Linköping University Medical Dissertations No. 967 Proislet Amyloid Polypeptide (proIAPP): Impaired Processing is an Important Factor in Early Amyloidogenesis in Type 2 Diabetes Johan F Paulsson Division of Cell Biology Department of Biomedicine and Surgery Faculty of Health Sciences, Linköping University SE-581 85 Linköping, Sweden Linköping 2006

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Linköping University Medical Dissertations No. 967
Proislet Amyloid Polypeptide (proIAPP):
Impaired Processing is an Important Factor in Early Amyloidogenesis in Type 2
Diabetes
Johan F Paulsson
Division of Cell Biology
Department of Biomedicine and Surgery
Faculty of Health Sciences, Linköping University
SE-581 85 Linköping, Sweden
Linköping 2006

Cover:
Confocal micrograph of an islet of Langerhans from a +hIAPP/-mIAPP transgenic mouse with islet amyloid deposits seeded by proIAPP amyloid-like fibrils. The tissue is stained with Congo red that binds to amyloid deposits and fluoresce red at 545 nm and erythrocytes auto-fluoresce green at 488nm. During the course of the research underlying this thesis, Johan F Paulsson was enrolled in Forum Scientium, a multidisciplinary doctoral programme at Linköping University, Sweden.
© Johan Paulsson, 2006
All rights reserved
ISSN 0345-0082
ISBN 91-85643-59-9
Published articles have been reprinted with the permission from the
publishers.
Paper I © American Diabetes Association 2005.
Paper II © Springer-Verlag 2006.
Printed in Sweden by LiU-Tryck, Linköping, Sweden, 2006

This thesis is dedicated to my family:
Anki, Göran, Anna and David


ABSTRACT Amyloid is defined as extracellular protein aggregates with a characteristic fibrillar ultra-structure, Congo red affinity and a unique x-ray diffraction pattern. At present, 25 different human amyloid fibril proteins have been identified, and amyloid aggregation is associated with pathological manifestations such as Alzheimer’s disease, spongiform encephalopathy and type 2 diabetes. Amyloid aggregation triggers apoptosis by incorporation of early oligomers in cellular membranes, causing influx of ions. Amyloid is the only visible pathological islet alteration in subjects with type 2 diabetes, and islet amyloid polypeptide (IAPP) is the major islet amyloid fibril component. IAPP is produced by beta-cells and co-localized with insulin in the secretory granules. Both peptides are synthesised as pro-molecules and undergo proteolytic cleavage by the prohormone convertase 1/3 and 2. Although IAPP is the main amyloid constituent, both proIAPP and proIAPP processing intermediates have been identified in islet amyloid.
The aim of this thesis was to study the role of impaired processing of human proIAPP in early islet amyloidogenesis. Five cell lines with individual processing properties were transfected with human proIAPP and expression, aggregation and viability were studied. Cells unable to process proIAPP into IAPP or to process proIAPP at the N-terminal processing site accumulated intracellular amyloid-like aggregates and underwent apoptosis. Further, proIAPP immunoreactivity was detected in intracellular amyloid-like aggregates in beta-cells from transgenic mice expressing human IAPP and in transplanted human beta-cells. ProIAPP was hypothesized to act as a nidus for further islet amyloid deposition, and to investigate this theory, amyloid-like fibrils produced from recombinant IAPP, proIAPP and insulin C-peptide/A-chain were injected in the tail vein of transgenic mice expressing the gene for human IAPP. Pancreata were recovered after 10 months and analysed for the presence of amyloid. Both IAPP and proIAPP fibrils but not des-31,32 proinsulin fibrils, caused an increase in affected islets and also an increase of the amyloid amount. This finding demonstrates a seeding capacity of proIAPP on IAPP fibrillogenesis. IAPP has been known for some time to trigger apoptosis in cultured cells, and a novel method for real time detection of apoptosis in beta-cells was developed. Aggregation of recombinant proIAPP and proIAPP processing intermediates were concluded to be inducers of apoptosis as potent as IAPP fibril formation.
From the results of this study, a scenario for initial islet amyloidogenesis is proposed. Initial amyloid formation occurs intracellularly as a result of alterations in beta-cell processing capacity. When the host cell undergoes apoptosis intracellular proIAPP amyloid becomes extracellular and can act as seed for further islet amyloid deposition.


PREFACE This thesis is based on the following papers, which is referred to in the text by their Roman numerals: I Paulsson JF, Westermark GT. Aberrant Processing of
Human Proislet Amyloid Polypeptide Results in Increased Amyloid Formation. Diabetes. 2005 54:2117-25.
II Paulsson JF, Andersson A, Westermark P, Westermark GT.
Intracellular amyloid-like deposits contain unprocessed pro-islet amyloid polypeptide (proIAPP) in beta cells of transgenic mice overexpressing the gene for human IAPP and transplanted human islets. Diabetologia. 2006 49:1237-46.
III Paulsson JF, Schultz SW, Saraiva MJ, Kapurniotu A,
Westermark GT. There is a role for proislet amyloid polypeptide in islet amyloid fibrillogenesis. Manuscript.
IV Paulsson JF, Schultz SW, Köhler M, Leibiger I, Berggren PO,
Westermark GT. Real-time monitoring of apoptosis by caspase 3-like protease induced FRET reduction triggered by amyloid aggregation. Manuscript.


ABBREVIATIONS Aβ A-beta protein AEF Amyloid Enhancing Factor AGE Advanced Glycation End-products ANP Atrial Natriuretic Peptide AP/NTS Area Postrema/Nucleus of the Solitary Tract ApoAII Apolipoprotein A-II BSE Bovine Spongiform Encephalopathy CJD Creutzfeldt-Jakob Disease CGRP Calcitonin Gene-Related Peptide CPE Carboxypeptidase E CT Calcitonin CTR Calcitonin Receptor ECFP Enhanced Cyan Fluorescent Protein EYFP Enhanced Yellow Fluorescent Protein ER Endoplasmic Reticulum FAP Familial Amyloid Polyneuropathy FRET Fluorescence Resonance Energy Transfer GAG Glycosaminoglycan GST Glutathione S-Transferase HDL High Density Lipoprotein HSPG Heparan Sulphate Proteoglycan hproIAPP Human proIAPP IAPP Islet Amyloid Polypeptide IDE Insulin Degrading Enzyme IGT Impaired Glucose Tolerance LSCM Laser Scanning Confocal Microscopy mproIAPP Murine proIAPP N+IAPP N-terminal flanking peptide+IAPP NEFA Non-Esterified Fatty Acids PAM Peptidyl Amidating Monooxygenase complex PDX-1 Pancreatic Duodenal Homeobox-1 PC Prohormone Convertase PEI Polyethyleneimine proIAPP Proislet Amyloid Polypeptide PrP Prion Protein PS Phosphatidylserine RAMPs Receptor Activity Modifying Proteins recC-peptide/A-chain Recombinant human proinsulin fragment
C-peptide/A-chain recIAPP Recombinant human IAPP recIAPP+C Recombinant IAPP+C-terminal flanking peptide

recN+IAPP Recombinant N-terminal flanking peptide+IAPP recproIAPP Recombinant human proIAPP SAA Serum Amyloid A TGN Trans-Golgi Network ThT Thioflavin T TTR Transthyretin

TABLE OF CONTENTS
ABSTRACT.............................................................................................. 5 PREFACE................................................................................................. 7 ABBREVIATIONS.................................................................................. 9 TABLE OF CONTENTS ...................................................................... 11 BACKGROUND .................................................................................... 13
AMYLOID AND AMYLOIDOSIS ................................................................ 13 History and definitions...................................................................... 13 The amyloid forming process............................................................ 14 Seeding effect and transmissibility ................................................... 15 Toxic effect of amyloid forming process ........................................... 17 Functional amyloid ........................................................................... 17
ISLET AMYLOID POLYPEPTIDE ................................................................ 18 Introduction....................................................................................... 18 Tissue expression .............................................................................. 18 Embryogenesis .................................................................................. 18 Genetics............................................................................................. 19 Prohormone processing .................................................................... 20 Biological function............................................................................ 21 IAPP receptors.................................................................................. 22 IAPP degradation ............................................................................. 22
TYPE 2 DIABETES AND ISLET AMYLOID .................................................. 23 Introduction to type 2 diabetes ......................................................... 23 Islet amyloid and amyloidogenic sequences..................................... 24 Transgenic animal models ................................................................ 25 Beta-cell dysfunction and death........................................................ 25 IAPP and cytotoxicity ....................................................................... 27
ISLET FIBRILLOGENESIS: INITIATION AND LOCATION.............................. 27 Basal membrane components ........................................................... 27 Hyperglycemia and islet amyloid ..................................................... 28 Beta-cell granule components........................................................... 28 IAPP fibrillogenesis and non-esterified fatty acids (NEFA) ............ 29 Cell membrane components and fibrillogenesis............................... 29 Aberrant processing of proIAPP ...................................................... 30

AIM OF THE THESIS.......................................................................... 31 MATERIAL AND METHODS ............................................................ 33
IMMUNO-DETECTION ............................................................................. 33 Immunohistochemistry: tissue sections............................................. 33 Immunohistochemistry: cultured cells .............................................. 34 Immunoelectron microscopy............................................................. 34
PRODUCTION OF RECOMBINANT PEPTIDES ............................................. 35 PRODUCTION AND CHARACTERISATION OF MONOCLONAL ANTIBODIES . 35
Antigen production............................................................................ 35 Production of monoclonal antibodies............................................... 36 Characterization of antibodies ......................................................... 36
CELL TRANSFECTION PROTOCOLS .......................................................... 37 Calcium-phosphate transfection ....................................................... 37 DOTAP liposomal transfection......................................................... 37 Electroporation ................................................................................. 37 Polyethyleneimine (PEI) transfection............................................... 38
LASER SCANNING CONFOCAL MICROSCOPY (LSCM) ............................ 38 Basic concept of LSCM..................................................................... 38 LSCM and fluorophores.................................................................... 39
THIOFLAVIN T (THT) ASSAY.................................................................. 40 RESULTS AND DISCUSSION ............................................................ 41
INTRACELLULAR AMYLOID-LIKE AGGREGATES (PAPER I AND II) ........... 41 EXTRACELLULAR FIBRILLOGENESIS (PAPER III AND IV)........................ 46
GENERAL DISCUSSION .................................................................... 51 ACKNOWLEDGMENTS..................................................................... 55 REFERENCES....................................................................................... 59

BACKGROUND
- 13 -
BACKGROUND
Amyloid and amyloidosis
History and definitions The term amyloid was introduced in medicine in 1854 by the German physician Rudolph Virchow (1). He stained human tissue with iodine and sulphuric acid and found typical staining for cellulose in corpora amylacea and named it amyloid from amylum, the Latin word for starch. The difference between starch and cellulose and their restricted existence to plants was unknown at that time, and five years later Friedreich and Kekulé demonstrated that amyloid deposits from spleen consisted mainly of protein (2). Staining with the cotton dye Congo red is the most commonly used procedure for amyloid detection. Congo red tinctorial features with the amyloid specific apple green birefringence was first described by Divry and Florkin in 1927 (3). The widely used protocol for Congo red detection of amyloid was introduced by Puchtler et al. in 1962 (4). Cohen and Calkins used electron microscopy and showed that at high resolution amyloid consists of unbranched fibrils of variable length approximately 10nm in diameter (5). The appearance of the fibril does not depend on the amyloid protein (6). Amyloidosis is a pathological manifestation where normally soluble proteins or peptides undergo conformational changes that results in the formation of intermolecular hydrogen bonds, beta sheet conformation and fibril formation. Today, 25 different amyloid proteins or peptides have been identified from human amyloid disorders (7). Amyloid diseases are divided into systemic or local forms depending on the deposition pattern. In systemic forms, the precursor proteins are derived from circulating proteins and deposited at various locations (8, 9). In local forms, the amyloid precursor is generally derived from locally produced proteins and the amyloid is deposited at that location (10, 11) . Criteria for definition of amyloid are:
• Extracellular protein deposits that stain with Congo red and reveal a green birefringence when viewed in polarized light.
• Characteristic fibrillar ultra-structure. • An amyloid specific X-ray diffraction pattern.

BACKGROUND
- 14 -
All of above stated criteria should be fulfilled for the definition of amyloid (7). Synthetic or recombinant peptides that form fibrils should not be considered amyloid since they are not of cellular origin. Instead these should be referred to as amyloid-like fibrils or amyloid-like aggregates. Intracellular aggregates recognized by Congo red are not at present considered to be amyloid.
The amyloid forming process Aggregation of amyloid requires that the protein partially unfolds from its native state. Why this occurs is not known, but destabilizing mutants of amyloidogenic proteins are characteristic in aggressive forms of familial types of amyloid diseases (12). Small amyloidogenic peptides such as islet amyloid polypeptide (IAPP) and A-beta protein (Aβ) are considered to be natively unfolded and to be in random coil state (13). These peptides can adopt a partially structured conformation which can further be stabilized by the formation of oligomers (14) . The amyloid-like aggregation process in vitro occurs via nucleation-dependent oligomerization and can be divided into three phases (14, 15). First, at a critical concentration, peptide monomers self-assembly and form prefibrillar oligomeric species. This process is referred to as the lag phase and is thermodynamically unfavourable and the rate-limiting step of fibril formation. The second phase is the extension phase, where a rapid elongation of the amyloid-like fibrils occurs, and when most of the molecules are transformed into fibrils a final plateau phase is reached (Fig. 1).
Figure 1. Illustration of amyloid formation and the seeding effect.

BACKGROUND
- 15 -
Seeding effect and transmissibility A reduction or an elimination of the lag phase occurs if preformed fibrils of the same protein are added to a monomeric solution. This process is referred to as the seeding mechanism and has been shown in vitro for many of the amyloidogenic proteins (15-18). The seeding phenomenon has also been described in vivo in several animal models. There is a well characterized mouse model for secondary amyloidosis where AA amyloid is deposited secondary to chronic inflammation. Reactive amyloid appears after five or six weeks, but the induction time is shortened to 2 days if the animal receives a water extract from an AA-amyloid containing mouse organ. This extract is referred to as amyloid enhancing factor (AEF) (19, 20). Recent studies have suggested that the active component in AEF is the amyloid fibril itself and in the experimental model, AEF activity was independent of the route of administration (injection, inhalation or oral) (21, 22). In another study, brain homogenate containing Aβ amyloid from human or monkey with Alzheimer’s disease was injected into the brain of marmosets (small primates) (23). Cerebral Aβ-amyloid developed in 89% of these animals as compared to 12% in the uninjected group. Prion diseases are characterised by accumulations of an amyloidogenic isoform of the prion protein called PrPSc (PrP scrapie) which is derived from a normal cellular membrane glycoprotein PrPC (PrP cellular) (24, 25). Amyloid accumulations of PrPSc in the brain cause spongiform encephalopathy which is a form of lethal neurodegenerative disease affecting both humans and animals. The term prion stands for “protein infectious particle” and refers to the protein conformation, which is the transmissible agent causing this group of diseases. Human forms of prion disease are Creutzfeldt-Jakob disease (CJD), fatal familial insomnia, Gerstmann-Sträussler-Scheinker disease and Kuru (26, 27). CJD is an extremely rare age related disease that develops sporadically but, after the bovine spongiform encephalopathy (BSE) outbreak in Great Britain, a new form of human CJD appeared in the British population affecting younger individuals which was called variant CJD. A form of spongiform encephalopathy called Kuru affected tribes with ritual cannibalism in Papua New Guinea, but since cannibalistic feasts were prohibited the disease has virtually ceased to exist (27, 28). Both of these diseases are believed to be caused by ingestion of prions, and transmissibility of prion disease is evident both within and across species (29).

