Quantitative Proteome Analysis of Streptomyces coelicolor Nonsporulating Liquid Cultures Demonstrates a Complex Differentiation Process Comparable to That Occurring in Sporulating Solid Cultures Angel Manteca,* ,†,‡ Hye R. Jung, † Veit Schwa ¨mmle, † Ole N. Jensen,* ,† and Jesus Sanchez ‡ Department of Biochemistry and Molecular Biology, University of Southern Denmark, Campusvej 55, Odense M, DK-5230, Odense, Denmark, and Area de Microbiologia, Departamento de Biologia Funcional and IUBA, Facultad de Medicina, Universidad de Oviedo, 33006 Oviedo, Spain Received May 21, 2010 Streptomyces species produce many clinically important secondary metabolites and present a complex developmental cycle that includes programmed cell death (PCD) phenomena and sporulation. Industrial fermentations are usually performed in liquid cultures, conditions in which Streptomyces strains generally do not sporulate, and it was traditionally assumed that no differentiation took place. Recently, the existence of an early compartmentalized mycelium (MI) and a later multinucleated mycelium (MII) were described in solid and liquid cultures. The aim of this work was to compare the proteomes of the different developmental stages in liquid and solid S. coelicolor cultures, in order to give new insights in Streptomyces biology, and improve industrial fermentations. Using iTRAQ labeling and LC-MS/MS analysis of peptides, we demonstrate that differentiation in S. coelicolor liquid cultures is comparable to solid cultures. Eighty-three percent of all the identified proteins showed similar abundance values in MI and MII from liquid and solid cultures. Proteins involved in secondary metabolism (actinorhodin and type II polyketide biosynthesis, -lactamases, epimerases) were up-regulated in MII. Proteins involved in primary metabolism (ribosome, Krebs cycle, and energy production) were detected in greater abundance in MI. The most remarkable protein abundance differences between MII from solid and liquid cultures were associated with the final stages of hyphae compartmentalization and spore formation. Keywords: proteome • iTRAQ • differentiation • Streptomyces coelicolor Introduction Approximately two-thirds of industrial antibiotics and large numbers of eukaryotic cell differentiation inducers and inhibi- tors are synthesized by members of the Streptomyces genus. 1–4 Streptomycetes undergo a complex developmental cycle, which includes sporulation in solid cultures; however, most Strepto- mycetes do not sporulate in liquid cultures. Industrial processes for secondary metabolite production are performed in liquid cultures (large bioreactors), and it is generally assumed that differentiation processes are absent under these conditions. 5–9 The classical Streptomyces developmental model for conflu- ent solid cultures assumes that differentiation processes take place along the transversal axis of the cultures (bottom-up): Completely viable vegetative mycelia (substrate) grow on the surface and inside agar until they undergo a programmed cell death process (PCD), after which hyphae differentiate to a reproductive (aerial) mycelium characterized by the presence of hydrophobic covers, giving it a characteristic grayish ap- pearance. 10 Substrate and aerial mycelia are multinucleated, but at the end of the cycle, aerial hyphae form septa and spore chains (Figure 1) (reviewed in Fla ¨rdh and Buttner 10 ). Recently, we have refined this developmental cycle describing novel aspects during the presporulation phases in liquid and solid cultures. 8,11–17 We have characterized the existence of a previ- ously unidentified compartmentalized mycelium (MI) that initiates the developmental cycle after spore germination. 11,12 The MI suffers a highly ordered PCD, and the remaining viable segments of this mycelium begin to enlarge as a multinucleated mycelium (MII). In solid cultures, two types of MII have been defined, based on the absence (in early development) or presence (in late development) of the hydrophobic layers characteristic of aerial hyphae. 18,19 The traditionally denomi- nated substrate mycelium corresponds to MII lacking hydro- phobic layers, and the aerial mycelium to MII coated with these hydrophobic layers (Figure 1). 15 The only mycelial phases * Correspondence: Dr. Angel Manteca, Area de Microbiologia, Departa- mento de Biologia Funcional and IUBA, Facultad de Medicina, Universidad de Oviedo, 33006 Oviedo, Spain. E-mail: [email protected]; phone, (34) 985103000, ext 5289; fax, (34) 985103148. Prof. Ole Nørregaard Jensen. Department of Biochemistry and Molecular Biology, University of Southern Denmark, Campusvej 55, Odense M, DK-5230, Odense, Denmark. E-mail, [email protected]; phone, (45) 65502368; fax, (45) 65502467. † University of Southern Denmark. ‡ Universidad de Oviedo. 10.1021/pr100513p 2010 American Chemical Society Journal of Proteome Research 2010, 9, 4801–4811 4801 Published on Web 07/19/2010

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Quantitative Proteome Analysis of Streptomyces coelicolor
Nonsporulating Liquid Cultures Demonstrates a Complex
Differentiation Process Comparable to That Occurring in Sporulating
Solid Cultures
Angel Manteca,*,†,‡ Hye R. Jung,† Veit Schwammle,† Ole N. Jensen,*,† and Jesus Sanchez‡
Department of Biochemistry and Molecular Biology, University of Southern Denmark, Campusvej 55, OdenseM, DK-5230, Odense, Denmark, and Area de Microbiologia, Departamento de Biologia Funcional and IUBA,
Facultad de Medicina, Universidad de Oviedo, 33006 Oviedo, Spain
Received May 21, 2010
Streptomyces species produce many clinically important secondary metabolites and present a complexdevelopmental cycle that includes programmed cell death (PCD) phenomena and sporulation. Industrialfermentations are usually performed in liquid cultures, conditions in which Streptomyces strainsgenerally do not sporulate, and it was traditionally assumed that no differentiation took place. Recently,the existence of an early compartmentalized mycelium (MI) and a later multinucleated mycelium (MII)were described in solid and liquid cultures. The aim of this work was to compare the proteomes of thedifferent developmental stages in liquid and solid S. coelicolor cultures, in order to give new insightsin Streptomyces biology, and improve industrial fermentations. Using iTRAQ labeling and LC-MS/MSanalysis of peptides, we demonstrate that differentiation in S. coelicolor liquid cultures is comparableto solid cultures. Eighty-three percent of all the identified proteins showed similar abundance valuesin MI and MII from liquid and solid cultures. Proteins involved in secondary metabolism (actinorhodinand type II polyketide biosynthesis, �-lactamases, epimerases) were up-regulated in MII. Proteinsinvolved in primary metabolism (ribosome, Krebs cycle, and energy production) were detected in greaterabundance in MI. The most remarkable protein abundance differences between MII from solid andliquid cultures were associated with the final stages of hyphae compartmentalization and sporeformation.
Keywords: proteome • iTRAQ • differentiation • Streptomyces coelicolor
Introduction
Approximately two-thirds of industrial antibiotics and largenumbers of eukaryotic cell differentiation inducers and inhibi-tors are synthesized by members of the Streptomyces genus.1–4
Streptomycetes undergo a complex developmental cycle, whichincludes sporulation in solid cultures; however, most Strepto-mycetes do not sporulate in liquid cultures. Industrial processesfor secondary metabolite production are performed in liquidcultures (large bioreactors), and it is generally assumed thatdifferentiation processes are absent under these conditions.5–9
The classical Streptomyces developmental model for conflu-ent solid cultures assumes that differentiation processes takeplace along the transversal axis of the cultures (bottom-up):Completely viable vegetative mycelia (substrate) grow on the
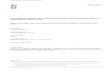
surface and inside agar until they undergo a programmed celldeath process (PCD), after which hyphae differentiate to areproductive (aerial) mycelium characterized by the presenceof hydrophobic covers, giving it a characteristic grayish ap-pearance.10 Substrate and aerial mycelia are multinucleated,but at the end of the cycle, aerial hyphae form septa and sporechains (Figure 1) (reviewed in Flardh and Buttner10). Recently,we have refined this developmental cycle describing novelaspects during the presporulation phases in liquid and solidcultures.8,11–17 We have characterized the existence of a previ-ously unidentified compartmentalized mycelium (MI) thatinitiates the developmental cycle after spore germination.11,12
The MI suffers a highly ordered PCD, and the remaining viablesegments of this mycelium begin to enlarge as a multinucleatedmycelium (MII). In solid cultures, two types of MII have beendefined, based on the absence (in early development) orpresence (in late development) of the hydrophobic layerscharacteristic of aerial hyphae.18,19 The traditionally denomi-nated substrate mycelium corresponds to MII lacking hydro-phobic layers, and the aerial mycelium to MII coated with thesehydrophobic layers (Figure 1).15 The only mycelial phases
* Correspondence: Dr. Angel Manteca, Area de Microbiologia, Departa-mento de Biologia Funcional and IUBA, Facultad de Medicina, Universidadde Oviedo, 33006 Oviedo, Spain. E-mail: [email protected]; phone,(34) 985103000, ext 5289; fax, (34) 985103148. Prof. Ole Nørregaard Jensen.Department of Biochemistry and Molecular Biology, University of SouthernDenmark, Campusvej 55, Odense M, DK-5230, Odense, Denmark. E-mail,[email protected]; phone, (45) 65502368; fax, (45) 65502467.
