of reaction only if it involves gases.The rate of reaction of solids and liquids are not effected by
changes in pressure.A higher pressure can increase the rate of reaction.Example:
Haber process Nitrogen and hydrogen combine to form ammonia when heated, but at
low pressure, the reaction very slow. At a pressure of 250 atm, the rate of reaction is higher.

Continue…

Assignment
Group 1To investigate the effect of the size of the reactant on the rate
of reaction.Your presentation should include the following aspect:
Aim All the variables Hypothesis List of substances and apparatus Procedure Tabulation of data Discussion Conclusion

Assignment
Group 2To investigate the effect of concentration on the rate of
reaction.Your presentation should include the following aspect:
Aim All the variables Hypothesis List of substances and apparatus Procedure Tabulation of data Discussion Conclusion

Assignment
Group 3To investigate the effect of temperature on the rate of
reaction.Your presentation should include the following aspect:
Aim All the variables Hypothesis List of substances and apparatus Procedure Tabulation of data Discussion Conclusion

Assignment
Group 4To investigate the effect of catalyst on the rate of reaction.
Your presentation should include the following aspect: Aim All the variables Hypothesis List of substances and apparatus Procedure Tabulation of data Discussion Conclusion

Assignment
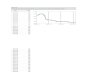
Group 5Solve the following problem:
100
Volume (cm3)
Time (s)

Continue…
1 g of calcium carbonate powder in excess is added to 50 cm3 of 0.1 mole/dm3 hydrochloric acid. The volume of gas produced is recorded at fixed points of time. A graph, showing volume against time as given in figure above is obtained. The experiment is repeated, using the same quantity of calcium carbonate and 50cm3 of 0.3 mole/dm3 hydrochloric acid.
Calculate the maximum volume of gas that is produced. Draw the graph of volume against time on the same axes of the
earlier experiment.
Related Documents