BACKGROUND
- 16 -
Apolipoproteins are the protein components in the lipoprotein complex. The amyloidogenic apolipoprotein A-II (ApoAII) is abundant in serum high density lipoproteins (HDL) and is deposited in aging mice having the amyloidogenic apoAII-C gene (30). Systemic ApoAII amyloidosis is induced in young mice by intravenous injections and also by oral administration of ApoAII amyloid fibrils. This finding suggests a prion-like mechanism of transmissibility (31, 32). In contrast to the above mentioned examples, injections of preformed transthyretin (TTR) fibrils in transgenic mice expressing human wild-type TTR do not result in enhancement of amyloid deposition in these animals (33). Cross-seeding occurs when amyloid or amyloid-like fibrils from one specific protein has the ability to accelerate fibril aggregation of another protein. In vitro studies on fibril formation have shown that Aβ1-40 fibrils are potent seeds and can cross-seed IAPP fibril formation, but inversely IAPP fibrils do not seed Aβ1-40 fibril formation (17). A sequence similarity between two proteins with cross-seeding properties seems to be important. When Aβ1-40 and IAPP were sequenced aligned an overall sequence identity of 25% and a sequence similarity of 50% was recognized. Cross-seeding between almost identical proteins with the discrepancy of amino acid substitutions or differences in total number of amino acid residues like Aβ1-40 and Aβ1-42 have been reported (17, 34, 35). Seeding of a protein in solution with amyloid-like fibrils from the same protein originating from a different species with a high sequence homology has also been reported (35). Hen lysozyme fibril formation was accelerated by seeds from human lysozyme fibrils with 60% identity to hen lysozyme. In the same study, fibrils from bovine insulin having 0% sequence identity with hen lysozyme did not enhance the aggregation process. Cross-seeding has also been demonstrated in vivo. Amyloid-like fibrils that consist of approximately 10 amino acid residues from amyloidogenic TTR and IAPP sequences have been able to accelerate experimental AA amyloidosis in mice (21, 36). Further, experimental AA amyloidosis was also enhanced by administration of silk, Sup 35 and curli which are amyloid-like fibrils found in nature (37).

BACKGROUND
- 17 -
Induction of ApoAII amyloid deposition in the ApoAII-C mouse was accelerated by injections of human and murine amyloid-like fibrils from various recombinant and synthetic peptides like TTR, Serum Amyloid A (SAA) and Aβ1-40 (38). Injection of these peptides in monomeric form did not enhance deposition of ApoAII amyloid in these mice. In humans, amyloid depositions in the heart of patients with familial amyloid polyneuropathy (FAP) that were heterozygous for mutant forms of TTR were composed of approximately 50% wild-type TTR (39). In one of these FAP patients that had undergone a liver transplantation which is the site of TTR production, 80% of the cardiac amyloid was concluded to be wild-type TTR. This finding suggests that fibrillar forms of mutant TTR can act as seed for wild-type TTR amyloidogenesis.
Toxic effect of amyloid forming process Amyloid cytotoxicity is believed to occur via a common mechanism independent of protein or peptide (40, 41). Spherical particles of approximately 3-10nm have been identified with electron and atomic force microscopy at an early phase of fibril formation and to disappear as mature fibrils appear. These aggregates are referred to as oligomers or proto fibrils (42). Oligomers can be incorporated into and form pores or channels in cellular membranes, resulting in cell leakage and influx of cations which can trigger the apoptosis cascade (43-45). Therefore, the amyloid fibril itself is considered to be a non-toxic end product of the aggregation process. Aggregation of IAPP has been shown to trigger apoptosis in cultured beta-cells and toxic oligomers of IAPP are estimated to consist of 25-6000 molecules (46-50).
Functional amyloid Amyloid is not only considered to be a product of a pathological manifestation but has also been shown to have a functional role in nature (51). As an example, certain bacteria such as some E. coli and Salmonella express extracellular fibers named curli which are considered to be a virulence factor (52). Curli consist of the protein curlin and have all the properties of amyloid-like fibrils including binding of Congo red (53). Melanocytes and retinal pigment epithelium produce the melanosome organelle which has a core structure containing a proteolytic fragment of Pmel17 protein called Mα. Mα was recently found to self-assemble and form an amyloid-like structure in the melanosome (54). The function for aggregated Mα is to bind and orientate melanin precursors. Finally, silk from the silk worm Bombyx mori and spider silk have a beta-sheet rich fibrillar ultra-structure which have been proposed to have amyloid-like properties (37, 55).

BACKGROUND
- 18 -
Islet amyloid polypeptide
Introduction Islet amyloid was described in 1901 by Eugene Opie who reported that the parenchyma of the pancreatic islets of Langerhans was replaced by a hyaline substance in autopsy material from a patient with diabetes (56). The peptide content of islet amyloid were for a long time unidentified, but the amyloid deposit was known to be associated with type 2 diabetes (57-59). The deposited peptide was identified 1986 and fully characterized in 1987 as a 37 amino acid residue polypeptide and given the name islet amyloid polypeptide (IAPP) (11, 60).
Tissue expression IAPP is produced by the beta-cells in the islets of Langerhans and IAPP is stored and secreted together with insulin upon stimulation (61, 62). IAPP is located in the halo region together with C-peptide while insulin is stored in the dense core of the secretory granule (63). The intragranular concentration of IAPP is approximately 1-4mM and the molar ratio to granular insulin is 1-10% (63-65) . Human plasma levels of IAPP at fasting state are normally between 2-10 pM (66-68). IAPP is well preserved phylogenetically and has been detected in the pancreas of all studied mammals, chicken and in the insulin producing Brockmann body of sculpin and salmon (69-76). IAPP expression has also been reported in a subfraction of somatostatin producing pancreatic delta-cells in rats, in enteroendocrine cells in human fundus, and in the gastrointestinal tract of rodents (77, 78). Also sensory neurons in rats have been reported to express IAPP (79).
Embryogenesis The pancreas develops from the ventral and dorsal buds of the primitive gut epithelium at the foregut/midgut junction and in the fetal mouse pancreas, and IAPP and insulin are both detected at embryonic day 10.5-12 (80, 81). Glucagon producing cells are distinguished at embryonic day 9.5 and make up the majority of the endocrine cells until the day 13.5. An interesting observation is that glucagon expressing cells also produce IAPP at embryonic day 12.5 and, as embryonic development precedes the numbers of IAPP expressing alpha-cells falls and no IAPP reactivity can be detected in alpha-cells in the adult murine pancreas. In human fetal pancreas, insulin expressing cells emerge at 9-12 weeks of gestational age while no IAPP reactivity can be identified in the tissue at this time

BACKGROUND
- 19 -
(81, 82). IAPP expression can be detected at week 13 of gestation in cells situated in duct walls and scattered between acinar cells (82). After week 14 a small number of IAPP positive cells can be detected in islet-like clusters, and most of the IAPP positive cells are centrally located in islets by week 24 (82). Co-expression of IAPP and glucagon in fetal pancreatic endocrine cells have been reported at weeks 18 and 22 of gestational age, but contradictory results exist where double labeling for Insulin/IAPP, glucagon/IAPP and somatostatin/IAPP provided convincing IAPP staining of insulin producing cells only at week 19-24 of gestational age. In general, IAPP expression is apparent in pancreatic pluripotent endocrine stem cells during human embryological development and the early onset of IAPP expression suggests that IAPP might have a role in fetal development (83).
Genetics The human IAPP gene was isolated and characterized in the late 80´s (84-86). The gene is composed of 3 exons situated on the short arm of chromosome 12 where exon 1 is non-coding, exon 2 codes for the signal peptide and 5 residues of the N-terminal proIAPP and exon 3 encodes the remaining part of the pre-proIAPP molecule (87). Transcription of the IAPP gene is controlled by a promoter region located from -2798 to+450bp relative to the transcriptional start. Insulin and IAPP have similar promoter regions, and both genes are activated in response to glucose by the transcription factor called pancreatic duodenal homeobox-1 (PDX-1) (88-90). IAPP is a member of the calcitonin gene peptide family together with calcitonin (CT), calcitonin gene-related peptide (CGRP) and adrenomedullin (91). CGRP is a 37 amino acid neuropeptide which is a potent vasodilator, and receptors for CGRP are widely distributed in the body (92, 93). CT is a 32 amino acid peptide hormone produced by the C-cells of the thyroid gland and is a potent inhibitor of osteoclast-mediated bone resorption (94). IAPP shares 43-46% residue homology with CGRP-I and II and 20% with human CT (85, 95). CT and CGRP-I are products from the CT/CGRP gene and alternative splicing leads to the translation of the CGRP and CT peptides in a tissue-specific manner (91, 96). CGRP-II is a product of a separate gene but differs only at 3 out of 37 residues from CGRP-1 (97).

BACKGROUND
- 20 -
Prohormone processing Pre-proIAPP consists of 89 amino acid residues, and a 22 residue signal peptide is cleaved after entrance into the endoplasmic reticulum (ER) (98). The proIAPP molecule of 67 residues is transported through the golgi network and is subsequently packed in secretory granules (62, 99). ProIAPP is first processed at the C-terminal processing site by the prohormone convertase (PC) 1/3 at the basic amino acid residues lysine and arginine (Lys50 –Arg51 ) (Fig. 2) (100). Some studies indicate that this process may start in the late part of the trans-golgi network (TGN) since PC1/3 exists in a partially active form at this location (101, 102). PC1/3 is produced as a precursor molecule proPC1/3 and its activation is initiated by autocatalytic cleavage at the N-termini in the ER, followed by C-terminal cleavage in the mature secretory granule where full processing activity is gained (102-104). At the N-terminus, proIAPP is processed by PC2 after basic residues lysine and arginine (Lys10-Arg11) (105). PC2 also has the ability to process proIAPP at the C-termini in the absence of PC1/3 (100). Maturation of proPC2 is initiated in the ER where the 7B2 protein binds to and facilitate proPC2 transport to the TGN (106, 107). ProPC2 is then activated by auto-enzymatic cleavage in the characteristic milieu of the secretory granule with low pH and a high calcium concentration (102, 108). In contrast to PC1/3, no enzymatically active PC2 precursors exist. Carboxypeptidase E (CPE) is responsible for the removal of the dibasic residues lysine and arginine in the C-termini of processed proIAPP exposing a glycine used for carboxyamidation by the peptidyl amidating monooxygenase complex (PAM) (109, 110). Formation of a disulfide bond between cysteine 2 and 7 is required to gain full biological activity (98, 111). A second dibasic cleavage site is found in the C-terminal flanking peptide of proIAPP but no processing has been described at this site.

BACKGROUND
- 21 -
Figure 2. Schematic image of IAPP and insulin processing Proinsulin is processed by the same prohormone convertases as proIAPP and PC1/3 initially cleaves proinsulin at the B-chain/C-peptide dibasic junction (Arg31-Arg32) with the formation of the proinsulin processing intermediate des-31,32 proinsulin (Fig. 2) (112, 113). PC2 cleaves proinsulin at the C-peptide/A-chain junction after dibasic residues (Lys64-Arg65), and this results in equimolar production of insulin and C-peptide (114). CPE is responsible for the removal of the basic residues from the cleavage sites (115).
Biological function A large range of physiological effects have been ascribed to IAPP, and the most important will be reviewed here. Autocrine and paracrine effects of IAPP IAPP act on beta-cells and suppress glucose and arginine stimulated insulin secretion via a negative feedback mechanism (116, 117). However, recent findings have suggested that IAPP exert a dual action and act via positive feedback on basal insulin secretion (118). IAPP would by this mean act as a modulator of insulin fluctuations. An increased insulin secretion was detected when isolated rat islets were studied in a perfusion system with the presence of IAPP antagonist (IAPP 8-37), and elevated insulin levels were noted in glucose challenged transgenic male mice deficient of IAPP compared to wild-type littermates (111, 119). IAPP has been demonstrated to exert an inhibitory effect on alpha-cells and suppresses glucagon secretion. A similar negative effect on somatostatin release from delta-cells has been reported (118, 120). Anorectic effect

BACKGROUND
- 22 -
IAPP has a hormonal role in the control of food intake, as both acute and chronic infusions of IAPP have resulted in anorectic effects in rats (121, 122). IAPP can cross the blood-brain barrier, and systemic or local administration of IAPP impact food intake by reducing meal sizes (121, 123-125). Area postrema/nucleus of the solitary tract (AP/NTS) in the brain is an important site for mediating the satiety effect of IAPP, and damage in this area reduces the anorectic effect of administered IAPP in rats. Effect on calcium homeostasis IAPP have been suggested to have a regulatory role in calcium homeostasis, and infusion of IAPP decrease circulating levels of calcium in human subjects (126). IAPP can stimulate proliferation of rat and human osteoblasts and reduce bone resorption by inhibition of osteoclast motility (126-128). In a recent study, a 50% reduction of bone mass were detected in IAPP deficient mice when compared to wild-type littermates and increased bone resorption was determined to be the cause of the low bone mass (129). IAPP is believed to exert its effects on calcium homeostasis through activation of the calcitonin receptor (130).
IAPP receptors High affinity receptors for IAPP are generated from the calcitonin receptor (CTR) and the receptor activity modifying proteins (RAMPs) (131). RAMPs exists in three forms designated RAMP-1, -2 and -3 and they are single trans-membrane proteins with a short intracellular domain and a relative long extracellular domain (132). The CTR exists in several isoforms due to alternative splicing, and the most common are CTR-1 and CTR-2 (133). A combination of CTR-2/RAMP-1 or CTR-2/RAMP-3 generate receptors with high affinity for IAPP when co-expressed in cell lines from monkey, hamster, rabbit and frog but other combinations of CTR/RAMP receptors with high affinity for IAPP have been described (131, 134-136). The CTR-2/RAMP-1 and CTR-2/RAMP-3 IAPP receptors have been identified to occur naturally on murine beta-cells (137).
IAPP degradation The enzyme responsible for intracellular degradation of insulin and IAPP is insulin degrading enzyme (IDE) (138). IDE is a ubiquitously expressed protein with high expression in liver, testes, muscle and brain, and the enzyme has a molecular mass of 110 kDa and is suggested to exist in an active form as a dimer or trimer (139, 140). In the cell, IDE is found in the cytosol, peroxisomes, rough ER, and cell membrane and has also