† University of Southern Denmark.‡ Universidad de Oviedo.
10.1021/pr100513p 2010 American Chemical Society Journal of Proteome Research 2010, 9, 4801–4811 4801Published on Web 07/19/2010
present in liquid cultures were MI and MII without hydropho-bic layers8,9 (Figure 1).
Streptomyces biology has been studied using proteomicsapproaches in various cellular contexts, including PCD,14
germination,20,21 variations in the proteome of the bald Amutant,22–24 primary response to phosphate limitation,25 di-auxic lag phase,26 and Streptomyces griseus A-factor mutant.27,28
Recently, we reported the first quantitative mass spectrometry-driven proteome analysis of Streptomyces differentiation in solidcultures,17 demonstrating the switch from primary to secondarymetabolism in MI and MII.
System biology experiments (transcriptomics, proteomics,etc.) in liquid cultures are imperative in order to understandStreptomyces differentiation in these conditions, and eventuallyto improve industrial fermentations. Recently, two independenttranscriptomic analyses of Streptomyces gene expression levelsas a function of time in liquid cultures were performed.29,30
These studies demonstrated the existence of a transition fromprimary to secondary metabolism during development in theseconditions. In the present work, we go one step further bydesigning a protocol to fractionate the MI and MII develop-mental stages in liquid cultures, comparing the differences intheir proteomes and also with the proteomes of analogousstructures from solid cultures. This first comparative study ofproteomes from Streptomyces hyphae in solid and liquidcultures revealed similarities and distinct differences betweenthe MI and MII.
Experimental Procedures
Bacterial Strains and Media. S. coelicolor M145 strain wasused in this study. Liquid cultures were maintained in R5Aliquid media.8 Flasks of 100 mL with 20 mL of culture mediumwere inoculated directly with spores (1 × 107 spores/mL) andincubated at 200 rpm and 30 °C. For solid cultures, Petri dishes
(8.5 cm) with 25 mL of solid GYM medium (glucose, yeast/malt extract)31 were covered with cellophane disks, inoculatedwith 100 µL of a spore suspension (1 × 107 viable spores/mL),and incubated at 30 °C. This medium promotes the rapiddevelopment of a lawn that differentiates readily and yieldsabundant sporulation.
Sampling and Fractioning of S. coelicolor Cells throughoutthe Differentiation Cycle. Cells from S. coelicolor grown in liquidcultures were processed at different developmental time points(14 and 90 h). The 14-h time point corresponds to the firstcompartmentalized mycelium (MIL) and 90 h to the secondmultinucleated mycelium (MIIL). Two independent cultureswere prepared and processed (biological replicates). Samples(100 mL from 14 h culture and 20 mL from 90 h culture) werecentrifuged at 5000g (10 min at 4 °C). Mycelial pellets weremechanically disaggregated (vigorous vortexing for 1 min) in40 mL of A buffer (50 mM Tris-HCl, pH 7, 150 mM NaCl, 10mM MgCl2, 1 mM EDTA, 7 mM �-mercaptoethanol, and 0.5mM PMSF), precooled to 0 °C, and centrifuged for 10 min at7740g and at 4 °C, 5000g. Usually, two washing steps are enoughto separate the extracellular protein in Streptomyces,13 but werepeated the mechanical disaggregation and washing steps 8times, in order to improve reproducibility. In the case of solidcultures, the mycelium lawn of S. coelicolor M145 grown oncellophane disks was scraped off at different time points (12and 72 h) using a plain spatula. The 12-h time point corre-sponds to MIS and 72 h, to MIIS (Figure 1). As in liquid cultures,two independent cultures were prepared. At 12 h, the firstcompartmentalized mycelium was separated from the non-septated mycelium by conversion of the cell compartments toprotoplast forms.17 Samples of MII were obtained duringphases in which MI had died (72 h). Mycelial pellets weremechanically disaggregated (vigorous vortexing for 1 min) inA buffer (2.5 g of mycelium in 10 mL) precooled to 0 °C, and
Figure 1. Streptomyces coelicolor development stages and sample preparation. (a) Cell-cycle features of Streptomyces development.Mycelial structures (MI, first compartmentalized mycelium; MII, second multinucleated mycelium), liquid and solid cultures, the classicalnomenclature of substrate and aerial mycelium, and the hydrophobic layers are indicated. (b) Confocal laser-scanning fluorescencemicroscopy pictures of the different types of mycelia: left, MI stained with the membrane stain FM4-64; middle, MIIL stained with thecell wall stain WGA; right, sporulating hyphae of MIIS stained with WGA. Developmental time points are indicated. Arrows indicatesepta. See text for details.
research articles Manteca et al.
4802 Journal of Proteome Research • Vol. 9, No. 9, 2010
washed as previously described.17 In all cases, samples wereobserved under confocal laser-scanning fluorescent microscopyafter staining with vital dyes, as detailed below. Cells werebroken up in an MSE soniprep 150, in 4 cycles of 10 s, on ice.The unbroken cells and cellular debris were eliminated bycentrifugation (7740g) at 4 °C for 15 min.
Cytosolic and membrane fractions were obtained accordingto Quiros et al.32 by ultracentrifugation at 100 000g in aBeckman LB-70 M ultracentrifuge. The resulting supernatantconstitutes the cytosolic fraction, whereas the sediment rep-resents the membrane fraction, which included membrane-anchored proteins (intrinsic and extrinsic), as well as peripheralproteins weakly bound to the membranes. Membranes wereresuspended in buffer A and incubated at 0 °C for 30 min withperiodic vortex shaking. They were subsequently ultracentri-fuged again at 100 000g. This process was repeated 3 times,discarding the supernatants. Membranes were later resus-pended in 100 mM Na2CO3 (pH 11) and washed two moretimes. These three supernatants were collected and cor-responded to the extrinsic membrane proteins. Finally, themembranes were washed two times with buffer A without salt.These membranous pellets corresponded to the intrinsicmembrane proteins, which were not delipidated. Supernatantsderived from the washing steps in Na2CO3 (extrinsic membraneproteins) were collected and dialyzed (Sigma D7884 benzoy-lated cellulose tubing) against buffer A at 4 °C for 1 h with fourbuffer changes. Membrane fractions were stored at -80 °C.
Viability, Membrane and Cell Wall Staining. The perme-ability assay previously described for Streptomyces was usedto stain the samples.33 This involves staining the dead cells withpropidium iodide and viable cells with SYTO 9 green fluores-cent nucleic acid stain (LIVE/DEAD Bac-Light Bacterial ViabilityKit, Molecular Probes, L-13152). Equal volumes of the mixtureof the stains and the mycelial sample were incubated in thedark for at least 10 min. The samples were observed under aLeica TCS-SP2-AOBS confocal laser-scanning microscope at awavelength of 488 and 568 nm excitation and 530 nm (green)or 640 nm (red) emissions. Images were mixed using the LeicaConfocal Software.
Membranes were stained with the Lipophilic styryl dye, N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexatri-enyl) pyridinium dibromide (FM 4-64) (Invitrogen, T-3166).Streptomyces cells were collected at various time points.Harvested mycelium was processed as described elsewhere.11
Cells were fixed in 2.8% paraformaldehyde and 0.0045% glu-taraldehyde in PBS (0.14 M NaCl, 2.6 mM KCl, 1.8 mM KH2PO4
and 10 mM Na2HPO4) for 15 min at room temperature, andwashed twice with PBS. FM 4-64 was added to a final concen-tration of 1 mg mL-1 and incubated at room temperature for3 h. Samples were washed twice and observed under theconfocal laser-scanning microscope at wavelengths of 550 nmexcitation and 700 nm emission.
