-
5/25/2018 Quy Lu t Th Vng Benzen
1/17
QUY LUT TH ELECTROPHINVO VNG BENZEN
Ngibin son:Hoavang06
-
5/25/2018 Quy Lu t Th Vng Benzen
2/17
BENZEN (C6H6)
Vng benzen l mtvng hpbi6 nhm C-H, minguyn tCcn limtha tr;
Cc nguyn tC sdngha trny tothnh 3 niigingnhxiclohexadien.
3 niiny khng cnhm c schuynch thng xuyngiacc ninv niitothnh vng khp kn.
t ng ng v i
Cng thcKekule(1865):
-
5/25/2018 Quy Lu t Th Vng Benzen
3/17
BENZEN (C6H6)
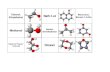
Quan nimhinivcutrc cabenzen:Benzen c cutovng ngphng,6 nguyn tC v 6 nguyn tH
nmtrn mtphngl lcgic u.Su nguyn tC trong benzen utrngthi lai ha sp2tothnh
mtphngs. Minguyn tC cn mtelectron p chalai ha xen phhai pha vimcnhnhau tothnh hthngelectron pgiitoutrn ton bphn t. Vng benzen l mth lin hpbnvngvmt
nhitnghc(nnglngthmha E = 36 kcal/mol).
+
-
+
-
+
-
+
-+-
-
5/25/2018 Quy Lu t Th Vng Benzen
4/17
Phn ng th electrophin vng benzen (SEAr)
Benzen tiu biucho cc hthngthm,cimvcutrc cabenzen thhincc cimvtnh cht:
- Khuynh hng i vo phn ng th chim u th do phn ng th hthnglin hpcboton.
- Khuynh hngivo phnngcnggimdo phnngcnghthnglinhpbph v.
Phn ng th electrophin SEAr l phn ng c trng quan trng ca vngbenzen.Phnngtngqut:
xc t c
H
+ E Y
-ss
+
E
+ H Y
Tc nhn electrophin l cation E+hoc phn t E-Y.Cc phn ng th SEAr u cn s c mt ca cc cht xc tc.
Vai tr ca cht xc tc l lm tng qu trnh to tc nhn electrophin.
-
5/25/2018 Quy Lu t Th Vng Benzen
5/17
C ch phn ng th SEAr
Phnngxyra theo 2 giai on:Giai on1: Tc nhn electrophin tncng vo h thngelectron pcanhn thm
tothnh mtphcp; tipphcpchuynthnh phcs:
E
(tr ng th i chuyn tip)
E
phc p phcs
HE
+
Giai on2: phcsl mtcation khng bnvi4 electron pgiitontrn 5 nguyntC; nguyn tC thsu cavng lin ktviE trngthi lai ha sp3. Phcscbnha theo 2 cch:
+ Cnganion Y-tothnh hthngvng xiclohexadien (tosnphmcng)+ Tch proton tolihthngelectron pcavng thm(tosnphmth).
H
H
E
Y(sn phm cng)
E
1.(+ Y )
2.(- H )
(sn phm th)phcs
HE
+
Khuynh hngtch proton H+c u tin hndo c mc nnglngthphn.
-
5/25/2018 Quy Lu t Th Vng Benzen
6/17
Mt s phn ng th SEAr
Halogen ha
Benzen
Br2/FeBr3
Brom benzen
Br
+ HBr
Br2 + FeBr3 Br + [FeBr4]C ch:
+ H+Br
Br
phc p phc s
HBr
+
Br
Nitro ha C ch:
Benzen Nitro benzen
NO2
+ H2OHNO3/H2SO4
HNO3 + H2SO4 NO2 + 2HSO4 + H2O
NO2
N
O
O
+ N
O
O
HSO4-
phc p phcs
+
HNO2
Sunfo ha C ch:
+ H2O
Axit benzensunfonic
SO3H
Benzen
H2SO4
H2SO4 SO3 + H3O + HSO4
O+ S
O
O
-s:
:
:
:
:
:
:
s+
-s
-s
SO3
phc s
+
HSO3
HSO4
SO3H
H+
-
5/25/2018 Quy Lu t Th Vng Benzen
7/17
Mt s phn ng th SEAr
Ank yl ha theoFriedel-Crafts
C ch:
+ R - X
Benzen(R : gc ankyl X : halogen) Alkyl benzen
R
AlX3khan
R X AlX3..........s+
s-
R
+ H - X + AlX3AlX4+
RH
R - X + AlX3 R X AlX3..........s+
s-
Dng ankyl halogenua lm tc nhn ankyl ho
di s c mt ca cht xc tc Liuyt:
C ch:Ax yl ha theo
Friedel-Crafts
+ HX
Benzen(R : gc ankyl X : halogen)
+ X - C - R
O
AlX3khan C R
O
Aryl alkyl xeton
s+
s-
R - C - X + AlX3
O
R C O AlX3.....