BACKGROUND
- 23 -
been found in extracellular locations such as the cerebrospinal fluid (139). IDE has highly restricted substrate specificity, but amino acid sequence comparisons reveal no similarity between different IDE substrates. It is believed that the enzyme recognizes elements of secondary or tertiary structure (141). IDE has been shown to degrade several amyloidogenic peptides such as Aβ-peptide, atrial natriuretic peptide (ANP), calcitonin and IAPP and has been suggested to function as a scavenger for amyloidogenic peptides (141). Reduction of IDE activity in cultured rat beta-cells exposed to human IAPP resulted in impaired IAPP degradation, intracellular Congo positive material and increased IAPP induced cytotoxicity (142). IDE is secreted at high levels from microglia cells and has been shown to degrade Aβ peptide extracellularly (143). These findings suggest that IDE has a role in the clearance and prevention of amyloid aggregation. Circulating IAPP is eliminated from plasma by renal excretion (144).
Type 2 diabetes and islet amyloid
Introduction to type 2 diabetes There are two distinct forms of diabetes, type 1 and type 2. Type 1 diabetes usually debuts at an early age and is an autoimmune disease that result in a total loss of beta-cells and insulin production (145). Regular administration of exogenous insulin is essential for survival of type 1 diabetic individuals. Type 2 diabetes is a multifactorial disease related to genetic predisposition, lifestyle and age (146, 147). The disease is characterised by a peripheral insulin resistance in muscle and fat tissue in combination with a large reduction of beta-cells. A high insulin demand will initially be compensated for by an increase in insulin production that will result in beta-cell stress and as the disease progress, beta-cell failure will occur with hyperglycemia as a result. Today, the number of people affected with diabetes world wide is approximately 150-200 million (148-150). Predictions state that the number of affected persons will rise to 366 million by the year 2030, and the word epidemic have been used for the increasing numbers of type 2 diabetic subjects (148). It has been debated whether the escalated numbers of diabetes cases is derived from an increased world population, longer life expectancy and earlier onset of the disease, but, when accounted for, these factors are merely contributing and do not give a complete explanation for the global rise of diabetes (151). More then a

BACKGROUND
- 24 -
billion of the world’s population are overweight and factors such as obesity, food consumption and lack of exercise are important factors closely associated to the development and pathogenesis of type 2 diabetes (152). Type 2 diabetes is a progressive disease and strategies of treatment changes as the course of the disease advance. Early changes in lifestyle with increased physical activity and more healthy dietary habits can improve peripheral insulin resistance and thereby reduce insulin demand (153). Pharmaceutical improvement of peripheral insulin sensitivity can be obtained with thiazolidinediones such as rosiglitazone, or with metformin that in addition to enhanced insulin sensitivity also suppresses hepatic glucose output and has a beta-cell protective feature (154-156). Other strategies are sulfonylurea treatment that increase insulin secretion from beta-cells, and, in late stages of the disease, administration of exogenous insulin is necessary to keep the patient normoglycemic.
Islet amyloid and amyloidogenic sequences Islet amyloid is the only microscopically detectable pathological feature of type 2 diabetes, and islet amyloid depositions have been found in 60-95% of type 2 diabetic individuals at autopsy (59, 157, 158). In non-diabetic age matched individuals, islet amyloid was found in 15% of subjects with less degree of amyloid load. Islet amyloid depositions have also been reported in 50% of patients suffering from insulinomas (159). Monkeys and cats also deposit islet amyloid in parallel to diabetes and insulinomas (160, 161). The residues at position 20-29 of the polypeptide chain have been determined to be the amyloidogenic region of the peptide and proline substitutions in the 24-29 region of rodent IAPP abolish amyloid fibril formation completely (85, 162, 163). Proline is a beta-sheet breaker and when single proline substitutions in the human wild-type 20-29 region was performed, a total inhibition of amyloid formation was seen when proline was substituted at position 22, 24, 26, 27 or 28 (164). Other regions of the IAPP molecule have been proposed to have amyloidogenic properties (30-37 and 8-20), but their significance is difficult to evaluate since they only form amyloid as short synthesized peptides and not when they are in the IAPP molecule (165, 166). A serine to a glycine substitution at position 20 (S20G) of the IAPP molecule have been reported in Asian individuals with early onset of type 2 diabetes, and this mutation has been associated with increased risk of development of the disease (167, 168). The mutant S20G peptide are more fibrillogenic in vitro than wild-type human IAPP (169, 170).

BACKGROUND
- 25 -
Transgenic animal models Rodents do not develop islet amyloid due to the proline substitutions in the IAPP molecule, and several transgenic mice strains expressing human IAPP have been established as models for islet amyloidogenesis. Strategies for generation of transgenic animals have been to express the human IAPP gene driven by the rat insulin I or II promoter or link cDNA for human IAPP to the rat insulin II promoter (171-174). One strain expresses the human IAPP gene driven by the human insulin promoter (175). Studies on the transgenic mouse strains showed that an over-production of IAPP did not result in amyloid formation with the exception of one of the developed strains (171-175). Factors other than over-expression had to be involved in islet amyloidogenesis. Islet amyloid was reported in transgenic mice fed a diet high in fat or treated with growth hormone and dexamethasone (176, 177). Induction of human IAPP in mouse strains with diabetic traits did also result in formation of islet amyloid (178, 179). A transgenic mouse strain expressing human but not murine IAPP (+hIAPP/-mIAPP) was created by crossbreeding with a mIAPP knockout mouse, but islet amyloid was only deposited in male mice fed a diet high in fat (180). Islet amyloid is an uncommon finding in female transgenic mice, but when oophorectomized, islet amyloid occurs and ovarian products are suggested to have a protective role for islet amyloidogenesis (181). IAPP knock-out mice have a normal phenotype and have normal blood glucose and insulin fasting levels but increased insulin response following glucose administration (119). In recent years, transgenic rats expressing human IAPP have been created and islet amyloid deposition together with beta-cell reduction and increase of fasting blood glucose have been reported (182).
Beta-cell dysfunction and death Beta-cell dysfunction is present in type 2 diabetes and is initiated years before clinical symptoms appear. Insulin secretion from beta-cells can be divided into two phases (183, 184). The first phase is a direct response to increased blood glucose where secretory granules in the very proximity of the plasma membrane dock and release their content. This response lasts for approximately 10 minutes. The second phase is more prolonged and continues until the blood glucose is normalized. During this phase, secretory granules are being translocated to the cell membrane from a central pool of secretory granules, and some of these carry newly synthesized insulin (185). The first phase of glucose induced insulin secretion is defective in impaired glucose tolerant (IGT) subjects without fasting hyperglycemia, and both phases are reduced in individuals with

BACKGROUND
- 26 -
type 2 diabetes (186, 187). Also, spontaneous pulses of insulin release occur every 12-15 min in healthy individuals and this pulsatile insulin secretion is impaired in type 2 diabetic individuals, indicating aberrant beta-cell function (188). Studies have shown that increased plasma levels of proinsulin or the processing intermediate des 31,32 proinsulin in initial stages of the disease and an increased demand of insulin that exceed the rate limiting step of the PC´s are possible explanations (189-191). However, an aberrant processing of proinsulin may also be a response to beta cell dysfunction caused by elevated plasma non-esterified fatty acids (NEFA). Dyslipidemia is a hallmark of type 2 diabetes, and chronic exposure of NEFA to rat beta-cells resulted in down regulation of biosynthesis, or reduced auto-catalytic activation of, PC2, PC1/3 and 7B2 leading to elevated secretion of proinsulin (192). Beta-cell mass is adaptive, and a balance between insulin supply and metabolic demand is maintained by adjustments in beta-cell growth and survival (193). An increase in beta-cell mass will compensate for higher insulin demand, and insulin resistance when gaining weight (158). This mechanism eventually fails and type 2 diabetic individuals have a 60% reduction of beta-cell mass compared to health individuals; this reduction is most likely caused by high levels of apoptosis (158). Normal beta-cell replication and neogenesis could not compensate for the cell loss, and islet amyloid was found in more than 80% of diabetic subjects included in this study (158). A study by the same authors performed on obese transgenic mice expressing human IAPP showed an 80% reduction in beta-cell mass in parallel with deposition of islet amyloid and development of diabetes (194). This finding is consistent with the proposal that amyloid aggregation causes beta-cell apoptosis (194). An increase of alpha-cells has been observed in conjunction with beta-cell reduction and may contribute to hyperglucagonaemia and hyperglycaemia (195-197).

BACKGROUND
- 27 -
IAPP and cytotoxicity Apoptosis seems to be the major route for the beta-cell reduction in type 2 diabetes and IAPP fibril formation has been suggested to be the cause of cell death. Cultured beta-cells undergo apoptosis when incubated with human IAPP but not with the non-amyloidogenic murine IAPP, and the cytotoxic effect is ascribed to the formation of ion leaking pores in cell membranes (47, 48, 198). Replicating beta-cells in culture are more sensitive to IAPP fibrillization induced apoptosis than non dividing cells (199). Intracellular apoptosis pathways triggered by IAPP aggregation is not fully understood, but the common downstream protease caspase 3 with upstream activated JNK pathway (c-jun NH2-terminal kinase/stress-activated protein kinase) have been observed in two independent studies (46, 50). Zhang et al. also reported activated caspase 8 and caspase 1 proteases upstream of caspase 3.
Islet fibrillogenesis: initiation and location
Production, secretion and degradation of IAPP occur in healthy individuals without aggregation and deposition of islet amyloid. The amyloid criteria state that amyloid deposits should be extracellularly located but factors or events that trigger initiation of IAPP fibrillization may be of intracellular origin. Here, extra and intracellular mechanisms suggested to initiate fibrillogenesis are described.
Basal membrane components Heparan sulphate proteoglycans (HSPG´s) are important components of extracellular matrix and basement membranes and are present in islet amyloid deposits (200). HSPG´s consist of a protein core with one or several negatively charged glucoseaminoglycans (GAG´s) side chains. The heparan sulphate GAG side chains of the HSPG perlecan are able to enhance IAPP fibril formation in vitro (201). A heparin binding site has been identified at the N-terminus of proIAPP and processing of proIAPP by PC2 will remove this positive charged site (202, 203). This observation suggests that secreted proIAPP could be bound to the proteoglycans present in the basement membranes, giving rise to a local increase of peptide concentration and thereby facilitating amyloid formation. Amyloid is often seen in the peri-vascular area of the islet.

BACKGROUND
- 28 -
Hyperglycemia and islet amyloid Chronic hyperglycemia results in a non-enzymatic glycation of proteins referred to as advanced glycation end-products (AGE) (204). It is most unlikely that a monomeric form of AGE-IAPP exists in vivo, since secreted IAPP has a half-life of approximately 30 minutes, and AGE conversion is associated with low turn-over rate proteins such as haemoglobin and collagen (205). Instead, glycation of deposited islet amyloid might occur and in vitro studies have shown that AGE-IAPP amyloid-like fibrils have a more potent seeding capacity than IAPP amyloid-like fibrils (206). This finding suggests that glycation of islet amyloid may enhance further deposition, but it is not important for initial fibril formation since amyloid deposition also occurs in hypo and normoglycaemic subjects such as patients with insulinoma (60).
Beta-cell granule components In addition to IAPP, the beta-cell granule hosts a wide range of proteins and peptides such as insulin, C-peptide, transthyretin, chromogranin A, chromogranin B, synaptophysin, parathyroid hormone-like peptide, PCs, CPE and many more (64, 207-209). The mature granule has an acidic environment with a pH of 5.2 and high concentrations of Ca2+ and Zn2+ which are required for prohormone processing and insulin crystallisation (210). Mechanisms that protect against the strong amyloidogenicity of IAPP must exist in the secretory granule where IAPP is present at millimolar concentrations. In vitro studies on the beta-cell granule components and their effect on IAPP fibril formation revealed that insulin and proinsulin can act as inhibitors and prevent fibril formation (63, 65, 211). C-peptide, Ca2+ and Zn2+ individually enhanced the formation of fibrils, while the combination of C-peptide and Ca2+ lead to an inhibitory effect (63). These results point to the complexity of the granule environment and highlight the importance to maintain a delicate balance in the secretory granule in order to avoid fibril formation (212, 213). Intra granular fibrils have been recognized in the halo region of beta-cells from cultured islets and transgenic mice (175, 214, 215). Overproduction of IAPP in relation to insulin has also been implicated as a factor for early amyloid formation. Obese patients with IGT had significantly higher fasting levels of IAPP than normal control subjects, while a progression to a diabetic state reduces the level of plasma IAPP (216). Mice fed a long term high fat diet developed glucose intolerance accompanied by an increased beta-cell mass, hyperinsulinemia and a 50% increase in plasma level of IAPP (217). In parallel to these

BACKGROUND
- 29 -
observations, a reduced cellular insulin mRNA expression was detected while mRNA levels of IAPP were unaltered, suggesting a change of intracellular insulin/IAPP ratio during long time exposure to a high fat diet (217).
IAPP fibrillogenesis and non-esterified fatty acids (NEFA) NEFA can act directly on beta-cells through the cell surface receptor GPR-40 and amplify glucose stimulated insulin secretion (218, 219). Type 2 diabetic subjects have elevated NEFA levels in plasma, and a linear correlation with levels of blood glucose is observed (220, 221). Transgenic mice that over-express human IAPP do not spontaneously develop islet amyloid. Instead, amyloid developed after an extensive period on high fat diet (176, 180). Islets from theses transgenic mice were cultured in the presence of different NEFA´s, and formation of intragranular fibrils immunoreactive for IAPP were observed in beta-cells (215). IAPP fibrillogenesis was studied in vitro in presence of NEFA and all investigated NEFA´s catalyzed fibril formation without being incorporated into the fibril itself (215, 222).
Cell membrane components and fibrillogenesis Cellular membrane components and their role in fibrillogenesis are of interest, since the cytotoxicity of human IAPP has been linked to the formation of pore structures in membranes. Phospholipids extracted from pancreas from a diabetic subject enhanced fibrillogenesis of synthetic IAPP in vitro and additional studies demonstrated that negatively charged synthetic liposomes were potent enhancers of IAPP fibril formation (223). The authors suggest that the N-terminal part of human IAPP interacts with negatively charged phospholipids and that the assembly of IAPP oligomers/protofibrils occurs on the cell membrane. The most abundant anionic phospholipid in cell membranes, phosphatidylserine (PS), has in the form of synthetic liposomes been able to accelerate IAPP fibrillogenesis in vitro (224). It is difficult to evaluate these findings since PS is restricted to the cytosolic surface of the plasma membrane of mammalian cells and is only exposed extracellularly during early apoptosis (225).