Cell walls were stained with wheat germ agglutinin (WGA)conjugated with Texas red (Invitrogen W-21405), which bindsselectively to N-acetylglucosamine and N-acetylneuraminicacid. Fixed cells were incubated for 1 min in 2 mg mL-1
lysozyme in glucose/Tris/EDTA (GTE: 50 mM glucose, 20 mMTris/HCl, pH 8, 10 mM EDTA). The samples were washed againwith PBS and blocked in 2% BSA in PBS for 5 min. WGA wasadded to a concentration of 100 mg mL-1 in 2% BSA in PBS,and incubated at room temperature for 3 h. Finally, the sampleswere washed eight times with PBS and observed under the
confocal microscope at wavelengths of 595 nm excitation and615 nm emission.
Protein Separation and iTRAQ Labeling of Peptides. Proteinquantification was performed using the Bradford34 and Lowry35
assays with a bovine serum albumin standard (Sigma). Proteinswere separated by SDS-PAGE (50 µg per lane) using precastPAGEr 4-20% Tris-Glycine gels (Lonza) and stained withCoomassie Blue (Coomassie Brilliant Blue G-250). For intrinsicmembrane proteins, membranes containing 50 µg of proteinwere boiled in the SDS loading buffer for 5 min and loadeddirectly in the gel. The four samples (MIS, MIIS, MIL, and MIIL)of each subcellular fraction and the two biological replicatesanalyzed were loaded in six different gels, which were used forsix independent iTRAQ experiments. Each gel-lane was dividedinto six slices with a scalpel. Gel slices were cut into smallpieces, washed with distilled water, and shrunk with acetoni-trile. Cys residues were reduced with DTT and S-alkylated withiodoacetamide, swelled with a solution of 10 ng/µL trypsin(Promega), 50 mM triethylammonium bicarbonate (TEAB)digestion buffer, and incubated overnight at 37 °C. Afterdigestion, supernatants were recovered and peptide extractionsfrom remaining gel fragments were performed with a volumeof 5% formic acid for 30 min; subsequently, an equal volumeof pure acetonitrile was added and incubated for an additional30 min at room temperature. Extracts obtained from each gelslice were vacuum-dried. For quantification, peptides werelabeled with iTRAQ 8plex reagent (Applied Biosystems, FosterCity, CA) as per our previously reported protocol:17,36 113-, 114-,and 115-iTRAQ tags for 12-, 24-, and 72-h time points fromsolid samples, respectively, and 116 and 117 for liquid cultures,at 14 and 90 h of culture (Figure 2). After labeling for 2 h atroom temperature (RT), samples were combined (6 samplescorresponding to the original gel pieces). The concentrationof organic solvent was reduced using a vacuum concentrator,and peptide desalting was performed using GELoader micropi-
Figure 2. Overview of the mass spectrometry based proteomicsstrategy. The iTRAQ-reagent methodology used for multiplexedcomparative analysis of Streptomyces proteins isolated by SDS-PAGE during development. The six steps performed in parallelfor the six gel slices are indicated by arrows. Developmental timepoints and subcellular fractions are indicated.
Streptomyces Proteome Variations during Differentiation research articles
Journal of Proteome Research • Vol. 9, No. 9, 2010 4803
pet tips (Eppendorf) prepared with C18 (Empore extractiondisks, 3M) and R3 (Poros Oligo R3).
Analysis of iTRAQ Labeled Peptides by NanoLC-TandemMass Spectrometry. Tryptic peptides were separated using aNanoAcquity UPLC system (Waters Corporation) modified witha 2.6 µL PEEKSIL/sample loop (SGE, Darmstadt, Germany).Mobile phase A was 0.1% formic acid in ddH2O and mobilephase B was 0.1% formic acid in 90% acetonitrile (FisherScientific). A 2.5 µL sample was injected and loaded into theBEH C18, 1.7 µm, 15 cm × 75 µm analytical reversed phasecolumn (Waters Corporation) in a direct injection mode with3% B for 10 min at 400 nL/min. Peptides were eluted from thecolumn with a linear gradient of 3-7% B for 4 min, 7-30% Bfor 60 min, 30-60% B for 15 min, 60-90% for 5 min, at a flowrate of 300 nL/min. The column was washed with 90% B for10 min followed by equilibration for 14 min at a flow rate of400 nL/min. The column temperature was maintained at 36°C. The lock mass solution for MS and MS/MS was composedof 500 fmol/µL of (Glu1)-fibrinopeptide B (Sigma) and deliveredby the auxiliary pump of the nanoAcquity equipment to thereference sprayer of the NanoLockSpray source of the massspectrometer at a constant flow rate of 500 nL/min.
The UPLC system was interfaced to a Q-TOF tandem massspectrometer (Synapt, Waters Corporation, Manchester, U.K.).The mass spectrometer was operated in positive ion mode ata mass resolution of approximately 10 000 full width at half-maximum (fwhm). The TOF analyzer (v-mode) of the massspectrometer was externally calibrated with (Glu1)-fibrinopep-tide B fragment ions from m/z 50 to 1500. Acquired data werepostcalibrated using the doubly protonated precursor ion of(Glu1)-fibrinopeptide B. The reference sprayer was sampledevery 120 s. LC-MS/MS data were acquired using a data-dependent acquisition method. MS survey analysis was per-formed for 0.48 s with an interscan delay of 0.02 s followed bytwo MS/MS cycles. The fragment ions from the two mostabundant multiply charged precursor ions (+2, +3, and +4)were detected at an integration rate of 0.48 s with a 0.02 sinterscan delay. The collision energy ramped from 20 to 45 eV.Dynamic exclusion of precursors was set to 60 s. Each samplewas analyzed twice. The precursor ions selected for thefragmentation during the first LC-MS/MS analysis were ex-cluded in the second LC-MS/MS analysis.
LC-MS/MS Data Analysis. The ProteinLynx Global server(PLGS) program version 2.3 was used to convert LC-MS/MSraw data into pkl files. Pkl files were submitted for search bythe MASCOT search engine (version 2.2) against the NCBInrdatabase with taxonomy limited to S. coelicolor (22 Jan. 2009,8537 entries). The following MASCOT search parameters wereused: peptide mass tolerance 10 ppm, fragment mass tolerance0.1 Da, trypsin cleavage with a maximum of 2 missed cleavages.Fixed modification: S-carbamidomethyl on cysteine; iTRAQ onlysine residues and N-termini of peptides. Variable modifica-tions: oxidation on methionine. The annotated mass spectrumof individual MS protein identification was shown in theSupporting Information. Peptide false positive rates werecalculated using the decoy option provided by MASCOT (usingthe combined pkl file).
Relative quantification was performed using PLGS (WatersCorp.) with automatic normalization. The PLGS quantificationalgorithm uses Bayesian Markov Chain Monte Carlo methodsto explore the subsequent probability, and takes the differentscores of individual peptides from a protein into account inorder to quantify changes in expression. Results obtained from
PLGS were exported into MS-Excel for further computationaland bioinformatic data analysis. Proteins that were not repre-sented by any peptide above the MASCOT homology thresholdwere discarded. When a protein was detected more than oncein the same biological replicate (six gel slices), we retained theone having the highest MASCOT score.
For proteins identified in the two biological replicatesanalyzed, iTRAQ ratios were considered as significant if theiraverage in both replicates (( standard deviation) was greateror lower than one. With respect to the remaining proteins,iTRAQ ratio values were considered significant if their coef-ficient of variation (CV) was less than 0.25. Consequently, weretained protein abundance values with good reproducibilitybetween biological replicates (CV < 0.25), as well as those withCVs exceeding 0.25, but with averaged iTRAQ ratios that variedsignificantly between mycelial stages (average iTRAQ ratios (SD above or below one); we discarded the remaining proteins(protein abundance values without good reproducibility be-tween biological replicates).
The ProteinCenter 2.0 software (Proxeon, Odense, Denmark)was used to conduct the computational and bioinformatic dataanalyses and protein classification. Proteins were classified intofunctional categories according to their annotated functionsin the Gene bank database and by homology/functions ac-cording to the Gene Ontology, the Conserved Domain, and theKEGG Pathway Databases.