X
::
C
+ H - X + AlX3
s+
s-
R C O AlX3.....
X
:
:
R O
+
CH
O
R
AlX4
Tc nhn axyl ha thng dng l cc axylhalogenua RCOX hoccc anhydrit axit (RCO)2O:
-
5/25/2018 Quy Lu t Th Vng Benzen
8/17
Quy lut th SEAr
1. nh hng ca nhm th n s th SEAr:
Mt nhm th R c sn nhn thm c nh hng n v tr ca cc nhmvo sau gi l nhm nh hng.
xc t c
R
+
R
E E
Sdnhvyl:
+ Khi benzen chab th,mtelectron 6 nguyn tC trong vng lnhnhau vo chthu cmtsnphmkhi thlnu.
+ Khi th lnthhai, nhm thmic thnhhngvo cc v tr ortho,metavpara so vinhm nhhng,sthu cmthnhp3 snphmngphn c tlphntrmkhc nhau.
-
5/25/2018 Quy Lu t Th Vng Benzen
9/17
Quy lut th SEAr
Khi nhn thmc snmtnhm th:Da trn c s tc dng nh hng ngi ta chia lm 2 loinhm th: Nhm thloiI:Tc dngnhhngnhm thvo sau utin vo vtr ortho vparaso vinhm thnhsn; ngthias cc nhm thny kch hots th: gm cc nhm thhotha nhn thm(O-, -NR2, NH2, -OH, -OR, -NHCOCH3, -CH3, -C(CH3), C6H5-) v mtsnhm thphnhotha nhn
thm(halogen, -CH2Cl,) Nhm thloiII:Tc dngnhhngnhm thvo sau utin vo v tr meta so vi nhm th nh sn; ng thi ccnhm th ny th ng ha s th: gm cc nhm th phnhotha nhn thm(-NO2, -NR3, CF3, -CN, -COOH, -COOR, -SO3H, -SO2R, -CHO, -COR.)
ortho
para
I
meta
II
OCH3
ortho ortho
para
Loi I
CH3
ortho ortho
para
Loi I Loi I I
NO2
meta
V d:
-
5/25/2018 Quy Lu t Th Vng Benzen
10/17
Quy lut th SEAr
Kh i nhn thmc snnhiunhm th:Trnghp nhn thm c snnhiunhm th th v tr nhm thvo sau c
quytnhbinhm thc tc dngnhhngmnhhn.
Trnghpnhm thnhsncng loi:Davo thcnghimlpctrttnhhngcc nhm thnhsncng loitheo dy sau:
Nhm thloiI:
O-> -NR2> -NHR > NH2> -OH > -OR > -NHCOR > halogen (F > Cl > Br > I) >> - OCOR > - R > -ankenyl
Nhm thloiII:-NO2> -NR3> -CN > -COOH > -COOR > -SO3H > -CHO> -COR
OCH3
Loi I
ClLoi Iortho
para
Loi I I
NO2
COOH
meta
Loi I IOCH3
Loi I
CH3Loi I
para
ortho
V d:
-
5/25/2018 Quy Lu t Th Vng Benzen
11/17
Quy lut th SEAr
Trng hp nhm th nh sn khc loi:Trng hp nhm th nh sn khc loi: nhm th loi I ng vai tr
nhhngnhm thvo sau.
V d:
NO2
NHCOCH3
Loi I
Loi I Iortho
para
COOH
Loi I I
ClLoi Iortho
para
ortho
para
Loi I
OH
SO3H
Loi I I
NO2
CH3
Loi I
Loi I I
ortho
para
CH3
Loi I
NO2Loi I I
para
CH3
Loi I
NO2Loi I I
Tuy nhin quy lut thchc tnh tngiv ngoi yu tin tcn phthuc vo cc yu t khng gian, iu kin phn ng, bn cht ca nhm
th
-
5/25/2018 Quy Lu t Th Vng Benzen
12/17
Gii thch quy lut th SEAr
Quy lut thcxy dng trn cs thcnghim. Tuy nhin c thgiithch quy lutthdatrn csyutintv nghc.