BACKGROUND
- 30 -
Aberrant processing of proIAPP Aberrant prohormone processing appears in the early stage of type 2 diabetes, as detected by an increased level of proinsulin and the des-31,32 proinsulin processing intermediate in plasma (189, 190). A subsequent change in proIAPP processing is expected since the prohormones are processed by the same convertases and at the same location. Some evidence for this hypothesis was presented by Percy et al. who detected increased plasma levels of IAPP immunoreactive material in individuals with IGT and molecular mass analysis of the IAPP peptides revealed masses corresponding to proIAPP and or proIAPP processing intermediates (68). Prolonged exposure of human beta-cells to high glucose showed increased intracellular proportion of proIAPP and its processing intermediate N-terminal+IAPP (N-IAPP) (213). Islet amyloid deposits consist primarily of fully processed IAPP but immunoreactivity against proIAPP and/or N-IAPP intermediate has been described in islet amyloid deposits from human and mouse (226, 227).

AIM OF THE THESIS
- 31 -
AIM OF THE THESIS The general aim of this thesis was to investigate the occurrence and impact of aberrant processing of human proIAPP on early islet amyloidogenesis. This was performed by:
- Study processing and aggregation of human proIAPP in endocrine cell lines with individual processing properties.
- Investigation of human proIAPP immunoreactivity in intracellular
amyloid-like deposits in human and murine beta-cells.
- Production and characterisation of recombinant proIAPP, proIAPP processing intermediates and IAPP.
- Investigation of the seeding effect of proIAPP amyloid-like fibrils
in vivo and in vitro.
- Development of an assay for real-time detection of beta-cell apoptosis and the study of the cytotoxic effect of proIAPP and the processing intermediates.

AIM OF THE THESIS
- 32 -

MATERIAL AND METHODS
- 33 -
MATERIAL AND METHODS Some key methods and techniques used for this thesis are described.
Immuno-detection (paper I, II and III)
Immunohistochemistry: tissue sections Tissue were fixed in 10% neutral buffered formalin and embedded in paraffin and sections were placed on glass slides (plus slides; Histolab, Gothenburg, Sweden) deparaffinised and rehydrated. Antigen retrieval was performed by placing sections in preheated 0.2M sodium citrate pH 6.0 and left to cool to room temperature (paper I), heat treatment in 121°C for 20 minutes in 10mM citric acid pH 6.0 (paper II) or 1 minute incubation in formic acid (paper III). Primary antibodies used in this thesis are listed below (Table 1). Visualisation of immunoreactivity with horse radish peroxidase (HRP)/diaminobenzidine (DAB) reaction required inactivation of endogenous peroxidase in 0.3% hydrogen peroxidise in TBS (50mM Tris-HCl, 150mM NaCl pH 7.6) for 30 minutes prior to immunolabeling (paper I and II). Visualisation of peroxidase rich erythrocytes was performed by incubation of tissue sections in 0.7mM DAB in TBS, prior to immunolabeling with alkaline phosphatise linked secondary antibodies and Fast red substrate (Sigma-Aldrich, St. Louis, MA, USA) (paper III). Slides were counter stained with Mayer’s hematoxylin, dehydrated and mounted. Table 1. Primary antibodies used for immuno-detection.

MATERIAL AND METHODS
- 34 -
Immunohistochemistry: cultured cells Cells cultured on cover slips were fixed in 2% paraformaldehyde in PBS (137mM NaCl, 2.7mM KCl, 4.3mM Na2HPO4, 1.4mM KH2PO4, pH 7.4) for 30 minutes and incubated with diluted IAPP polyclonal rabbit antibodies in BSS-HEPES buffer (137mM NaCl, 5.36mM KCl, 0.8mM MgSO4*7H20, 0.44mM KH2PO4, 1.4mM NaHPO4, 1% HEPES) with 0.1% saponin at +4°C overnight. Cells were rinsed 3 times in BSS-HEPES-saponin buffer and incubated with Alexa conjugated secondary antibodies (Molecular probes, Eugene, OR, USA) and diluted 1:1000 in BSS-HEPES-saponin for 2 hours at room temperature. Cells were washed 3 times in BSS-HEPES-saponin and 3 times in BSS-HEPES buffer and incubated with 25μg/ml propidium iodide with 250μg/ml RNAse A in BSS-HEPES buffer for 15 minutes for nuclear staining. Cover slips were mounted with 50/50 PBS/glycerol and studied in a confocal microscope.
Immunoelectron microscopy Tissue was fixed in 2% paraformaldehyde with 0.25% glutaraldehyde in PBS and embedded in epon or unicryl (Ladd Research Industries, Burlington, VT, USA). Part of the tissue was post-fixed in OsO4. Ultra-thin sections were placed on formvar coated nickel grids and antigens were retrieved by incubation of sections in sodium periodate (NaIO4) saturated aqueous solution for 10 minutes. Grids were thoroughly washed in deionised water and background staining was blocked in 3% BSA in TBS for 30 minutes followed by overnight incubation with primary antibody diluted in 1% BSA in TBS. In the detection step, sections were incubated with secondary antibodies or protein A conjugated with 10nm colloidal gold particles diluted 1:200 in 1% BSA in TBS for 2 hours. After being rigorously washed, specimens were contrasted with 2% uranyl acetate in 50% ethanol for 10 minutes and in Reynold´s lead citrate (120mM sodium citrate, 25mM lead citrate, pH 12) for 1.5 minutes.

MATERIAL AND METHODS
- 35 -
Production of recombinant peptides (paper I, III and IV)
Recombinant protein production and purification was performed by blunt end insertion of PCR amplified fragments corresponding to cDNA of desired peptides into the multiple cloning site of pGEX 2TK expression vector (GE healthcare, Uppsala, Sweden). The pGEX vector contains a glutathione S-transferase (GST) in front of the multiple cloning site, and peptides are expressed as GST-fusion proteins. High protein yield bacteria Y1090 E. coli were transformed and cultured at +37°C until the OD600 reached 0.8 and protein synthesis was induced with 3mM isopropyl β-D-1 thiogalactopyranoside (IPTG) (Fermentas, St Lenon Rot, Germany) for 3 hours at +25°C. Bacteria were harvested and resuspended in TEDG buffer (50mM Tris-HCl, 1.5mM EDTA, 400mM NaCl, 10% Glycerol, pH 7.4), sonicated and ultra centrifuged at 100000g in a SW41 Ti rotor for 30 minutes at +4°C. The supernatant was transferred to sepharose 4B beads (GE healthcare) and incubated rotating for 2 hours at +4°C. Sepharose beads were washed three times in NET-N buffer (50mM Tris-HCl, 150mM NaCl, 5mM EDTA, 0.5% NONIDET-NP 40, pH 7.4) followed by three times wash in PBS. While still bound to the beads, the GST-tag was enzymatically removed by thrombin protease (GE healthcare), 20U/mg expected peptide, in PBS (paper III and IV) or the fusion protein was recovered by boiling the beads (paper I).
Production and characterisation of monoclonal antibodies
(paper I)
Antigen production A 71 amino acid residue sequence corresponding to position 397-467 of mouse PC1/3 were chosen as immunogen for production of monoclonal PC1/3 antibodies. This sequence has 98.6% and 97.2% identity with corresponding sequences of rat and human PC1/3 respectively. A peptide sequence of 81 residues corresponding to position 376-456 of rat PC2 was selected as immunogen for production of monoclonal PC2 antibodies. This peptide sequence has 98.8% and 97.5% identity with mouse and human PC2 respectively. mRNA was isolated from AtT-20 mouse pituitary cells and GH3 rat pituitary cells and cDNA libraries were made and used for PCR amplification of DNA fragments corresponding to the immunogens were inserted into the pGEX 2TK (GE healthcare) vector and expressed as fusion proteins. Expressed GST-PC1/3 and GST-PC2 were suspended in X2 tricine sample buffer (0.1M Tris-HCl, 25%

MATERIAL AND METHODS
- 36 -
Glycerol, 8 % SDS, 0.2M DTT, 0.02% Coomassie blue G-250), heated and separated by tricine-SDS-polyacrylamide gel electrophoresis as described by Schagger el al (228). Peptide bands corresponding to the theoretical molecular masses of GST-PC1/3 (35 kDa) and GST-PC2 (36 kDa) were excised from the gel and extracted in isotonic sodium chloride solution.
Production of monoclonal antibodies Twelve BALB/c mice (Scanbur-BK, Sollentuna, Sweden) were immunized intraperitoneal with GST-PC1/3 or GST-PC2 peptides mixed 1:1 with Freund´s complete adjuvant (Difco Laboratories, Detroit, MI, USA). For the subsequent immunisations Freund´s incomplete adjuvant was used. The presence of antibodies reactive against islet cells was verified in blood taken from the retro orbital plexa. Three days prior to hybridisation, animals received a booster injection. The spleen was recovered after cervical dislocation and the splenocytes were isolated. Splenocytes were mixed 10:1 with non secreting SP2/0 mouse myeloma cells and fused by addition of 50% polyethylene glycol in PBS (229). Cells were incubated for 2 hours in RPMI medium supplemented with 10% fetal bovine serum (FBS), 1mM sodium pyrovate, 100IU/ml penicillin, 100mg/ml streptomycin and 50µM β-mercaptoethanol at +37 °C in a humidified milieu containing 5% CO2. The cell suspension was seeded into 96-well microtiter plates (Costar, Cambridge, MA, USA) with peritoneal mouse macrophages as feeder-cells. Cells were cultured for two weeks in the presence of HAT supplemented medium (0.1mM hypoxanthine, 0.4μM aminopterin and 16μM thymidine) for selection of hybridomas.
Characterization of antibodies Medium was collected from wells with cell growth and screened for PC1/3 and PC2 antibody producing clones by immunohistochemistry on formalin fixed human pancreas sections. Positive clones were collected, and limited-dilutions were performed twice. Specificity for the antigen and absence of cross reactivity between the PC antibodies were analysed with western blot technique, and the immunoreaction was abolished by absorption with respective peptide. The isotypes of the monoclonal PC1/3 and PC2 antibodies were determined with a mouse monoclonal antibody isotyping test kit (Serotech, Oxford, UK).

MATERIAL AND METHODS
- 37 -
Cell transfection protocols (paper I and IV)
Cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 medium with 11mM D-glucose containing 10% FBS, 100IU/ml penicillin and 100μg/ml streptomycin (Sigma-Aldrich) in humidified air with 5% CO2 at +37°C. The medium of insulin producing Beta-TC-6 (B-TC-6) cells was supplemented with 50µM β-mercaptoethanol. Cells were cultured to 80% confluency on 14mm diameter cover glasses (Menzel GmbH, Braunschweig, Germany). Medium containing 0.4mg/ml G-418 selection antibiotics (GE healthcare) was added to cells 24 hours after transfection.
Calcium-phosphate transfection For 20ml transfection medium, 50μg DNA in 1ml 0.25M CaCl2 was mixed with 1ml HEPES-buffered saline X2 (0.28M NaCl, 0.05M HEPES, 1.5mM NaHPO4, pH 7.05) and left to precipitate for 10 minutes. The precipitate was mixed with 18ml culture medium which was subsequently added to cells. After 5 hours incubation, medium was substituted to 10% glycerol in HEPES-buffered saline, and the cells were incubated for 2 min. After a rinse in PBS, new medium was added to the cells.
DOTAP liposomal transfection Preparation of 20ml transfection medium required 20μg DNA in a 480μl HEPES-buffered saline mixed with 120μl DOTAP (Roche, Basel, Switzerland) followed by 10 minutes of incubation. The DNA-DOTAP solution was added to RPMI medium and rigorously mixed before adding to cells. After 5 hours of incubation, the medium was replaced with new culture medium.
Electroporation Cells were trypsinized and resuspended in PBS and 10μg of DNA was added to a volume of 400μl cell suspension containing 100000 cells and transferred to an electroporation cuvette. Electropermeabilization of the cell membrane was generated with 4 pulses at 0.6kV and 25μF using a gene pulser electroporator (Bio-Rad, Hercules, CA, USA). Cells were transferred to wells with 14mm ∅ cover glasses and culture medium was added after 1 hour of incubation.

MATERIAL AND METHODS
- 38 -
Polyethyleneimine (PEI) transfection One hour prior to transfection, cell culture medium was changed to serum free medium. A solution of 40µg DNA, 10mM PEI (Sigma-Aldrich) and 5% sucrose in a total volume of 175µl was mixed and left to incubate for 10 minutes and then added to 9 ml serum free medium. After 6 hours of incubation, FBS was added to a final concentration of 10%.
Laser scanning confocal microscopy (LSCM) (paper I, III
and IV)
Basic concept of LSCM The concept of confocal microscopy was developed in 1957 by Marvin Minsky, a post-doctoral student at Harvard University (230). His invention remained mainly unnoticed due to the absence of an intensive light source and techniques required for imaging. It was not until 1987 that the first commercial instrument appeared and extensive technical progress has led to the development of the widely used LSCM imaging technique. The advantage of LSCM over fluorescence microscopy is the use of an aperture pinhole which only lets emitted light from one focal plane to pass through and reach a photo detector. This excludes secondary fluorescence in areas apart from the focal plane and results in very sharp images. A powerful light source is essential and lasers are used for the confocal imaging technique. Lasers produce monochromatic light with the advantage of highly parallel beams which can travel a long distance and be focused into a small spot with high intensity. The laser beam is scanned with the help of a dichromatic mirror across the specimen in a raster. If emitted from the focal plane the light from the specimen passes the dichromatic mirror and the pinhole aperture and hits the photo detector (Fig. 3). The detector registers photons and is connected to a computer that generates an image of light intensity for each point of the scanned area. No colour information exists in a confocal image and is solely generated by the computer. Several lasers are usually connected to the microscope for detection of multiple fluorophores. The fluorophores needs to be very resistant to photo bleaching because of the exposure of the specimen to high intensity light. Since a confocal image only shows information from one focal plane, it is possible to virtually slice the specimen by moving it in the y-axis while taking images at different levels, a so called Z-stack. It is possible to create a 3-dimensional volume render of the specimen from the collected information.

MATERIAL AND METHODS
- 39 -
Figure 3. Illustration of confocal microscopy theory. In A, emitted light from the specimen located in the focal plane will pass through the pinhole aperture and hit the photo detector while in B, emitted light is away from the focal plane and can not pass.
LSCM and fluorophores All confocal microscopy studies were performed in a Nikon eclipse E600 microscope connected to a Nikon C1 confocal unit with argon 488 nm, HeNe 543nm and HeNe 633 nm lasers (Nikon, Kawasaki, Japan). Digital images were obtained with an EZ-C1 detector connected to a computer with software version 1.0 for Nikon C1 confocal microscopy. Alexa 488nm and 594nm conjugated to secondary antibodies were used for visualisation of primary antibodies and propidium iodide (543nm) or To-PRO-3 (633nm) (Molecular probes) served as nuclear markers. Congo red dye fluoresces red when bound to amyloid and gave a strong signal when analyzed with the 543nm laser (231). Erythrocytes auto-fluoresce when tissue was investigated with the 488nm laser. This phenomenon was taken advantage of when pancreas sections of transgenic mice were investigated for localization of amyloid deposits (paper III).