Cluster Analysis of Protein Expression Profiles. The aver-aged iTRAQ values obtained in two biological replicates for eachprotein at all four phases analyzed (MIS, MIIS, MIL, MIIL) werelog2-transformed. Data were normalized to obtain a mean valueof zero and a standard deviation of one, ensuring that proteinswith similar expression patterns can be easily comparedwithout taking into account their absolute values. For cluster-ing, we used the fuzzy c-means algorithm with a Euclideandistance matrix.37 This method groups the data into c proteinclusters with most similar patterns by minimizing an objectivefunction. The results provide c membership values for eachprotein. A membership value gives a measure in the range (0,1) of how closely a protein’s expression pattern follows that ofthe cluster center. We have considered clusters with member-ship values greater than 0.5 as significant. We associated eachprotein to the cluster for which it had the highest membershipvalue. The parameter for the fuzzyness of the data was set tom ) 2. The optimum value for the other parameter, the numberof clusters c, was determined by comparing the Xie-Beniindex38 and the minimum centroid distance39 calculated fromthe corresponding results.
Results and Discussion
Identification and Quantification of Streptomyces Proteins.Streptomyces is a mycelial bacterium with a complex develop-mental cycle which makes it difficult to fractionate differentmycelial phases. We recently developed and reported a meth-odology to overcome this impediment in solid cultures.17 Inthe present study, we applied this methodology to obtain MIand MII phases from solid cultures (MIS, MIIS; 12 and 72 h,respectively). MI in liquid cultures (MIL) was obtained at earlytime points (14 h); MII in liquid cultures (MIIL) was obtainedduring phases after the death of MIL (90 h).8 In both cases,mechanical disaggregation of mycelial pellets combined withintensive washing removed the protein released by secretionor lysis. Samples were further fractionated into three subcellularfractions: cytosolic, membrane-anchored proteins (intrinsic
research articles Manteca et al.
4804 Journal of Proteome Research • Vol. 9, No. 9, 2010
membrane proteins), and peripheral proteins bound to themembrane by weak bonds (extrinsic membrane proteins).17
Proteins were further processed for quantitative proteomicsusing isobaric tags (iTRAQ) and LC-MS/MS as previouslyreported (Figure 2).17 A total of 642 proteins (8.3% of S.coelicolor proteome) were identified from peptide MS/MSspectra that scored above the peptide MASCOT homologythreshold value (false positive rates of 1.36% and 1.08% for eachbiological replicate) (Figure 3). In two biological replicates, 361proteins were detected, and 359 were quantified in at least oneof the developmental phases analyzed (MIS, MIIS, MIL, MIIL)(Figure 3a). These 359 proteins were distributed among thedifferent subcellular fractions analyzed (Figure 3b) and werethe only ones considered for further analyses and presentedin the Tables and Figures included in this manuscript. The fold-change of all the quantified proteins were consistent across thebiological replicates analyzed (Figure 3c). The analysis of theindividual protein abundance values (average log10 iTRAQ ratiosfrom two biological replicates) between different developmentalstages (MIL/MIS, MIIL/MIIS, MIIL/MIL) (blue, red, and green linesin Figure 3d) demonstrates that they were far more variablethan those acquired from a methodological replicate of thesame developmental stage (MIL/MIL, orange line in Figure 3d).Moreover, disparities in the iTRAQ ratios between MII and MIin liquid conditions (MIIL/MIL; green line) (Figure 3d) weregreater than those between the same mycelial stage obtained
from liquid or solid cultures (MIIL/MIIS, red line; or MIL/MIS,blue line) (Figure 3d).
Clustering of Proteins with Similar Abundance Profiles.With the aim of identifying the proteins with similar abun-dances along the four developmental phases analyzed, we usedthe fuzzy c-means algorithm33 with a Euclidean distance matrix(Figure 4). We clustered proteins that were detected in twobiological replicates and only in one subcellular fraction(cytosolic or membranes) (223 proteins) (Figure 3). Onehundred and forty-seven proteins were assigned to 6 specificclusters, each of which exhibited distinct protein profiles(Figure 4). Most of the proteins involved in primary metabolismwere included in clusters 1 (13 proteins up-regulated in MIL
and MIS) and 2 (26 proteins up-regulated in MIS): ribosomalproteins (SCO4711, SCO4719, SCO3909, SCO4718) (MII/MIratios between 0.2 and 0.5), proteins of the Krebs cycle andenergy production (SCO4475, SCO3092) (MII/MI ratios between0.4 and 0.7), or proteins involved in lipid metabolism (SCO1815)(MII/MI ratio of 0.6) (Table 1). By contrast, proteins involvedin secondary metabolism, as well as regulatory proteins, wereincluded in clusters 3 and 4 (17 proteins up-regulated duringthe MII phases) and cluster 5 (4 proteins up-regulated duringMIIL): proteins involved in actinorhodin biosynthesis (ActVAand ActVA4) (MII/MI ratios between 2 and 8), proteins impli-cated in the biosynthesis of type II polyketides (SCO5086) (MII/MI ratios of 28 and 6), an epimerase (SCO0395) (MII/MI ratios
Figure 3. Presentations of the quantitative proteomics data set obtained for developmental stages of S. coelicolor. Number of identifiedproteins, subcellular location, and quantitative proteomic data analysis are shown. (a) Venn diagram showing overlap of proteinsidentified in each of the two biological experiments. Number of proteins significantly quantified according to criteria described inExperimental Procedures is indicated. (b) Venn diagram of protein distribution in the subcellular fractions. These proteins correspondto those detected in two biological replicates. (c) Correlation of the values for biological replicates significantly quantified (seeExperimental Procedures for details). Cytosolic, membrane, and extrinsic proteins were combined. (d) Variation of iTRAQ ratios (averagefrom two biological replicates) of different developmental phases (blue, red and green), with respect to methodological variation (orange);proteins significantly quantified in all the conditions analyzed were shown (105 proteins); iTRAQ ratio values for each protein weresorted in increasing order. Cytosolic, membrane, and extrinsic proteins were pooled.
Streptomyces Proteome Variations during Differentiation research articles
Journal of Proteome Research • Vol. 9, No. 9, 2010 4805
of 1.9 and 2.2), a �-lactamase (SCO2380) (MII/MI ratios of 1.6and 2.5), a histidine kinase (SCO4677) (MII/MI ratio of 3), andso forth. Proteins included in cluster 6 (Figure 4) displayed themaximum normalized protein abundance values in MI or MII,depending on the developmental conditions (liquid or solidcultures), and could not be unambiguously assigned to eitherMI or MII. These clusters were used to organize the proteinsin Supporting Information Tables 1-3, and the same meth-odology was used to identify proteins with similar or differentabundance patterns in the cytosolic or membrane fractions(Supporting Information Table 4).
Overall, protein functions associated with primary andsecondary metabolism clustered together, and their abundancevalues correlated well with Streptomyces differentiation. Pro-teins with similar functions are clustering together, and con-sequently, this knowledge will facilitate the interpretation offurther experiments designed to define the biological role ofproteins with putative or unknown function.
Similarities and Differences between MI and MII Proteomesfrom Liquid and Solid Cultures. Next, we compared the MIand MII proteomes from solid and liquid cultures byconsidering the abundance values of the main functionalprotein groups (Figure 5). With few exceptions, the differ-entially expressed proteins showed similar relative abun-dances in solid and liquid conditions, that is, the same
proteins were up- or down-regulated in MIIS and MIIL withrespect to MI (83%) (Figure 5).
The proteins with greatest divergence in their iTRAQ ratiosbetween MI and MII in solid and liquid cultures (MII/MI logiTRAQ ratios greater than 0.2 or less than -0.2) are shown inFigure 6. Twenty of these proteins had similar abundancevalues in liquid and solid cultures (-0.2 < log iTRAQ MIL/MISand MIIL/MIIS < 0.2) (Figure 6a, right panel), and can beconsidered as reliable markers of MI and MII (Figure 6a). Theproteins detected in greater abundance in MI were involvedin the Krebs cycle and energy production (SCO4475, SCO3092)(MII/MI ratios between 0.4 and 0.7); lipid metabolism (SCO1815)(MII/MI ratio of 0.6) (Table 1); a putative transport associatedprotein (SCO1903); aldehyde dehydrogenase (SCO4913); aputative TetR transcriptional regulator (SCO1691), and hypo-thetical proteins (SCO5414, SCO4179, SCO4033, SCO2067)(Table 1). SCO1691 is a very intriguing transcriptional regulator,which was only detected in MI (Table 1). Several TetR familytranscriptional regulators have been described in Streptomycesas repressors of antibiotic biosynthesis and export,40–42 so aputative role of SCO1691 repressing the onset of antibioticproduction in the MI might be feasible.