Tuthucvo bnchtnhm thm mtelectron vng tngln hocgimi,do khnngphnng thSEAr sddng hnhockh hnkhikhng c nhm th.
Phn loinhm thnhvng:Nhm thhotha nhn thm:gmcc nhm ththhincc hiung
dng(+C, +I, +H) yelectron vo vng lm tngkhnngtham gia phnngSE cavng benzen thunlicho phnngSEAr.
V d: cc nhm thO-, -NR2, NH2, -OH, -OR > -NHCOR
Nhm thphnhotha nhn thm:gmcc nhm th thhincchiungm (-C, -I) ht electron vo vng lm gimkhnngtham gia phnng
SEcavng benzen kh khncho phnngSEAr.V d: cc nhm thNO2, COOR, -CN
Trnghpnhm thc cc hiungI v C tc dngtheo chiuhngngcnhau(v dhalogen, -OH, -NR2(-I, +C) th davo trsscaphngtrnh Hammet: cc halogen c trsHammet sdngth phnhothanhn thm,cn nhmOH, -NR2c trssm th hotha nhn thm.
-
5/25/2018 Quy Lu t Th Vng Benzen
13/17
Gii thch quy lut th SEAr
trngthi tnh(trngthi cbn)
Nhm thnhsnloiI c hiung+I, +C: yelectron vo vng. Do slin hpelectron trong vng nn cc vtr orthovparamtelectron tnghn:
ortho
para
I I
-
-
-
III
Nhm thnhsnloiII c hiung-I, -C : ht electron tvng ra. Do s lin hpelectron trong vng nn cc vtr orthovparaxuthinintch dng,vtr metacintch dngnhnht:
meta
II
II II II II
Nhm thc hiung-I, +C(v dcc halogen F, Cl, Br, I) ht electrontvng ra lm xuthinin tch dng lnnhtv tr orthov para
nn tc nhn electrophin utin vo vtr meta (l vtr c intch dngnhnht.
(X: halogen)
:
X
-
5/25/2018 Quy Lu t Th Vng Benzen
14/17
Gii thch quy lut th SEAr
trngthi ng
Tc nhn electrophin tn cng to phcs. Nu vng benzen c sn mtnhm thY th c 3 phnngcnhtranh nhau vo cc vtr ortho, meta,para.V vycnxt mcnnglngcaphcsdo phnngsutin tao phccnnglngthphn.
Do phcs l mt tiu phn giu nng lng nn mc nng lng ca ncng thpnuintch dnggiitocng nhiu. Bnchtcanhm thnh
snY ngvai tr lntrong vicgiitointch dngtrn phcs.
ortho
para
Y
E+meta
so
sm
Y
E
H
Y
EH
sp
YH
E
+
+
+
-
5/25/2018 Quy Lu t Th Vng Benzen
15/17
Gii thch quy lut th SEAr
trngthi ng
Y l nhm thloiI (c hiung+I, +C):gy hiungdngyelectron vo vng
benzen lm gimintch dngtrn phcs, cbitgimnhiucc phcsov spdointch cahai phcnmgnnhm thY nhtv nnglnghotha cc phcso v spnhNhm thE utin vo vtr orthovpara.
Y l nhm th loi II (c hiu ng -I, -C): gy hiu ng m ht electron ca vngbenzen, v thkhng nhnggiitointch dngm cn lm tngintch dngcaphcs, m tngnhiunhtphcsov sp(do intch dngcahai phcnmgn
nhm thY nht). vtr metakhng nhvynn nnglnghotha caphcspnhhnNhm thE utin vo vtr meta.
ortho meta
+
o p m>=
Y
H
E
EH
Y
+
para
+
E
H
Y
(Y: nhm thlo i I I)
Y
(Y: nhm thlo i I)
Y
ortho meta
+
Y
H
E
EH
Y
+
para
E
H
Y
+
o p m
-
5/25/2018 Quy Lu t Th Vng Benzen
16/17
Gii thch quy lut th SEAr
trngthi ng
Y l nhm thc hiung-I, +C nhhalogen:
trngthi ngphcsc tnh electrophin mnh, l mththiuhtelectron, dosc mtcacpelectron chasdng lun chimu thnn halogen thhinhiung+C> -I, chyuyelectron vo vng benzen nhcc loinhm thloi I khc. Do trng thi ng (phcs) l giai onquytnhcaphnngnn
halogen tuy l nhm thphnhotha nhn thmnhngvnu tin nhhngvo cc vtr orthovpara.
ortho meta
+
o p m
-
5/25/2018 Quy Lu t Th Vng Benzen
17/17