- 40 -
Thioflavin T (ThT) assay (paper III and IV)
Kinetic studies of fibril formation were performed in Sigmacote (Sigma-Aldrich) treated 96 well black plates (Thermo Labsystems, Stockholm, Sweden) in a sample volume of 100μl. Synthetic and recombinant peptides were kept in DMSO stock solutions and diluted in assay buffer (50mM glycine, 25mM sodium phosphate buffer, pH 7.0 and 10μM ThT) prior to experiments. Fluorescence was measured at 442 nm excitation and 486 nm emission wavelengths in a Wallac 1420 multilabel counter (Perkin Elmer, Turku, Finland) with WorkOut software version 1.5 (Perkin Elmer).

RESULTS AND DISCUSSION
- 41 -
RESULTS AND DISCUSSION
Intracellular amyloid-like aggregates (paper I and II)
Immunoreactivity for proIAPP has previously been described in islet amyloid, and a more profound investigation of the role for proIAPP processing in the initial phase of fibril formation was performed by expression of human proIAPP in five cell lines with individual processing properties (Table 2). Table 2. Cell line characteristics.
PCR on reverse transcribed mRNA from each cell line was performed for verification of PC1/3 and/or PC2 expression. A housekeeping gene was included in the analysis so a semi-quantification of PC expression was enabled. B-TC-6 cells expressed mRNA for both PC1/3 and PC2 while no expression of the processing enzymes was detected in GH4C1 and COS-7 cells. The level of PC1/3 mRNA in AtT-20 cells was 80% of the expression detected in B-TC-6 cells and no PC2 mRNA was detected in AtT-20 cells. PC1/3 mRNA expression was not detected in GH3 cells while PC2 mRNA levels corresponded to 20% of PC2 expression in B-TC-6 cells. An immunohistochemical analysis of prohormone expression was performed with the previously described monoclonal antibodies. B-TC-6 and AtT-20 cells immunolabeled with antibodies against PC1/3 and B-TC-6 and GH3 cells were confirmed to express PC2. COS-7 and GH4C1 cells did not immunolabel with antibodies against either of the two convertases, in accordance with the mRNA expression analysis. There are some contradictory findings on PC2 expression in GH3 cells, and it has even been implied that subclones of this cell line with different PC2 expression exists (232-234). GH3 cells used in this thesis were purchased from ATCC and PC2 mRNA and protein expression was demonstrated in these cells, though considered to be quite low when compared to B-TC-6 cells.

RESULTS AND DISCUSSION
- 42 -
Plasmid constructs for eukaryotic expression of human preproIAPP and murine preproIAPP were generated in pcDNA 3. The signal peptide is essential for protein expression directed to the regulated secretory pathway and will be removed upon entrance of the ER. Vector preproIAPP expression in transfected cells will be referred to as human proIAPP (hproIAPP) or murine proIAPP (mproIAPP) expression. The transfection efficiency of four different methods was compared and the results are presented below (Table 3). Transfection with PEI was the only procedure where all cell lines were transfected and was therefore used throughout this thesis. Table 3. Efficiency of different transfection methods on five cell lines.
B-TC-6, GH4C1, COS-7, GH3 and AtT-20 cells were transfected with human or murin proIAPP, and expression was verified with antisera raised against murine IAPP 1-37, an antiserum which cross-reacts with human IAPP. Expression of mproIAPP appeared as a granular deposition evenly distributed throughout the cytoplasm in all studied cell lines. No amyloid-like depositions appeared in cells after transfection with mproIAPP. This is in accordance with earlier findings that mouse IAPP is non-amyloidogenic. Expression of hproIAPP in B-TC-6 and GH3 cells also showed a granular pattern, but in some transfected GH3 cells accumulation of large immunoreactive material was present in the cytoplasm. Expression of hproIAPP in GH4C1, COS-7, and AtT-20 cells resulted in the formation of intracellular immunoreactive aggregates and Congo red staining verified the presence of amyloid-like aggregates in GH4C1, COS-7, and AtT-20 cells but not in B-TC-6 and GH3 cells. Processing of hproIAPP was further characterized with antibodies against proIAPP processing sites. These antibodies cover the di-basic PC cleavage site at the N-terminus (AA 169) or the C-terminus (AA 165) and loss of epitope occurs if proIAPP is processed at these specific sites. After immunolabeling of B-TC-6 and GH3 cells with these antibodies, a very weak reaction was observed as a result of proIAPP processing. PC2 were concluded to process the expressed proIAPP both at the N- and C-terminus in GH3 cells since only a weak reactivity with antibodies

RESULTS AND DISCUSSION
- 43 -
against these sites were detected. The ability of PC2 to process proIAPP both N- and C-terminally in the absence of PC1/3 has previously been described (100). The intracellular amyloid-like aggregates in COS-7 and GH4C1 were determined to consist of proIAPP since the aggregates were recognized by antibodies specific for the N- and C-terminal processing sites. No reactivity against the C-terminal cleavage site was detected in AtT-20 cells which were concluded to be a result of PC1/3 processing at this terminus. Intracellular aggregates in AtT-20 cells were reactive against the antiserum specific for the N-terminal cleavage site and determined to consist of N-terminal flanking peptide+IAPP (N+IAPP). Cell viability after transfection with hproIAPP and mproIAPP was monitored for five consecutive days to investigate if the process of intracellular amyloid-like aggregation was cytotoxic. No discrepancy in degree of transfection was observed between the hproIAPP and mproIAPP vectors, but cell death occurred at higher rates in GH4C1, COS-7 and AtT-20 cells transfected with hproIAPP compared to the non-amyloidogenic mproIAPP. Interestingly, viability in GH3 cells transfected with hproIAPP did not decline faster than control cells though IAPP aggregates were found in some of the GH3 cells. However, these deposits were not identified as amyloid-like aggregates by Congo staining. Instead, we regard them as transient formations that result from low PC2 content. B-TC-6 cells were not included in this study since they expresses mproIAPP endogenously. A decrease in cell viability was identified only in those cells with impaired proIAPP processing in conjunction with amyloid-like aggregation. The result of the time study clearly indicates that intracellular fibril formation as a result of aberrant processing is a cytotoxic process. To further investigate the occurrence of intracellular amyloid-like aggregates and their content of IAPP precursor molecules, a morphological and immunohistochemical study of beta-cells from transgenic mice and human transplanted islets were performed. Transgenic +hIAPP/-mIAPP mice were fed a diet of standard chow and lard ad libitum and killed after 11 months. Pieces of pancreas were fixed for light and electron microscopy and studied immunohistochemically. Out of 330 investigated islets, 24 had beta-cells containing intracellular amyloid and the total number of amyloid containing beta-cells within each islet was very low ranging from 1-4. Investigations of islets with the M30 antibody detecting apoptosis together with Congo red staining showed that cells with congophilic intracellular aggregates were also apoptotic. Intracellular fibrillogenesis that triggers apoptosis is probably

RESULTS AND DISCUSSION
- 44 -
a phenomenon that can be detected during a short time period in early islet amyloidogenesis, since the apoptosis process is relative fast. Ultra-structurally, fibrils in the halo region of the secretory granules were observed in beta-cells from mice fed lard ad libitum but not in beta-cells from mice fed standard chow. These fibrillar structures labelled with antibodies raised against processing sites of proIAPP but no reactivity against insulin or C-peptide were detected. Larger intracellular amyloid-like deposits were found in some beta-cells of the transgenic mice. These deposits had a fibrillar appearance and were recognized by antibodies specific for the N- and C-terminal processing sites and an antibody specific for the C-terminus of proIAPP. Reactivity against insulin or C-peptide was not detected within the fibrillar deposits. Intra-cellular amyloid-like material was also apparent in beta-cells of human islets transplanted under the renal capsule of non-diabetic nude mice. These deposits labelled with antibodies reactive against the proIAPP processing sites but to a lesser extent and with a more patch-like appearance than the mouse deposits and no intragranular fibrils were detected in the human beta-cells. From these results we conclude that intracellular IAPP amyloid-like aggregates in beta-cells of mouse and man consists at least partially of proIAPP or proIAPP processing intermediates. Unprocessed promolecules have been described in other endocrine amyloid deposits where proA-type natriuretic peptide (proANP) have been reported in atrial amyloid and procalcitonin in amyloid deposits associated with medullary carcinoma of the thyroid (235, 236) . The presence of proIAPP in intracellular amyloid-like deposits suggests that initiation of islet amyloid occurs at this location. Expression of proIAPP in cell lines possessing different processing properties put emphasis on the importance of processing to prevent aggregation. Absence of proIAPP processing resulted in accumulation of intracellular aggregates and increased cell death. Processing at the C-terminal flanking region was not sufficient to prevent fibrillization since congophilic deposits made up by N+IAPP were found in AtT-20 cells. In my study the N-terminal processing of proIAPP seems to be important for prevention of intracellular aggregation of amyloid-like material. These results were confirmed in a recent published paper where GH3 cells (with low PC2 expression) were virally transduced with human proIAPP and PC1/3 and/or PC2 (232). Results from that study showed that the apoptotic rate of GH3 cells expressing high levels of proIAPP decreased when cells were co-transduced with PC2 but not with PC1/3. This was also demonstrated in cultured islets from a transgenic mouse strain that express human IAPP but lack PC2 (+hAIPP/-PC2) (232). Viral

RESULTS AND DISCUSSION
- 45 -
transduction of these islets with PC2 restored N-terminal processing of proIAPP and amyloid formation and number of apoptotic cells were decreased. My results together with the results from the aforementioned study demonstrates that impaired processing of proIAPP leads to amyloid formation and cell death by accumulation of proIAPP or proIAPP intermediates. Impaired processing of proIAPP has been identified in beta-cells cultured in high glucose levels and in subjects with IGT (68, 213). In our transgenic +hIAPP/-mIAPP mouse strain, a diet high in fat is necessary for development of islet amyloid but the exact mechanism for this observation is not really known (180). One can speculate that fibrillogenesis may be triggered by direct effects on beta cells such as increased receptor-mediated insulin release and reduced activation of the processing enzymes, as well as indirect effects such as peripheral insulin resistance. Islet amyloid deposition was reduced but not abolished in transgenic hIAPP mice given rosiglitazone and metformin, suggesting that mechanisms other than peripheral insulin resistance and thereby reduced beta-cell secretory demand are involved in initiation of IAPP fibrillization. I have studied amyloid deposition in transgenic mice fed a diet high in fat and in human islets transplanted under the renal capsule of nude mice. Intracellular amyloid deposits were found in the mouse model and had reactivity for both N and C-terminal processing sites and the C-terminal flanking peptide of proIAPP. Different sizes of amyloid-like aggregates were observed ranging from the shape and size of a single secretory granule to much larger deposits involving the major part of the cytoplasm. This finding might reflect different stages in the intracellular amyloid forming process suggesting the secretory granule to be the location of initial fibrillogenesis. Fibrils that consist of proIAPP was detected in the halo region of the secretory granules in the +hIAPP/-mIAPP mice and fibril formation in this space is interesting for several reasons. Oligomers are hypothesized to be the cytotoxic species in the amyloid forming process, since it has been shown that exogenous addition of human IAPP induces apoptosis in cultured cells under conditions where it is also able to permeabilize lipid bilayers (49). Amyloid-like fibrils were detected in the halo region of secretory granules, and the surrounding lipid membrane of the vesicles may function as a platform for induction of fibril formation. Studies on cell membrane components in vitro have shown that negatively charged phospholipids accelerate IAPP fibril formation and it has been proposed that IAPP molecules interact with and assemble into protofibrillar structures on, and elongate as fibrils from, the membrane surface (223, 224). It has also been hypothesised that it is the N-terminal of the IAPP

RESULTS AND DISCUSSION
- 46 -
molecule that interacts with cell membrane surfaces (223). If this assumption is correct, the N-terminal of proIAPP would be more favourable for electrostatic interactions with anionic cellular membranes which hold more positively charged amino acid residues such as the processing site of PC2. Irrespectively of which part of the molecule binds to lipid membranes, the pI value of proIAPP is 9.51 while that if IAPP is 8.90, making proIAPP a better candidate for membrane interaction. Pore formation in the secretory vesicle membrane may result in intra granular milieu changes that result in disturbances of PC auto-catalysis and/or reduction of the inhibiting effects of insulin or other granule components, thus further promoting fibril formation. Secretion of insulin and IAPP require that secretory granules dock to and fuse with the beta-cell membrane and if granular membranes contain amyloid pores, extracellular cationic influx may occur. Intragranular proIAPP fibrils might also be secreted from the cell and attach to HSPG in basal membranes and function as seeds for extra cellular amyloid deposition (203). Since the apoptosis marker M30 and Congo red staining was found within the same cell, it is most likely that the large amyloid-like aggregates eventually will be extracellular since the apoptotic beta-cell will be removed by macrophages and amyloid is known to be protease resistant (237, 238).
Extracellular fibrillogenesis (paper III and IV)
Islet amyloid consists largely of fully processed IAPP, but some proIAPP reactivity has been found within the amyloid mass located extracellularly. Therefore, a study of possible seeding mechanisms by proIAPP fibrils was set up. Attempts to express IAPP and proIAPP with systems such as the OmpA signal peptide directing the expressed peptide to the periplasmic space of E. coli has previous failed. Recombinant proIAPP, proIAPP processing intermediates and IAPP were expressed in a GST-fusion system where fibril formation was most likely prevented by steric hindrance of the 27 kDa GST-tag. When the recombinant peptides were enzymatically removed from the GST-tag, aggregates appeared in the solution and were harvested after an overnight incubation and identified as amyloid-like fibrils by Congo red affinity, apple green birefringence and fibrillar ultra-structure. Transgenic +hIAPP/-mIAPP male mice were divided into four different groups and injected in the tail vein with sonicated amyloid-like fibrils from recombinant human IAPP (recIAPP), recombinant human proIAPP (recproIAPP), recombinant human proinsulin fragment C-peptide/A-chain (recC-peptide/A-chain) or NaCl. These mice were fed lard ad libitum and killed after 10 months and