Several proteins were more abundant in MII. We detectedan epimerase (SCO0395) (MII/MI ratio of 2); proteins involvedin lipid metabolism (SCO5385) (MII/MI ratios of 5 and 3);
Figure 4. Cluster analysis (classification by fuzzy c-means approach) of protein expression patterns for S. coelicolor developmentalstages. Clusters include proteins detected in only one subcellular fraction (pooled cytosolic and membrane peptides) with similarexpression patterns along developmental time points. Number of proteins for each functional category, are indicated. Primary metabolism(DNA/RNA replication, aerobic and anaerobic energy production, glycolysis and glyconeogenesis, pentose phosphate pathway, aminoacid metabolism, nucleotide metabolism, translation, protein folding, RNA/protein processing, nucleases/RM methylases, lipidmetabolism); secondary metabolism (secondary metabolite synthesis and secreted proteins, DNA competence, TTA BldA targets, bldwhi proteins); transporters (ABC and others); proteins with unknown functions; stress and defense proteins; regulatory proteins(transcriptional regulators, kinases and phosphatases, other regulatory proteins); catabolism/proteases; cell wall/membrane/septation(cell division and septation proteins; proteins involved in cell wall and membrane synthesis). Proteins clustered were those significantlyquantified at least in one of the developmental phases analyzed (see Experimental Procedures).
research articles Manteca et al.
4806 Journal of Proteome Research • Vol. 9, No. 9, 2010
disulfide oxidoreductases (SCO2035) (MII/MI ratio of 2); acomponent of the SulfBCD complex, which is believed toparticipate in iron-sulfur cluster formation during oxidativestress (SCO1925) (MII/MI ratio of 2);43 transporters (SCO5580,SCO2008) (MII/MI ratios between 2 and 6); SCO2770, speB,agmatinase (MII/MI iTRAQ ratio of 3), and SCO6551, an aldo/keto reductase (MII/MI iTRAQ ratios of 1.6 and 1.9) (Table 1and Figure 6a). Several regulatory proteins were also detectedin greater relative abundance in MIIL and MIIS: SCO4920, aDeoR family transcriptional regulator (MII/MI ratio of 2);SCO5046, a whiB family sigma factor regulating the finalsporulation steps (MII/MI ratio of 1.92) (Table 1). The greaterabundance of SCO5046 in MII is consistent with its role inhydrophobic cover formation and sporulation in solid cultures,in addition to differentiation in liquid conditions.10
Only 5 of the proteins with the greatest differences in MI/MIIiTRAQ ratios exhibited inverse relative abundances in liquid andsolid cultures (Figure 6b). SCO1793 (sporulation Spo0M homolo-gous protein) and SCO5142, homologue to the Bacillus sporula-tion protein DivIVA,44 were more abundant in MIIS than in MIIL
(2.5-fold) (Table 1). It suggests that the most remarkable differ-ences between MIIS and MIIL emerge in the final stages of hyphaecompartmentalization and spore formation. The 50S ribosomalproteinL15(SCO4721),aputativessDNA-bindingprotein(SCO3907),and a putative transcriptional regulator (SCO0168) also showeddifferent abundances in MI and MII obtained in liquid and solid
culture conditions (Figure 6b and Table 1). The biological func-tions of these proteins have yet to be determined.
Relative Abundance of Proteins Detected in More thanOne Subcellular Fraction. Sixteen of the proteins detected inmore than one subcellular fraction (cytosolic, intrinsic orextrinsic membrane) presented great divergences in theirrelative abundances between MI and MII (MII/MI log iTRAQratios greater than 0.2 or less than -0.2). Among these, only 6proteins showed no variation between liquid or solid cultures(MIL/MIS and MIIL/MIIS log iTRAQ ratios between -0.2 and0.2) (Figure 7), which can be added to the 20 proteins describedabove (Figure 6). Five of these proteins were detected in greaterabundance in MII: a possibly secreted protein (SCO5995) witha TTA leucine codon (MII/MI ratios between 1.9 and 4), whichcould be one of the targets of the bldA developmental gene(the only S. coelicolor gene encoding tRNA for the TTA leucinecodon);45 a putative transcriptional regulator (SCO3571) (MII/MI ratios of 2); a DNA gyrase (SCO3873) (MII/MI ratios between1.9 and 4); a putative secreted protein (SCO2780) (MII/MI ratiosof 6), and an ABC transporter (SCO7677) (MII/MI ratiosbetween 1.6 and 1.9) (Table 2 and Figure 7). In contrast, 30Sribosomal protein S1 (SCO1998) was detected in greaterabundance in MI (MII/MI ratios between 0.2 and 0.6) (Table2). SCO5995 and SCO7677 had signal peptides; SCO1998 andSCO2780 had transmembrane domains; however, SCO3571,and SCO3873 did not harbor any transmembrane domains or
Table 1. Proteins Showing the Greatest Abundance Differences between MI and MII from Liquid and Solid Cultures (Figure 6)a
MIIS/MIS MIIL/MIL MIL/MIS MIIL/MIIS
function SCO no. Cl function C I E C I E C I E C I E
Krebs cycle andenergy metabolism
SCO3092 1 Oxidoreductase 0.4 0.7 0.7 1SCO4475 1 Cytochrome C assembly protein 0.6 0.7 1 1.4
Amino acid metabolism SCO2770 3 speB, agmatinase 3 2.7 0.9 0.9Lipid metabolism SCO1815 1 3-oxacyl-(ACP) reductase 0.6 0.6 0.9 1
SCO5385 3 3-hydroxybutyryl-CoA dehydrogenase 5 3 1.4 0.8Other anabolic enzymes SCO6551 3 Aldo/keto reductase family 1.6 1.9 0.8 1Translation, protein folding SCO4711 2 30S ribosomal protein S17 0.2 0.6 0.4 0.9
SCO4719 2 30S ribosomal protein S5 0.4 0.6 0.7 0.8SCO3909 2 50S ribosomal protein L9 0.4 0.5 0.7 0.7SCO4718 2 50S ribosomal protein L18 0.4 0.5 0.5 0.7SCO4721 2 50S ribosomal protein L15 0.4 1.1 0.5 1.3
Unknown SCO4033 1 Hypothetical protein 0.2 0.2 1 0.8SCO2067 1 Hypothetical protein 0.4 0.7 0.8 0.9SCO4179 1 Hypothetical protein 0.5 0.4 0.8 0.8SCO4913 1 aldehyde dehydrogenase 0.6 0.6 0.9 0.8SCO5414 1 Hypothetical protein 0.6 0.5 1.2 1
Stress/defense proteins SCO1903 1 Possible transport associated protein 0.6 0.6 0.8 0.8SCO1925 3 Component of SufBCD complex 2.1 2 1.2 1.2SCO2035 3 Protein Disulfide Oxidoreductases 2.2 2 1.2 1
ABC transporters SCO2008 3 ABC-type branched-chain amino acidtransport systems
3 3 1 0.9
Transporters/secreted SCO5580 3 ftsY, prokaryotic docking protein 2.2 2.3 1.1 1.2Secondary metabolites
synthesisSCO0395 3 Epimerase/dehydratase 1.9 2.2 1.2 1.5SCO5086 5 Ketoacyl reductase; Biosynthesis of
type II polyketide backbone1.9 4.3 1.3 2
SCO5079 3 ActVA4; Biosynthesis of actinorhodin 2.1 2.6 1.5 2SCO2380 3 Beta-lactamase 2.5 1.6 1 0.7SCO5077 3 ActVA; Biosynthesis of actinorhodin 8.4 3.8 2.2 0.9
Transcriptional regulators SCO1691 ns TetR transcriptional regulator MISb MIL
b 0.8 MISb
SCO0168 6 CAP family transcription factor 1.5 0.4 2.3 0.6SCO4920 3 DeoR transcriptional regulator 2.1 1.7 1.4 1.1SCO4895 ns RNA polymerase sigma factor 2.3 1.7 ns ns
Cell division/septation SCO5142 ns Possible secreted with DivIVA domain 2.3 0.7 1 0.4SCO1793 4 Spo0M homologue proteins 2.8 0.8 1.5 0.4
Kinases SCO4677 3 Histidine kinase-like ATPases 3.1 ns 1.1 nsOther regulatory proteins SCO3907 ns ssDNA-binding protein 0.4 2.1 0.8 3.5Bld Whi proteins SCO5046 ns Whib family sigma factor 2 2 0.7 0.7
a See Supporting Information Tables 1-3 for details. The second multinucleated mycelial stages with respect to the first compartmentalized mycelium(MIIS/MIS, MIIL/MIL) are shown. iTRAQ ratios are the average of two biological replicates. MIL/MIS and MIIL/MIIS ratios are also indicated. C, cytosolic; I,membrane intrinsic; E, membrane extrinsic. n.s., nonsignificant iTRAQ ratio values or clustering (see Experimental Procedures). Functions (according toGene bank, Gene Ontology, Conserved Domain, and KEGG); Cl, clusters of proteins with similar abundances (see Figure 5); C, cytosolic; I, membraneintrinsic; E, membrane extrinsic. b Proteins detected exclusively in MI.
