Quantum numbers and Periodic table II/

Quantum numbers and Periodic table II/. What is an orbital? It is the space around the nucleus in which the electron is found with a probability of 90%.
Dec 22, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
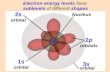
What is an orbital?
• It is the space around the nucleus in which the electron is found with a probability of 90%.
• The electron spends 90% of its time in that space.
90%
II/
university
Faculty of art
chem
Faculty of engineering
Faculty of science
phys MathMech civilArabic Engl.
D BM M MMBB M BM B B
1 2 3
a b c d e f g
Each student in the university is defined by a set of symbols:
A Math student in the master program has the set: 3 g
Mainshells
sub-shells
II/
Similarly, electrons in atom are specified by a set of numbers, the quantum numbers:
The Quantum Numbers
name symbol values Physical significance
Principal Quantum Number n 1, 2, 3, 4, …….
-Gives the main shell in which the electron exists.
-Determines largely the energy of the electron.
-Determines the size of the orbital.
Azimuthal (secondary, angular moment) Quantum
Numberℓ 0, 1, 2, …, (n-1)
-Gives the subshell in which the electron exists.
-contributes to energy of electron.
-Determines the shape of the orbital
Magnetic Quantum Number mℓ -ℓ, …, 0, … +ℓ -Determines the orientation of orbital in space
Spin Quantum Number ms +/- 1/2 -orientation of rotation of electron around itself.
II/
n ℓ(n-1)
mℓ
(-ℓ, …, +ℓ)
No. of orbitals
No. of electrons
1 0 (s) 0 1 2 2
2 0 (s)
1 (p)
0
-1, 0, 1
1
3
2
6
8
3 0 (s)
1 (p)
2 (d)
0
-1, 0, 1
-2, 1, 0, 1, 2
1
3
5
2
6
10
18
4 0 (s)
1 (p)
2 (d)
3 (f)
0
-1, 0, 1
-2, 1, 0, 1, 2
-3, -2, 1, 0, 1, 2, 3
1
3
5
7
2
6
10
14
32
2n2
II/
Nodes: Regions where electrons are not allowed to be present in. Electron Probability of being there is zero.
II/
Related Documents