RESULTS AND DISCUSSION
- 47 -
analysed for the occurrence of islet amyloid, islet size and amyloid load. Islet amyloid was detected in 20% of investigated islets from mice injected with recIAPP, in 10% of mice injected with recproIAPP and in 1-2% of those injected with recC-peptide/A-chain or NaCl fibrils. Islet amyloid found in mice injected with recombinant fibrils were localized to the perivascular space and were immunoreactive for IAPP. Labeling with antibodies reactive against both proIAPP processing sites were diffuse and IAPP was concluded to be the major constituent of the investigated islets amyloid. The number of islet affected with amyloid was not only greater in the group of animals receiving IAPP or proIAPP recombinant fibrils, but the amount of deposited amyloid was also greater (Table 4). Table 4. Data from in vivo seeding of islet amyloid deposition
One group of animals (n=6) were injected with amyloid-like fibrils and killed 24 hours after injection. Pancreas, liver, lung, spleen and kidney were analyzed for the presence of fibrils, and none were detected. This clearly demonstrates that the islet amyloid observed in the transgenic mice is endogenous deposits and not the injected amyloid-like fibrils. Seeding with recombinant amyloid-like fibrils in vitro was measured in a ThT assay. Lag phase reduction of IAPP fibril formation was observed with proIAPP fibrils while C-peptide/A-chain fibrils did not affect the rate of IAPP aggregation. These results clearly demonstrate a nidus effect of exogenously administered fibrillar IAPP and, even more importantly, a seeding effect of proIAPP fibrils on IAPP islet amyloid deposition. The sonication step of the fibril infusion liquid is important for generation of large amounts of free fibril ends and also to shatter large aggregates that may obstruct blood vessels. A considerable quantity of the injected fibrils must get entangled by the lung capillary network but obviously a fraction passes and continues through to the circulation where the fibrils are distributed and trapped in capillaries throughout the body. Fibrils trapped in the pancreas capillaries where IAPP levels are high can exert their seeding effects. The seeding phenomenon of endogenously administered amyloid-like fibrils has previously been demonstrated in other animal

RESULTS AND DISCUSSION
- 48 -
models for amyloidosis such as AA amyloidosis, ApoAII and Alzheimer’s (19, 23, 31). Here we have been able to demonstrate that proIAPP amyloid-like fibrils have the ability to seed IAPP fibril formation, and this finding has been verified both in vivo and in vitro. The entire IAPP sequence is located within the proIAPP molecule, and we propose that proIAPP fibrils can act as platforms for aggregation and elongation of IAPP molecules, making this seeding a semi cross-seeding. Very little is known about how the IAPP molecules are arranged in amyloid fibrils but a parallel arrangement of IAPP peptides within the amyloid fibril have been suggested, and position 1 to 8 are not believed to be involved in the fibril (239). A computer calculated model suggests 3 strands containing beta-sheet structure in the IAPP 1-37 molecule (fig 4A.) and either parallel (fig 4B) or anti-parallel interactions between IAPP molecules (240).
Figure 4. Models of IAPP folding and arrangements in amyloid fibrils. Insulin is an amyloidogenic peptide, and repeatedly heating and freezing in an acidic solution are required for fibril formation in vitro (241). In rare occasions, insulin can be iatrogenic deposited at the site of injection in insulin treated patients but the insulin amyloid in humans has no clinical relevance (242). Since injection of recombinant C-peptide/A-chain amyloid-like fibrils did not seed amyloidogenesis in the transgenic mice, insulin fibrils in humans are most unlikely to seed islet amyloid deposition. As reviewed in the introduction, cross-seeding between different amyloidogenic peptides has previously been confirmed and sequence similarity between the two interacting peptides seems to be an important factor. The finding that proIAPP fibrils have a seeding effect on IAPP fibrillogenesis goes well with this idea, since the IAPP sequence is included in proIAPP. A need for sequence similarity for cross seeding to occur can in part explain why a person with one form of amyloidosis does not develop other forms. Deposition of both apolipoprotein A-IV and transthyretin has been found in one subject with senile systemic

RESULTS AND DISCUSSION
- 49 -
amyloidosis, but the amyloid was not co-deposited and concluded to be two different forms of amyloidosis (243, 244). However, undetectable amounts of seeds could exist in the amyloid deposits. Another interesting finding is that Aβ1-40 fibrils seed IAPP fibril formation but IAPP fibrils do not enhance Aβ fibrillogenesis (17). These two peptides share a 50% sequence similarity, and the reason for the one way cross-seeding is not known. The fact that IAPP is more amyloidogenic than Aβ might make IAPP less sensitive to sequence homology or fibril structure. A novel method for real time measurement of beta-cell apoptosis was developed for the investigation and identification of cytotoxicity associated with the fibrillization process of synthetic and recombinant human IAPP. Beta-TC-6 cells were transfected with the pFRET2-DEVD vector which expresses a construct consisting of enhanced cyan fluorescent protein (ECFP) and enhanced yellow fluorescent protein (EYFP) linked by the amino acid residues DEVD (Asp, Glu, Val, Asp). This residue sequence is a specific substrate for caspase-3-like proteases and when ECFP and EYFP are linked by DEVD, fluorescence resonance energy transfer (FRET) will occur. If caspase 3 is activated in transfected cells, DEVD is cleaved, and the two fluorophores disperse and a loss of FRET can be monitored as a decrease of the 535nm /480nm fluorescence ratio. Evaluation of the method was performed with synthetic human IAPP, and the apoptotic agent staurosporine. A FRET reduction caused by the cleavage of the DEVD sequence by caspase-3-like activity was detected in cells incubated with staurosporine but also in cells incubated with seeded synthetic IAPP. Apoptosis is a transient event and the established assay allowed analysis on the same cell population over time. The developed apoptosis assay is highly reproducible, cost effective and easy to handle compared to other apoptosis assays. MTT assay is frequently used in cell studies but is not a proper apoptosis assay since it measures living cells. Assays where Ac-DEVD-AMC is used as substrate are labour intensive and reflect only one time point. It is important to consider factors such as cell density and rate of cell division when dealing with apoptosis assays. In the novel method described above, a ratio level of two fluorophores is measured so the number of cells does not have to be compensated for in the interpretation of the results. A weakness of the FRET assay is the poor medium in which the assay is performed. Further investigations of possible media that prolonged the survival of the beta-cells would be beneficial in allowing measurements over a longer time span.

RESULTS AND DISCUSSION
- 50 -
It has been suggested that proIAPP and proIAPP processing intermediates are cytotoxic and trigger apoptosis when deposited as intracellular amyloid-like aggregates. The apoptotic effect of these peptides was investigated in the FRET assay. Recombinant proIAPP (recproIAPP), N-terminal+IAPP (recN+IAPP), IAPP (recIAPP) and IAPP+C-terminal (recIAPP+C) were expressed and identified as amyloidogenic. A FRET reduction was observed in beta-cells incubated with fibril forming recombinant peptides that correspond to apoptotic rates of 49% for recproIAPP, 46% for recN+IAPP, 72% for recIAPP and 59% for recIAPP+C. No statistically significant differences in cell toxicity between the four recombinant peptides were observed. One research group has previously described production and characterisation of recproIAPP with a trx-his-tag expression system and these authors state that proIAPP is less amyloidogenic and less cytotoxic than IAPP (245). This finding is in contradiction to the results obtained by us where fibrillogenesis of proIAPP or IAPP was equally cytotoxic. Future prospects for this method are to study beta-cell apoptosis in the presence of anti-amyloidogenic or amyloid enhancing factors.

GENERAL DISCUSSION
- 51 -
GENERAL DISCUSSION IAPP is one of the most amyloidogenic peptides known in nature, and islet amyloid deposition of IAPP is a well described pathological characteristic of type 2 diabetes. However, most humans do not develop islet amyloid, and a sustained balance of production, processing and clearance must interplay with protective mechanisms to prevent fibril formation. Islet amyloid is an early event in the pathogenesis of type 2 diabetes and can be detected in transgenic mice and rats before hyperglycemia arises (246). This observation suggests that an increased demand of circulating insulin and thereby an elevated production of proIAPP/IAPP is a key factor for development of islet amyloid. In later stages of the disease, reduced insulin production, beta-cell reduction and hyperglycemia occur in close association with the degree of deposited islet amyloid (179). Islet amyloid appears to be a direct pathogenic factor of type 2 diabetes. The aim of this thesis was to study impaired proteolytic processing of proIAPP and its role in early islet amyloidosis. The intracellular consequence of this process was evaluated in cultured endocrine cell lines and in human and murine +hIAPP/-mIAPP beta-cells. Intracellular amyloid-like deposits were detected and identified as N+IAPP or proIAPP. Subjects with type 2 diabetes have disproportionate high levels of circulating proinsulin reflecting disturbances in the prohormone processing machinery influenced by factors such as hyperglycemia or hyperlipidemia. Our transgenic mice over-express human IAPP but do not develop islet amyloid unless they are fed a diet high in fat. This finding demonstrates that factors in addition to high levels of IAPP are necessary for the development of islet amyloidosis. The presented results suggest that aberrant processing of the precursor IAPP peptide in parallel with over-expression triggers intracellular fibrillogenesis and beta-cell apoptosis. To further investigate a role for proIAPP in islet amyloidogenesis, recombinant peptides of proIAPP, proIAPP processing intermediates and IAPP were produced. These amyloidogenic peptides were excellent tools for the study of seeded fibrillization both in vivo and in vitro and to study cytotoxicity effects on beta-cells. By injection of recproIAPP and recIAPP in fibrillar form, islet amyloid deposition was accelerated immensely in +hIAPP/-mIAPP mice and the load of deposited amyloid were also increased. This acceleration of islet amyloid formation may be described as prion-like mechanistically. The transgenic animals would

GENERAL DISCUSSION
- 52 -
have developed islet amyloid later in life irrespectively of fibril injections, and this can also be said about some humans, since we all have the amyloidogenic sequence of IAPP. For obvious reasons, ways of administration of IAPP or proIAPP fibrils do not exist and no seeding effect was evident for other fibrils such as the proinsulin fragment. Kuru was a form of spongiform encephalopathy affecting tribes in Papua New Guinea and was caused by ingestion of human brain but I doubt that pancreas is a delicacy though it got is name from Greek meaning all meat. These results should instead be seen as a demonstration of what happens when intra-cellular amyloid goes extracellular. I have been able to demonstrate that extracellular amyloid-like fibrils of proIAPP can trigger amyloid deposition of fully processed IAPP further strengthening my hypothesis that aberrant processing of proIAPP is an important factor for initiation and accumulation of islet amyloid. IAPP fibril formation has been shown to trigger apoptosis in cultured cells and the cytotoxic properties of proIAPP and proIAPP intermediates were investigated with a novel assay for real-time detection of beta-cell apoptosis. ProIAPP and its processing intermediates are able to form amyloid-like fibrils and have cell toxic features which were demonstrated by the FRET assay. The oligomerization process of the peptides was identified to trigger beta-cell apoptosis, while mature amyloid-like fibrils from the same peptides did not. These results demonstrate the importance of identifying where the initial fibril formation occurs in order to prevent beta-cell apoptosis. A scenario for initial islet amyloid formation based on results from us and others is proposed. Two routes of events are described which may occur in parallel. Electron micrographs from +hIAPP/-mIAPP male mice will illustrate the sequence of events.
A. An increased demand of insulin and/or a direct effect on the beta-cell results in impaired processing of proIAPP. Fibril formation of proIAPP or N+IAPP processing intermediate takes place intragranularly. Amyloid pores and protofibrils are formed in the secretory granule lipid bilayer that results in a disturbance of the granule milieu and disintegration of its structure.
B. Granules merge and form larger amyloid-like aggregates.
C. The growth of amyloid-like aggregates in the beta-cell triggers
apoptosis and cell debris is removed by macrophages.

GENERAL DISCUSSION
- 53 -
D. The amyloid remains since it is resistant to proteolytic degradation and is now extracellular and can act as nidus for IAPP secreted from surrounding beta-cells.
E. In the second suggested pathway, secretory granule with proIAPP
fibrils in the halo region and/or proIAPP/IAPP pore structures incorporated in the vesicle membrane fuses with the cell membrane to release its content. Now, the cell membrane is permeable for ions and fibrils are in the extracellular space acting as seeds.
Figure 5. Electron micrographs of islet amyloid fibrillization process.

GENERAL DISCUSSION
- 54 -
According to the presented hypothesis, intracellular events that result in formation of intracellular amyloid-like aggregates cause beta-cell death while the extracellular accumulation of islet amyloid occupy islet volume which may disturb normal cellular communication and regeneration. Therapeutic strategies should be aimed at prevention of initial fibrillogenesis and metformin and rosiglitazone have been shown to at least reduce the amount of deposited amyloid and to some degree prevent beta-cell reduction (247). Sulfonylurea increases secretory output of insulin and IAPP and would not be an optimal choice since a risk of further beta-cell stress occurs. Instead, insulin treatment at an early stage of the disease together with a change in diet and increased amount of exercise could be a way to reduce the strain on beta-cells, improve peripheral insulin resistance and prevent islet amyloid formation.

ACKNOWLEDGMENTS
- 55 -
ACKNOWLEDGMENTS The research presented in this thesis was financially supported by: The Swedish Research Council, Swedish Diabetes Association, Foundation in Memory of Lars Hierta, Lions Research Fund against Disease, and Östergötland County Council. I would also like to take the opportunity to thank the human resources behind this thesis, especially:
- Gunilla Westermark my supervisor. Thank you for introducing me to the fascinating field of amyloid and amyloidosis. You have been a great tutor and mentor and it is solely through your great expertise, never ending enthusiasm and good taste in Italian delicacies that made this thesis possible.
- Per Westermark for your great knowledge in the amyloid and
amyloidosis field and for giving me a job by discovering IAPP.
- Sofia Nyström, for being such a smart, fun and considerate co-worker. I have enjoyed your company in the lab and I wish you all the best in the future.
- Sebastian Schultz thank you for all scientific and private discussions
we have had during the last 2 years. Your “fika” has been overwhelming and I especially like your tiramisu. The conference and skiing in Colorado was excellent and I especially remember when we tried the national cuisine at Eric´s. A final advice from someone with experience, be careful when you resolve amyloid, you do not want TFA/HFIP on your fore….head!
- Jana Sponarova for your friendship and help in the lab. It has been
great to se you adapt to Swedish traditions such as picking mushrooms, fishing crayfish and eating “surströmming”. Always keep your guitar close.
- Marie-Louise Eskilsson for all the help and assistance throughout these
years and for keeping up with me when I have wished for tissue sections pronto.
- Gerd Mucciano and Katarzyna Lundmark for joy and company in
the lab.
- Gibbons G. Cornwell III for reviewing this thesis.