Streptomyces Proteome Variations during Differentiation research articles
Journal of Proteome Research • Vol. 9, No. 9, 2010 4807
signal peptide. Further work will be necessary to define thebiological significance of the presence of these proteins in morethan one subcellular fraction.
Several regulatory proteins were also identified among theproteins detected in more than one subcellular fraction (Figure3b and Supporting Information Table 4): two components ofthe bldk ABC transporter complex (bldkB and bldkD) weredown-regulated in MII with respect to MI (0.7-fold) (Table 2).The bald (bld) genes control the onset of aerial hyphaeformation by regulating the expression of genes involved in theproduction of SapB,46–48 rodlins,18 and chaplins.19 The BldKcomplex is located at the beginning of the bald signalingcascade and is a well-known oligopeptide transporter that actsas a differentiation signal for S. coelicolor.49,50 BldG (SCO3549)is a transcriptional regulator that constitutes one of the lattersteps of the bald cascade and participates in S. coelicolorsporulation.51 It has an intriguing expression pattern: it wasup-regulated in the intrinsic membrane fraction of MII (4-foldin solid and 2-fold in liquid) (Table 2); however, its abundancein cytosol is reduced in MII (0.6- and 0.9-fold) (Table 2). Onehistidine kinase (SCO1630) was detected in the intrinsic and
extrinsic membrane fractions in greater abundance in MII insolid and liquid (MII/MI ratios between 2 and 6) (Table 2).Further work will be necessary to characterize the biologicalsignificance of these proteins.
In summary, 83% of all identified proteins showed similarabundance values in MI and MII in solid and liquid cultures (MII/MI greater than or less than 1 in both conditions). MI should beconsidered as vegetative, since proteins governing primary me-tabolism were detected in greater abundance in this mycelium;MII corresponds to the differentiated Streptomyces hyphae in-volved in secondary metabolism synthesis, since it had greaterabundance values of secondary metabolism proteins. The mostremarkable differences between MII from solid and liquid culturesinvolved proteins regulating the final stages of hyphae compart-mentalization and spore formation. These proteins were clearlydown-regulated in the MIIL, which is consistent with the absenceof sporulation in these conditions (whiB family sigma factor,Spo0M homologous protein or DivVA homologue protein) (seeabove). In addition, several putative regulatory proteins (tran-scriptional regulators, kinases, etc.) (see above) were detected asdifferentially expressed during the MI and MII stages. These
Figure 5. Protein abundance values (logarithm of iTRAQ ratio values) for the main functional groups of proteins. Primary metabolism(DNA/RNA replication, aerobic and anaerobic energy production, glycolysis and glyconeogenesis, pentose phosphate pathway, aminoacid metabolism, nucleotide metabolism, translation, protein folding, RNA/protein processing, nucleases/RM methylases); secondarymetabolism (secondary metabolites synthesis, TTA BldA targets, bld whi proteins); transporters (ABC transporters, transporters andsecreted proteins); stress and defense proteins; regulatory proteins (transcriptional regulators, kinases, other regulatory proteins).Proteins presented here are those detected in only one subcellular fraction (cytosolic or membrane) and were quantified in alldevelopmental phases (see Experimental Procedures). MI, first compartmentalized mycelium; MII, second multinucleated mycelium;S, solid cultures, L, liquid cultures. Proteins labeled with asterisks are those included in Figure 6.
research articles Manteca et al.
4808 Journal of Proteome Research • Vol. 9, No. 9, 2010
proteins presumably regulate presporulation developmental phasesand current studies are aimed toward revealing their biologicalfunctions. This work revealed that differentiation in liquid culturesis much more similar to solid cultures than might be expected inthe context of the classical Streptomyces developmental model,demonstrating the switch from primary to secondary metabolismbetween the initial compartmentalized mycelium and the sub-sequent multinucleated hyphae in liquid cultures. More detailedknowledge of the differences between the MI (vegetative) and MII(reproductive) proteomes represents a huge advance in Strepto-
myces biology and enable future analyses of specific proteins thatinduce, maintain, and contribute to these differences. Suchexperiments will advance our understanding of the biomolecularpathways controlling the presporulation differentiation phases ofStreptomyces and will have implications for optimization ofindustry-scale fermentation processes.
Conclusion
The work presented here is pioneering in approachingStreptomyces industrial fermentations from the point of view
Figure 6. Differential protein abundance values (logarithm of iTRAQ ratio) for differentially expressed proteins between MI and MII.Proteins shown were those detected in only one subcellular fraction (cytosolic or membrane) and were quantified in all developmentalphases. (a) Proteins with similar abundances in liquid and solid cultures: MII/MI log iTRAQ ratios greater than 0.2 or less than -0.2 andMIL/MIS and MIIL/MIIS log iTRAQ ratios between -0.2 and 0.2. (b) Proteins with inverse logiTRAQ ratios in liquid and solid cultures:MII/MI, MIL/MIS, and MIIL/MIIS log iTRAQ ratios greater than 0.2 or less than -0.2. MI, first compartmentalized mycelium; MII, secondmultinucleated mycelium; S, solid cultures; L, liquid cultures.
Table 2. Distribution and Abundance Values of Proteins Detected in More than One Subcellular Fractiona
MIIS/MIS MIIL/MIL MIL/MIS MIIL/MIIS
function SCO no. function C I E C I E C I E C I E
Translation, protein folding SCO1998 30S ribosomal protein S1 0.6 0.5 0.2 0.6 0.7 0.3 1 1 0.6 1 1.4 1Transporters/Secreted SCO7677 Secreted solute-binding protein 1.6 1.9 1.6 ns 1.9 ns ns 0.8 1 0.8 0.8 0.8
SCO2780 Secreted protein 2.5 ns 1.7 3.1 ns 1.7 1.5 1 1.2 1.9 1 1.2Transcriptional regulators SCO3571 Transcriptional regulator 1 2 ns 1.9 0.7 0.8 ns 0.8Kinases SCO1630 Histidine kinase-like ATPases 3.7 2.2 2.6 ns 1.2 1.1 ns 0.8DNA/RNA replication SCO3873 DNA gyrase subunit A 6 1.2 3.1 5.6 1.4 1 0.7 4Bld Whi proteins SCO3549 BldG 0.6 4.8 0.9 2 ns 1.1 ns
SCO5115 BldKD 0.8 0.9 ns ns 1.1 0.6 1.3 0.7SCO5113 BldKB 0.9 ns 0.7 0.8 0.7 0.7 ns 1.1 ns 1.1 ns 1.2SCO5995 Secreted protein with TTA leucine codon 4 1.9 2.1 1.9 ns 1.2 0.5 1.3
a Proteins included here are those detected in more than one subcellular fraction and with greater divergences between their abundance values in MIand MII from liquid and solid cultures (Figure 7) (see Supporting Information Table 4 for details). Proteins encoded by the bald developmental genes arealso included. Labelling as in Table 1. Numbers in bold correspond to the abundance values included in Figure 7. Abbreviations as in Table 1.
Streptomyces Proteome Variations during Differentiation research articles
Journal of Proteome Research • Vol. 9, No. 9, 2010 4809
of differentiation. We demonstrated that morphological dif-ferentiation recently described by us in liquid cultures8 cor-relates with a change in mycelial physiology: the switch fromprimary to secondary metabolism between the MI and the MII.Several proteins were detected as differentially expressed duringStreptomyces development, including regulatory proteins whichwill constitute the targets for future experiments aimed to thecharacterization of biochemical pathways regulating Strepto-myces differentiation, especially the presporulation phases,which are the key to understand and improve industrialfermentations.