ACKNOWLEDGMENTS
- 56 -
- Peter Strålfors and his research group including:
- Nabila Aboulaich for your ultimate friendship and support during good
and bad times. We have shared a lot and it means much to me. I won our bet, though you are only one week behind and I know that your defending will be a piece of cake. Good luck in the states!
- Unn Kugelberg for your friendship, support and good taste in
decoration. I wish you and Fredrik all the best!
- Elisabeth, have you decided for a surname yet? Thank you for being an energy boost to level 12 and keep up the good work!
- Anita Öst, Siri Fagerholm and Anna Danielsson.
- Fredrik Nyström and his research group including: Lilian Sauma,
Niclas Franck and especially Karin Stenkula, we had a lot of fun in New Orleans!
- Mats Söderström and Sven Hammarström with their research groups
including: Tobias Strid, Kristina Loinder, Jesper Svartz for help with theory and practical advice on molecular biology.
- Veronica Brodin for friendship and late night discussions (not always
scientific) and for sharing your room with me.
- Joakim Bergström, Sewai Peng, Stina Enqvist and Annika Persson my colleagues from the Uppsala lab with interesting ideas about amyloid formation and amyloid researchers.
- Peter Gunnarsson for being a very good friend, scientist and gym
partner. A lot of research related frustration has been released in the weight lifting facility at Feelgood.
- Martin Tinnerfelt for your profound friendship, encouragement and
hospitality. Can you believe that we ran the marathon!
- Bengt-Arne Fredriksson for technical assistance with the electron microscopes.
- All project students over the years: Angelica S, Christopher E,
Christian R, Deepti V, Fredrik A, Hanna M, Jenny, Johan J, Karin E, Klara T, Lina J, Linda F, Linda G, Lisa, Magdalena E, Maria F, Marie O, Sofia F, and Ylva.

ACKNOWLEDGMENTS
- 57 -
I would also like to express my sincere gratitude and appreciation to:
- My sister Anna Wallgrund and her husband Marcus Wallgrund, thank you for your support and encouragements during these years.
- Linnéa Johansson, you will always have a special place in my heart
and I wish you all the best in life. - My parents Ann-Christine Paulsson and Göran Paulsson for always
being there for me and supporting me throughout my life. You have been the best of parents and I hope I make you proud.
- David Westin my soul mate and partner.

- 58 -

REFERENCES
- 59 -
REFERENCES 1. Virchow R 1854 Zur Cellulose-Frage. Virchows Arch Pathol Anat 6:416-426 2. Friedreich N, Kekulé A 1859 Zur Amyloidfrage. Virchows Arch Pathol Anat
16:50-65 3. Divry P, Florkin M 1927 Sur les propriétées optiques de lámyloide. CR Soc
Biol 97:1808-1810 4. Puchtler H, Sweat F, Levine M 1962 On the binding of congo red by
amyloid. J. Histochem. Cytochem. 10:355-364 5. Cohen AS, Calkins E 1959 Electron microscopic observations on a fibrous
component in amyloid of diverse origins. Nature 183:1202-3 6. Cohen AS, Shirahama T, Skinner M 1982 Electron Microscopy of amyloid.
Academic Press, New York 7. Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda
S, Masters CL, Merlini G, Saraiva MJ, Sipe JD 2005 Amyloid: toward terminology clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid 12:1-4
8. Costa PP, Figueira AS, Bravo FR 1978 Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A 75:4499-503
9. Benditt EP, Eriksen N, Hermodson MA, Ericsson LH 1971 The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett 19:169-173
10. Glenner GG, Wong CW 1984 Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885-90
11. Westermark P, Wernstedt C, Wilander E, Sletten K 1986 A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun 140:827-31
12. Saraiva MJ 1995 Transthyretin mutations in health and disease. Hum Mutat 5:191-6
13. Uversky VN, Fink AL 2005 Pathways to Amyloid Fibril Formation: Partially Folded Intermediates in the Fibrillation of Natively Unfolded Proteins. WILEY-VCH Verlag GmbH & Co, Weinheim, Germany
14. Rochet JC, Lansbury PT, Jr. 2000 Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 10:60-8
15. Jarrett JT, Lansbury PT, Jr. 1993 Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73:1055-8
16. Padrick SB, Miranker AD 2002 Islet amyloid: phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry 41:4694-703
17. O'Nuallain B, Williams AD, Westermark P, Wetzel R 2004 Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem 279:17490-9
18. Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV, Dobson CM 2004 Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci 13:1933-8

REFERENCES
- 60 -
19. Ranlov P 1967 The adoptive transfer of experimental mouse amyloidosis by intravenous injections of spleen cell extracts from casein-treated syngeneic donor mice. Acta Pathol Microbiol Scand 70:321-35
20. Werdelin O, Ranlov P 1968 Amyloidosis induced in mice by transplantation of casein-sensitized and not-sensitized spleen cells. Acta Pathol Microbiol Scand 72:13-22
21. Johan K, Westermark G, Engstrom U, Gustavsson A, Hultman P, Westermark P 1998 Acceleration of amyloid protein A amyloidosis by amyloid-like synthetic fibrils. Proc Natl Acad Sci U S A 95:2558-63
22. Lundmark K, Westermark GT, Nystrom S, Murphy CL, Solomon A, Westermark P 2002 Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci U S A 99:6979-84
23. Ridley RM, Baker HF, Windle CP, Cummings RM 2005 Very long term studies of the seeding of beta-amyloidosis in primates. J Neural Transm
24. Prusiner SB 1991 Molecular biology of prion diseases. Science 252:1515-22 25. Collinge J, Sidle KC, Meads J, Ironside J, Hill AF 1996 Molecular analysis
of prion strain variation and the aetiology of 'new variant' CJD. Nature 383:685-90
26. Collinge J 2005 Molecular neurology of prion disease. J Neurol Neurosurg Psychiatry 76:906-19
27. Collins S, McLean CA, Masters CL 2001 Gerstmann-Straussler-Scheinker syndrome,fatal familial insomnia, and kuru: a review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci 8:387-97
28. Gajdusek DC, Zigas V 1957 Degenerative disease of the central nervous system in New Guinea; the endemic occurrence of kuru in the native population. N Engl J Med 257:974-8
29. Prusiner SB 1998 Prions. Proc Natl Acad Sci U S A 95:13363-83 30. Higuchi K, Kitagawa K, Naiki H, Hanada K, Hosokawa M, Takeda T
1991 Polymorphism of apolipoprotein A-II (apoA-II) among inbred strains of mice. Relationship between the molecular type of apoA-II and mouse senile amyloidosis. Biochem J 279 (Pt 2):427-33
31. Higuchi K, Kogishi K, Wang J, Chen X, Chiba T, Matsushita T, Hoshii Y, Kawano H, Ishihara T, Yokota T, Hosokawa M 1998 Fibrilization in mouse senile amyloidosis is fibril conformation-dependent. Lab Invest 78:1535-42
32. Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, Li F, Guo Z, Hosokawa M, Mori M, Higuchi K 2001 Transmission of mouse senile amyloidosis. Lab Invest 81:493-9
33. Tagoe CE, French D, Gallo G, Buxbaum JN 2004 Amyloidogenesis is neither accelerated nor enhanced by injections of preformed fibrils in mice transgenic for wild-type human transthyretin: the question of infectivity. Amyloid 11:21-6
34. Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H 1999 Interaction between A beta(1-42) and A beta(1-40) in Alzheimer's beta-amyloid fibril formation in vitro. Biochemistry 38:15514-21
35. Morozova-Roche LA, Zurdo J, Spencer A, Noppe W, Receveur V, Archer DB, Joniau M, Dobson CM 2000 Amyloid fibril formation and seeding by wild-type human lysozyme and its disease-related mutational variants. J Struct Biol 130:339-51

REFERENCES
- 61 -
36. Ganowiak K, Hultman P, Engstrom U, Gustavsson A, Westermark P 1994 Fibrils from synthetic amyloid-related peptides enhance development of experimental AA-amyloidosis in mice. Biochem Biophys Res Commun 199:306-12
37. Lundmark K, Westermark GT, Olsen A, Westermark P 2005 Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc Natl Acad Sci U S A 102:6098-102
38. Fu X, Korenaga T, Fu L, Xing Y, Guo Z, Matsushita T, Hosokawa M, Naiki H, Baba S, Kawata Y, Ikeda S, Ishihara T, Mori M, Higuchi K 2004 Induction of AApoAII amyloidosis by various heterogeneous amyloid fibrils. FEBS Lett 563:179-84
39. Yazaki M, Tokuda T, Nakamura A, Higashikata T, Koyama J, Higuchi K, Harihara Y, Baba S, Kametani F, Ikeda S 2000 Cardiac amyloid in patients with familial amyloid polyneuropathy consists of abundant wild-type transthyretin. Biochem Biophys Res Commun 274:702-6
40. Glabe CG, Kayed R 2006 Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 66:S74-8
41. Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R 2005 Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A 102:10427-32
42. Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG 2003 Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486-9
43. Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT, Jr. 2002 Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418:291
44. Arispe N, Rojas E, Pollard HB 1993 Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A 90:567-71
45. Kagan BL, Azimov R, Azimova R 2004 Amyloid peptide channels. J Membr Biol 202:1-10
46. Zhang S, Liu J, Dragunow M, Cooper GJ 2003 Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem 278:52810-9
47. Lorenzo A, Razzaboni B, Weir GC, Yankner BA 1994 Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368:756-60
48. Mirzabekov TA, Lin MC, Kagan BL 1996 Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem 271:1988-92
49. Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC 1999 The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes 48:491-8
50. Rumora L, Hadzija M, Barisic K, Maysinger D, Grubiic TZ 2002 Amylin-induced cytotoxicity is associated with activation of caspase-3 and MAP kinases. Biol Chem 383:1751-8
51. Kelly JW, Balch WE 2003 Amyloid as a natural product. J Cell Biol 161:461-2
52. Romling U, Bian Z, Hammar M, Sierralta WD, Normark S 1998 Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol 180:722-31

REFERENCES
- 62 -
53. Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ 2002 Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851-5
54. Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW 2006 Functional amyloid formation within mammalian tissue. PLoS Biol 4:e6
55. Kenney JM, Knight D, Wise MJ, Vollrath F 2002 Amyloidogenic nature of spider silk. Eur J Biochem 269:4159-63
56. Opie EL 1901 The relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of the islands of Langerhans. J. Exp. Med.: 527 - 540
57. Westermark P, Wilander E 1983 Islet amyloid in Type 2 (non-insulin-dependent) diabetes is related to insulin. Diabetologia 24:342-6
58. Clark A, Holman RR, Matthews DR, Hockaday TD, Turner RC 1984 Non-uniform distribution of islet amyloid in the pancreas of 'maturity-onset' diabetic patients. Diabetologia 27:527-8
59. Westermark P, Grimelius L 1973 The pancreatic islet cells in insular amyloidosis in human diabetic and non-diabetic adults. Acta Pathol Microbiol Scand [A] 81:291-300
60. Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH 1987 Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A 84:3881-5
61. Westermark P, Wilander E, Westermark GT, Johnson KH 1987 Islet amyloid polypeptide-like immunoreactivity in the islet B cells of type 2 (non-insulin-dependent) diabetic and non-diabetic individuals. Diabetologia 30:887-92
62. Lukinius A, Wilander E, Westermark GT, Engstrom U, Westermark P 1989 Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia 32:240-4
63. Westermark P, Li ZC, Westermark GT, Leckstrom A, Steiner DF 1996 Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett 379:203-6
64. Nishi M, Sanke T, Nagamatsu S, Bell GI, Steiner DF 1990 Islet amyloid polypeptide. A new beta cell secretory product related to islet amyloid deposits. J Biol Chem 265:4173-6
65. Jaikaran ET, Nilsson MR, Clark A 2004 Pancreatic beta-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem J 377:709-16
66. Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA 1990 Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes 39:752-6
67. van Jaarsveld BC, Hackeng WH, Lips CJ, Erkelens DW 1993 Plasma concentrations of islet amyloid polypeptide after glucagon administration in type 2 diabetic patients and non-diabetic subjects. Diabet Med 10:327-30
68. Percy AJ, Trainor DA, Rittenhouse J, Phelps J, Koda JE 1996 Development of sensitive immunoassays to detect amylin and amylin-like peptides in unextracted plasma. Clin Chem 42:576-85
69. Nishi M, Chan SJ, Nagamatsu S, Bell GI, Steiner DF 1989 Conservation of the sequence of islet amyloid polypeptide in five mammals is consistent with its putative role as an islet hormone. Proc Natl Acad Sci U S A 86:5738-42

REFERENCES
- 63 -
70. Betsholtz C, Christmanson L, Engstrom U, Rorsman F, Jordan K, O'Brien TD, Murtaugh M, Johnson KH, Westermark P 1990 Structure of cat islet amyloid polypeptide and identification of amino acid residues of potential significance for islet amyloid formation. Diabetes 39:118-22
71. Jordan K, Murtaugh MP, O'Brien TD, Westermark P, Betsholtz C, Johnson KH 1990 Canine IAPP cDNA sequence provides important clues regarding diabetogenesis and amyloidogenesis in type 2 diabetes. Biochem Biophys Res Commun 169:502-8
72. Nishi M, Bell GI, Steiner DF 1990 Sequence of a cDNA encoding Syrian hamster islet amyloid polypeptide precursor. Nucleic Acids Res 18:6726
73. Johnson KH, Wernstedt C, O'Brien TD, Westermark P 1991 Amyloid in the pancreatic islets of the cougar (Felis concolor) is derived from islet amyloid polypeptide (IAPP). Comp Biochem Physiol B 98:115-9
74. Miyazato M, Nakazato M, Shiomi K, Aburaya J, Kangawa K, Matsuo H, Matsukura S 1992 Molecular forms of islet amyloid polypeptide (IAPP/amylin) in four mammals. Diabetes Res Clin Pract 15:31-6
75. Fan L, Westermark G, Chan SJ, Steiner DF 1994 Altered gene structure and tissue expression of islet amyloid polypeptide in the chicken. Mol Endocrinol 8:713-21
76. Westermark GT, Falkmer S, Steiner DF, Chan SJ, Engstrom U, Westermark P 2002 Islet amyloid polypeptide is expressed in the pancreatic islet parenchyma of the teleostean fish, Myoxocephalus (cottus) scorpius. Comp Biochem Physiol B Biochem Mol Biol 133:119-25
77. Ahren B, Sundler F 1992 Localization of calcitonin gene-related peptide and islet amyloid polypeptide in the rat and mouse pancreas. Cell Tissue Res 269:315-22
78. Mulder H, Ekelund M, Ekblad E, Sundler F 1997 Islet amyloid polypeptide in the gut and pancreas: localization, ontogeny and gut motility effects. Peptides 18:771-83
79. Mulder H, Leckstrom A, Uddman R, Ekblad E, Westermark P, Sundler F 1995 Islet amyloid polypeptide (amylin) is expressed in sensory neurons. J Neurosci 15:7625-32
80. Wilson ME, Kalamaras JA, German MS 2002 Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mech Dev 115:171-6
81. Rindi G, Terenghi G, Westermark G, Westermark P, Moscoso G, Polak JM 1991 Islet amyloid polypeptide in proliferating pancreatic B cells during development, hyperplasia, and neoplasia in humans and mice. Am J Pathol 138:1321-34
82. In 't Veld PA, Zhang F, Madsen OD, Kloppel G 1992 Islet amyloid polypeptide immunoreactivity in the human fetal pancreas. Diabetologia 35:272-6
83. Lukinius A, Korsgren O, Grimelius L, Wilander E 1996 Expression of islet amyloid polypeptide in fetal and adult porcine and human pancreatic islet cells. Endocrinology 137:5319-25
84. Mosselman S, Hoppener JW, Zandberg J, van Mansfeld AD, Geurts van Kessel AH, Lips CJ, Jansz HS 1988 Islet amyloid polypeptide: identification and chromosomal localization of the human gene. FEBS Lett 239:227-32
85. Betsholtz C, Svensson V, Rorsman F, Engstrom U, Westermark GT, Wilander E, Johnson K, Westermark P 1989 Islet amyloid polypeptide