Abbreviations: PCD, Programmed cell death; MI, first com-partmentalized mycelium; MII, second multinucleated myce-lium; GYM medium, glucose, yeast/malt extract; TEAB, trieth-ylammonium bicarbonate; fwhm, full width at half-maximum;PLGS, ProteinLynx Global server.
Acknowledgment. This research was funded by grantBIO2007-66313 from the DGI, Subdireccion General deProyectos de Investigacion, MEC, Spain. A.M. was supportedby a postdoctoral grant from the Ministerio de Ciencia eInnovacion, Spain and a short term fellowship from theFederation of European Microbiological societies (FEMS).H.R.J. was supported by a Ph.D.-fellowship from the DanishNational Research Foundation. V.S. was supported by apostdoctoral grant from the Danish Research Agency.Research in the O.N.J. laboratory was supported by theLundbeck Foundation and the Danish Research Agency. Theauthors would like to acknowledge the contribution of ErikoTakano from Microbial Physiology University of GroningenKerklaan 30 9751 NN Haren for providing the S. coelicolorM145 strain used in this work and Priscilla A. Chase forproofreading the text.
Supporting Information Available: Spectra of pep-tides from proteins identified with a single peptide, and Tableswith detailed quantitative data. This material is available freeof charge via the Internet at http://pubs.acs.org.
References(1) Tamaoki, T.; Nakano, H. Potent and specific inhibitors of protein
kinase C of microbial origin. Biotechnology 1990, 8 (8), 732–735.(2) Omura, S. The expanded horizon for microbial metabolitessa
review. Gene 1992, 115 (1-2), 141–149.(3) 3Umezawa, K. Induction of cellular differentiation and apoptosis
by signal transduction inhibitors. Adv. Enzyme Regul. 1997, 37,393–401.
(4) Champness, W. C. Prokaryotic Development; Brun, Y. V., Skimkets,L. J, Ed.; Actinomycete Development, Antibiotic Production andPhylogeny: Questions and Challenges; American Society for Mi-crobiology: Washington, DC, 2000; pp 11-31.
(5) Rueda, B.; Miguelez, E. M.; Hardisson, C.; Manzanal, M. B. Mycelialdifferentiation and spore formation by Streptomyces brasiliensisin liquid culture. Can. J. Microbiol. 2001, 47 (11), 1042–1047.
(6) Stocks, S. M.; Thomas, C. R. Viability, strength, and fragmentationof Saccharopolyspora erythraea in submerged fermentation. Bio-technol. Bioeng. 2001, 75 (6), 702–709.
(7) Pamboukian, C. R. D.; Guimaraes, L. M.; Candida, M. Applicationsof image analysis in the characterization of Streptomyces olindensisin submerged culture. Braz. J. Microbiol. 2002, 33, 17–21.
(8) Manteca, A.; Alvarez, R.; Salazar, N.; Yague, P.; Sanchez, J.Mycelium differentiation and antibiotic production in liquidcultures of Streptomyces coelicolor. Appl. Environ. Microbiol. 2008,74 (12), 3877–3886.
(9) Yague, P.; Manteca, A.; Simon, A.; Diaz-Garcia, M. E.; Sanchez, J.A new method for monitoring programmed cell death anddifferentiation in submerged cultures of Streptomyces. Appl. En-viron. Microbiol. 2010, 76 (10), 3401–3404.
(10) Flardh, K.; Buttner, M. J. Streptomyces morphogenetics: dissectingdifferentiation in a filamentous bacterium. Nat. Rev. Microbiol.2009, 7 (1), 36–49.
(11) Manteca, A.; Fernandez, M.; Sanchez, J. A death round affecting ayoung compartmentalized mycelium precedes aerial myceliumdismantling in confluent solid cultures of Streptomyces antibioti-cus. Microbiology 2005, 151 (11), 3689–3697.
(12) Manteca, A.; Fernandez, M.; Sanchez, J. Mycelium developmentin Streptomyces antibioticus ATCC11891 occurs in an orderlypattern which determines multiphase growth curves. BMC Micro-biol. 2005, 5, 51.
(13) Manteca, A.; Fernandez, M.; Sanchez, J. Cytological and biochemi-cal evidence for an early cell dismantling event in solid culturesof Streptomyces antibioticus. Res. Microbiol. 2006, 157 (2), 143–152.
(14) Manteca, A.; Mader, U.; Connolly, B. A.; Sanchez, J. A proteomicanalysis of Streptomyces coelicolor programmed cell death. Pro-teomics 2006, 6 (22), 6008–6022.
(15) Manteca, A.; Claessen, D.; Lopez-Iglesias, C.; Sanchez, J. Aerialhyphae in solid cultures of Streptomyces lividans and Streptomycescoelicolor originate from viable segments surviving an earlyprogrammed cell death event. FEMS Microbiol. Lett. 2007, 274 (1),118–125.
(16) Manteca, A.; Sanchez, J. Streptomyces development in coloniesand soils. Appl. Environ. Microbiol. 2009, 75 (9), 2920–2924.
(17) Manteca, A.; Sanchez, J.; Jung, H. R.; Schwammle, V.; Jensen, O. N.Quantitative proteomic analysis of Streptomyces coelicolor devel-opment demonstrates that onset of secondary metabolism coin-cides with hyphae differentiation. Mol. Cell. Proteomics 2010, 9(7), 1423–1436.
(18) Claessen, D.; Wosten, H. A. B.; van Keulen, G.; Faber, O. G.; Alves,A. M.; Meijer, W. G.; Dijkhuizen, L. Two novel homologous proteinsof Streptomyces coelicolor and Streptomyces lividans are involvedin the formation of the rodlet layer and mediate attachment to ahydrophobic solid. Mol. Microbiol. 2002, 44 (6), 1483–1492.
(19) Claessen, D.; Rink, R.; de Jong, W.; Siebring, J.; e Vreugd, P.;Boersma, F. G. H.; Dijkhuizen, L.; Wosten, H. A. A novel class ofsecreted hydrophobic proteins is involved in aerial hyphae forma-tion in Streptomyces coelicolor by forming amyloid-like fibrils.Genes Dev. 2003, 17 (14), 1714–1726.
(20) Bobek, J.; Halada, P.; Angelis, J.; Vohradsky, J.; Mikulık, K. Activationand expression of proteins during synchronous germination ofaerial spores of Streptomyces granaticolor. Proteomics 2004, 4 (12),3864–3880.
(21) Piette, A.; Derouaux, A.; Gerkens, P.; Noens, E. E.; Mazzucchelli,G.; Vion, S.; Koerten, H. K.; Titgemeyer, F.; De Pauw, E.; Leprince,P.; van Wezel, G. P.; Galleni, M.; Rigali, S. From dormant togerminating spores of Streptomyces coelicolor A3(2): new perspec-tives from the crp null mutant. J. Proteome Res. 2005, 4 (5), 1699–1708.
(22) Kim, D. W.; Chater, K.; Lee, K. J.; Hesketh, A. Changes in theextracellular proteome caused by the absence of the bldA gene
Figure 7. Differential protein abundance values (logarithm ofiTRAQ ratio) for differentially expressed proteins detected inseveral cellular locations. Proteins shown were those detectedin more than one subcellular fraction (cytosolic or membranes);quantified in all developmental phases; with different abun-dances in MI and MII (MII/MI logarithm iTRAQ ratio >0.2 or <-0.2);and without differences in liquid or solid cultures (-0.2 < MIL/MIS and MIIL/MIIS < 0.2). For subcellular location, see Table 2.Abbreviations as in Figure 6.
research articles Manteca et al.
4810 Journal of Proteome Research • Vol. 9, No. 9, 2010
product, a developmentally significant tRNA, reveal a new targetfor the pleiotropic regulator AdpA in Streptomyces coelicolor. J.Bacteriol. 2005, 187 (9), 2957–2966.
(23) Kim, D. W.; Chater, K. F.; Lee, K. J.; Hesketh, A. Effects of growthphase and the developmentally significant bldA-specified tRNA onthe membrane-associated proteome of Streptomyces coelicolor.Microbiology 2005, 151 (8), 2707–2720.
(24) Hesketh, A.; Bucca, G.; Laing, E.; Flett, F.; Hotchkiss, G.; Smith,C. P.; Chater, K. F. New pleiotropic effects of eliminating a raretRNA from Streptomyces coelicolor, revealed by combined pro-teomic and transcriptomic analysis of liquid cultures. BMC Ge-nomics 2007, 8, 261.
(25) Rodrıguez-Garcıa, A.; Barreiro, C.; Santos-Beneit, F.; Sola-Landa,A.; Martin, J. F. Genome-wide transcriptomic and proteomicanalysis of the primary response to phosphate limitation inStreptomyces coelicolor M145 and in a DeltaphoP mutant. Pro-teomics 2007, 7 (14), 2410–2429.
(26) Novotna, J.; Vohradsky, J.; Berndt, P.; Gramajo, H.; Langen, H.; Li,X. M.; Minas, W.; Orsaria, L.; Roeder, D.; Thompson, C. J.Proteomic studies of diauxic lag in the differentiating prokaryoteStreptomyces coelicolor reveal a regulatory network of stress-induced proteins and central metabolic enzymes. Mol. Microbiol.2003, 48 (5), 1289–1303.
(27) Akanuma, G.; Hara, H.; Ohnishi, Y.; Horinouchi, S. Dynamicchanges in the extracellular proteome caused by absence of apleiotropic regulator AdpA in Streptomyces griseus. Mol. Microbiol.2009, 73 (5), 898–912.
(28) Birko, Z.; Swiatek, M.; Szajli, E.; Medzihradszky, K. F.; Vijgenboom,E.; Penyige, A.; Keseru, J.; van Wezel, G. P.; Biro, S. Lack of A-factorProduction Induces the Expression of Nutrient Scavenging andStress-related Proteins in Streptomyces griseus. Mol. Cell. Proteom-ics 2009, 8 (10), 2396–2403.
(29) Jayapal, K. P.; Philp, R. J.; Kok, Y. J.; Yap, M. G.; Sherman, D. H.;Griffin, T. J.; Hu, W. S. Uncovering genes with divergent mRNA-protein dynamics in Streptomyces coelicolor. PLoS One 2008, 3,e2097.
(30) Nieselt, K.; Battke, F.; Herbig, A.; Bruheim, P.; Wentzel, A.;Jakobsen, O. M.; Sletta, H.; Alam, M. T.; Merlo, M. E.; Moore, J.;Omara, W.; Morrissey, E. R.; Hermosillo, M. J.; Rodriguez-Garcia,A.; Nentwich, M.; Thomas, L.; Legaie, R.; Gaze, W. H.; Challis, G. L.;Jansen, R. C.; Dijkhuizen, J.; Rand, D. A.; Wild, D. L.; Bonin, M.;Reuther, J.; Wohlleben, W.; Smith, M. C.; Burroughs, N. J.; Martin,J. F.; Hodgson, D. A.; Takano, E.; Breitling, R.; Ellingsen, T. E.;Wellington, E. M. The dynamic architecture of the metabolicswitch in Streptomyces coelicolor. BMC Genomics 2010, 11, 10.
(31) Novella, I. S.; Barbes, C.; Sanchez, J. Sporulation of Streptomycesantibioticus ETHZ 7451 in liquid culture. Can. J. Microbiol. 1992,38 (8), 769–773.
(32) Quiros, L. M.; Hardisson, C.; Salas, J. A. Isolation and propertiesof Streptomyces spore membranes. J. Bacteriol. 1986, 165 (3), 923–928.
(33) Fernandez, M.; Sanchez, J. Nuclease activities and cell deathprocesses associated with the developmenton solid cultures ofStreptomyces antibioticus ETH7451. Microbiology 2002, 148 (2),405–412.
(34) Bradford, M. M. A rapid and sensitive for the quantitation ofmicrogram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254.
(35) Lowry, O. H.; Rosenbrough, N. J.; Farr, A. L.; Randall, R. J. Proteinmeasurement with the Folin phenol reagent. J. Biol. Chem. 1951,193 (1), 265–275.
(36) Sachon, E.; Mohammed, S.; Bache, N.; Jensen, O. N. Phospho-peptide quantitation using amine-reactive isobaric tagging re-agents and tandem mass spectrometry: application to proteinsisolated by gel electrophoresis. Rapid Commun. Mass Spectrom.2006, 20 (7), 1127–1134.
(37) Bezdek, J. C. Pattern Recognition with Fuzzy Objective FunctionAlgorithms; Plenum Press: New York, 1981.
(38) Xie, X. L.; Beni, G. A validity measure for fuzzy clustering. IEEETrans. Pattern Anal. Mach. Intell. 1991, 13 (8), 841–847.
(39) Schwammle, V.; Jensen, O. N. A simple and fast method todetermine the parameters for fuzzy c-means cluster validation.Quant. Methods, submitted for publication, 2010. DOI: arXiv:1004.1307v1.
(40) Tahlan, K.; Yu, Z.; Xu, Y.; Davidson, A. R.; Nodwell, J. R. Ligandrecognition by ActR, a TetR-like regulator of actinorhodin export.J. Mol. Biol. 2008, 383 (4), 753–761.
(41) Ou, X.; Zhang, B.; Zhang, L.; Zhao, G.; Ding, X. Characterizationof rrdA, a TetR family protein gene involved in the regulation ofsecondary metabolism in Streptomyces coelicolor. Appl. Environ.Microbiol. 2009, 75 (7), 2158–216.
(42) Duong, C. T.; Lee, H. N.; Choi, S. S.; Lee, S. Y.; et al. FunctionalExpression of SAV3818, a Putative TetR-Family TranscriptionalRegulatory Gene from Streptomyces avermitilis, Stimulates Anti-biotic Production in Streptomyces Species. J. Microbiol. Biotechnol.2009, 19 (2), 136–139.
(43) Chahal, H. K.; Dai, Y.; Saini, A.; Ayala-Castro, C.; Outten, F. W.The SufBCD Fe-S scaffold complex interacts with SufA for Fe-Scluster transfer. Biochemistry 2009, 48 (44), 10644–10653.
(44) Errington, J. Septation and chromosome segregation during sporu-lation in Bacillus subtilis. Curr. Opin. Microbiol. 2001, 4, 660–666.
(45) Chater, K. F.; Chandra, G. The use of the rare UUA codon to define“expression space” for genes involved in secondary metabolism,development and environmental adaptation in Streptomyces. J.Microbiol. 2008, 46 (1), 1–11.
(46) Willey, J. M.; Santamaria, R. I.; Guijarro, J.; Geistlich, M.; Losick,R. Extracellular complementation of a developmental mutationimplicates a small sporulation protein in aerial mycelium forma-tion by S. coelicolor. Cell 1991, 65 (4), 641–650.
(47) Willey, J. M.; Schwedock, J.; Losick, R. Multiple extracellular signalsgovern the production of a morphogenetic protein involved inaerial mycelium formation by Streptomyces coelicolor. Genes Dev.1993, 7 (5), 895–903.
(48) Nguyen, K. T.; Willey, J. M.; Nguyen, L. D.; Nguyen, L. T.; Viollier,P. H.; Thompson, C. J. A central regulator of morphologicaldifferentiation in the multicellular bacterium Streptomyces coeli-color. Mol. Microbiol. 2002, 46 (5), 1223–1238.
(49) Nodwell, J. R.; McGovern, K.; Losick, R. An oligopeptide permeaseresponsible for the import of an extracellular signaling moleculegoverning aerial mycelium formation in Streptomyces coelicolor.Mol. Microbiol. 1996, 22 (5), 881–893.
(50) Nodwell, J. R.; Losick, R. Purification of an extracellular signalingmolecule involved in aerial mycelium formation by Streptomycescoelicolor. J. Bacteriol. 1998, 180 (5), 334–1337.
(51) Bignell, D. R.; Warawa, J. L.; Strap, J. L.; Chater, K. F.; et al. Studyof the bldG locus suggests that an anti-anti-sigma factor and ananti-sigma factor may be involved in Streptomyces coelicolorantibiotic production and sporulation. Microbiology 2000, 146 (9),2161–2173.
PR100513P
Streptomyces Proteome Variations during Differentiation research articles
Journal of Proteome Research • Vol. 9, No. 9, 2010 4811
Related Documents