REFERENCES
- 64 -
(IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res 183:484-93
86. Nishi M, Sanke T, Seino S, Eddy RL, Fan YS, Byers MG, Shows TB, Bell GI, Steiner DF 1989 Human islet amyloid polypeptide gene: complete nucleotide sequence, chromosomal localization, and evolutionary history. Mol Endocrinol 3:1775-81
87. Christmanson L, Rorsman F, Stenman G, Westermark P, Betsholtz C 1990 The human islet amyloid polypeptide (IAPP) gene. Organization, chromosomal localization and functional identification of a promoter region. FEBS Lett 267:160-6
88. Macfarlane WM, Shepherd RM, Cosgrove KE, James RF, Dunne MJ, Docherty K 2000 Glucose modulation of insulin mRNA levels is dependent on transcription factor PDX-1 and occurs independently of changes in intracellular Ca2+. Diabetes 49:418-23
89. Macfarlane WM, Campbell SC, Elrick LJ, Oates V, Bermano G, Lindley KJ, Aynsley-Green A, Dunne MJ, James RF, Docherty K 2000 Glucose regulates islet amyloid polypeptide gene transcription in a PDX1- and calcium-dependent manner. J Biol Chem 275:15330-5
90. Carty MD, Lillquist JS, Peshavaria M, Stein R, Soeller WC 1997 Identification of cis- and trans-active factors regulating human islet amyloid polypeptide gene expression in pancreatic beta-cells. J Biol Chem 272:11986-93
91. Muff R, Born W, Lutz TA, Fischer JA 2004 Biological importance of the peptides of the calcitonin family as revealed by disruption and transfer of corresponding genes. Peptides 25:2027-38
92. Petermann JB, Born W, Chang JY, Fischer JA 1987 Identification in the human central nervous system, pituitary, and thyroid of a novel calcitonin gene-related peptide, and partial amino acid sequence in the spinal cord. J Biol Chem 262:542-5
93. Steenbergh PH, Hoppener JW, Zandberg J, Visser A, Lips CJ, Jansz HS 1986 Structure and expression of the human calcitonin/CGRP genes. FEBS Lett 209:97-103
94. Findlay DM, Sexton PM 2004 Calcitonin. Growth Factors 22:217-24 95. Wimalawansa SJ 1997 Amylin, calcitonin gene-related peptide, calcitonin,
and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol 11:167-239 96. Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM 1982 Alternative
RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240-4
97. Brain SD, MacIntyre I, Williams TJ 1986 A second form of human calcitonin gene-related peptide which is a potent vasodilator. Eur J Pharmacol 124:349-52
98. Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF 1988 An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem 263:17243-6
99. Halban PA, Irminger JC 1994 Sorting and processing of secretory proteins. Biochem J 299 (Pt 1):1-18
100. Marzban L, Trigo-Gonzalez G, Zhu X, Rhodes CJ, Halban PA, Steiner DF, Verchere CB 2004 Role of beta-cell prohormone convertase (PC)1/3 in processing of pro-islet amyloid polypeptide. Diabetes 53:141-8

REFERENCES
- 65 -
101. Marzban L, Trigo-Gonzalez G, Verchere CB 2005 Processing of pro-islet amyloid polypeptide in the constitutive and regulated secretory pathways of beta cells. Mol Endocrinol 19:2154-63
102. Shennan KI, Taylor NA, Jermany JL, Matthews G, Docherty K 1995 Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. J Biol Chem 270:1402-7
103. Zhou A, Mains RE 1994 Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem 269:17440-7
104. Jutras I, Seidah NG, Reudelhuber TL, Brechler V 1997 Two activation states of the prohormone convertase PC1 in the secretory pathway. J Biol Chem 272:15184-8
105. Wang J, Xu J, Finnerty J, Furuta M, Steiner DF, Verchere CB 2001 The prohormone convertase enzyme 2 (PC2) is essential for processing pro-islet amyloid polypeptide at the NH2-terminal cleavage site. Diabetes 50:534-9
106. Zhu X, Lindberg I 1995 7B2 facilitates the maturation of proPC2 in neuroendocrine cells and is required for the expression of enzymatic activity. J Cell Biol 129:1641-50
107. Muller L, Zhu X, Lindberg I 1997 Mechanism of the facilitation of PC2 maturation by 7B2: involvement in ProPC2 transport and activation but not folding. J Cell Biol 139:625-38
108. Muller L, Cameron A, Fortenberry Y, Apletalina EV, Lindberg I 2000 Processing and sorting of the prohormone convertase 2 propeptide. J Biol Chem 275:39213-22
109. Marzban L, Soukhatcheva G, Verchere CB 2005 Role of carboxypeptidase E in processing of pro-islet amyloid polypeptide in {beta}-cells. Endocrinology 146:1808-17
110. Milgram SL, Kho ST, Martin GV, Mains RE, Eipper BA 1997 Localization of integral membrane peptidylglycine alpha-amidating monooxygenase in neuroendocrine cells. J Cell Sci 110 (Pt 6):695-706
111. Wang ZL, Bennet WM, Ghatei MA, Byfield PG, Smith DM, Bloom SR 1993 Influence of islet amyloid polypeptide and the 8-37 fragment of islet amyloid polypeptide on insulin release from perifused rat islets. Diabetes 42:330-5
112. Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF 2002 Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A 99:10299-304
113. Smeekens SP, Montag AG, Thomas G, Albiges-Rizo C, Carroll R, Benig M, Phillips LA, Martin S, Ohagi S, Gardner P, et al. 1992 Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci U S A 89:8822-6
114. Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF 1998 Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem 273:3431-7
115. Docherty K, Hutton JC 1983 Carboxypeptidase activity in the insulin secretory granule. FEBS Lett 162:137-41

REFERENCES
- 66 -
116. Silvestre RA, Peiro E, Degano P, Miralles P, Marco J 1990 Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas. Regul Pept 31:23-31
117. Degano P, Silvestre RA, Salas M, Peiro E, Marco J 1993 Amylin inhibits glucose-induced insulin secretion in a dose-dependent manner. Study in the perfused rat pancreas. Regul Pept 43:91-6
118. Akesson B, Panagiotidis G, Westermark P, Lundquist I 2003 Islet amyloid polypeptide inhibits glucagon release and exerts a dual action on insulin release from isolated islets. Regul Pept 111:55-60
119. Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Tornell J, Westermark P, Sundler F, Ahren B, Betsholtz C 1998 Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin). Biochem Biophys Res Commun 250:271-7
120. Wang F, Adrian TE, Westermark GT, Ding X, Gasslander T, Permert J 1999 Islet amyloid polypeptide tonally inhibits beta-, alpha-, and delta-cell secretion in isolated rat pancreatic islets. Am J Physiol 276:E19-24
121. Arnelo U, Blevins JE, Larsson J, Permert J, Westermark P, Reidelberger RD, Adrian TE 1996 Effects of acute and chronic infusion of islet amyloid polypeptide on food intake in rats. Scand J Gastroenterol 31:83-9
122. Arnelo U, Permert J, Adrian TE, Larsson J, Westermark P, Reidelberger RD 1996 Chronic infusion of islet amyloid polypeptide causes anorexia in rats. Am J Physiol 271:R1654-9
123. Chance WT, Balasubramaniam A, Zhang FS, Wimalawansa SJ, Fischer JE 1991 Anorexia following the intrahypothalamic administration of amylin. Brain Res 539:352-4
124. Lutz TA, Del Prete E, Scharrer E 1994 Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol Behav 55:891-5
125. Banks WA, Kastin AJ 1998 Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19:883-9
126. Gilbey SG, Ghatei MA, Bretherton-Watt D, Zaidi M, Jones PM, Perera T, Beacham J, Girgis S, Bloom SR 1991 Islet amyloid polypeptide: production by an osteoblast cell line and possible role as a paracrine regulator of osteoclast function in man. Clin Sci (Lond) 81:803-8
127. Cornish J, Callon KE, Lin CQ, Xiao CL, Mulvey TB, Coy DH, Cooper GJ, Reid IR 1998 Dissociation of the effects of amylin on osteoblast proliferation and bone resorption. Am J Physiol 274:E827-33
128. Alam AS, Moonga BS, Bevis PJ, Huang CL, Zaidi M 1993 Amylin inhibits bone resorption by a direct effect on the motility of rat osteoclasts. Exp Physiol 78:183-96
129. Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G 2004 Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol 164:509-14
130. Christmanson L, Westermark P, Betsholtz C 1994 Islet amyloid polypeptide stimulates cyclic AMP accumulation via the porcine calcitonin receptor. Biochem Biophys Res Commun 205:1226-35
131. Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, Main MJ, Foord SM, Sexton PM 1999 Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 56:235-42

REFERENCES
- 67 -
132. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM 1998 RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333-9
133. Moore EE, Kuestner RE, Stroop SD, Grant FJ, Matthewes SL, Brady CL, Sexton PM, Findlay DM 1995 Functionally different isoforms of the human calcitonin receptor result from alternative splicing of the gene transcript. Mol Endocrinol 9:959-68
134. Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM 2000 Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther 294:61-72
135. Armour SL, Foord S, Kenakin T, Chen WJ 1999 Pharmacological characterization of receptor-activity-modifying proteins (RAMPs) and the human calcitonin receptor. J Pharmacol Toxicol Methods 42:217-24
136. Muff R, Buhlmann N, Fischer JA, Born W 1999 An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology 140:2924-7
137. Martinez A, Kapas S, Miller MJ, Ward Y, Cuttitta F 2000 Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology 141:406-11
138. Bennett RG, Duckworth WC, Hamel FG 2000 Degradation of amylin by insulin-degrading enzyme. J Biol Chem 275:36621-5
139. Qiu WQ, Folstein MF 2006 Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging 27:190-8
140. Duckworth WC, Bennett RG, Hamel FG 1998 Insulin degradation: progress and potential. Endocr Rev 19:608-24
141. Kurochkin IV 2001 Insulin-degrading enzyme: embarking on amyloid destruction. Trends Biochem Sci 26:421-5
142. Bennett RG, Hamel FG, Duckworth WC 2003 An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes 52:2315-20
143. Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ 1998 Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem 273:32730-8
144. Leckstrom A, Bjorklund K, Permert J, Larsson R, Westermark P 1997 Renal elimination of islet amyloid polypeptide. Biochem Biophys Res Commun 239:265-8
145. Gillespie KM 2006 Type 1 diabetes: pathogenesis and prevention. Cmaj 175:165-70
146. DeFronzo RA, Bonadonna RC, Ferrannini E 1992 Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15:318-68
147. Ross SA, Gulve EA, Wang M 2004 Chemistry and biochemistry of type 2 diabetes. Chem Rev 104:1255-82
148. Wild S, Roglic G, Green A, Sicree R, King H 2004 Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047-53

REFERENCES
- 68 -
149. Zimmet P, Alberti KG, Shaw J 2001 Global and societal implications of the diabetes epidemic. Nature 414:782-7
150. Diamond J 2003 The double puzzle of diabetes. Nature 423:599-602 151. Colagiuri S, Borch-Johnsen K, Glumer C, Vistisen D 2005 There really is
an epidemic of type 2 diabetes. Diabetologia 48:1459-63 152. Haslam DW, James WP 2005 Obesity. Lancet 366:1197-209 153. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM,
Walker EA, Nathan DM 2002 Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393-403
154. Tack CJ, Smits P 2006 Thiazolidinedione derivatives in type 2 diabetes mellitus. Neth J Med 64:166-74
155. DeFronzo RA, Barzilai N, Simonson DC 1991 Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab 73:1294-301
156. Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patane G, Boggi U, Mosca F, Piro S, Del Prato S, Marchetti P 2002 Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes 51 Suppl 1:S134-7
157. Clark A, de Koning EJ, Hattersley AT, Hansen BC, Yajnik CS, Poulton J 1995 Pancreatic pathology in non-insulin dependent diabetes (NIDDM). Diabetes Res Clin Pract 28 Suppl:S39-47
158. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102-10
159. O'Brien TD, Butler AE, Roche PC, Johnson KH, Butler PC 1994 Islet amyloid polypeptide in human insulinomas. Evidence for intracellular amyloidogenesis. Diabetes 43:329-36
160. de Koning EJ, Bodkin NL, Hansen BC, Clark A 1993 Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta-cell population. Diabetologia 36:378-84
161. Westermark P, Wernstedt C, O'Brien TD, Hayden DW, Johnson KH 1987 Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol 127:414-7
162. Betsholtz C, Christmansson L, Engstrom U, Rorsman F, Svensson V, Johnson KH, Westermark P 1989 Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett 251:261-4
163. Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C 1990 Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A 87:5036-40
164. Moriarty DF, Raleigh DP 1999 Effects of sequential proline substitutions on amyloid formation by human amylin20-29. Biochemistry 38:1811-8
165. Nilsson MR, Raleigh DP 1999 Analysis of amylin cleavage products provides new insights into the amyloidogenic region of human amylin. J Mol Biol 294:1375-85
166. Jaikaran ET, Higham CE, Serpell LC, Zurdo J, Gross M, Clark A, Fraser PE 2001 Identification of a novel human islet amyloid polypeptide beta-sheet domain and factors influencing fibrillogenesis. J Mol Biol 308:515-25

REFERENCES
- 69 -
167. Sakagashira S, Sanke T, Hanabusa T, Shimomura H, Ohagi S, Kumagaye KY, Nakajima K, Nanjo K 1996 Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes 45:1279-81
168. Seino S 2001 S20G mutation of the amylin gene is associated with Type II diabetes in Japanese. Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus. Diabetologia 44:906-9
169. Ma Z, Westermark GT, Sakagashira S, Sanke T, Gustavsson A, Sakamoto H, Engstrom U, Nanjo K, Westermark P 2001 Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid 8:242-9
170. Sakagashira S, Hiddinga HJ, Tateishi K, Sanke T, Hanabusa T, Nanjo K, Eberhardt NL 2000 S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am J Pathol 157:2101-9
171. Fox N, Schrementi J, Nishi M, Ohagi S, Chan SJ, Heisserman JA, Westermark GT, Leckstrom A, Westermark P, Steiner DF 1993 Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM). FEBS Lett 323:40-4
172. de Koning EJ, Morris ER, Hofhuis FM, Posthuma G, Hoppener JW, Morris JF, Capel PJ, Clark A, Verbeek JS 1994 Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A 91:8467-71
173. D'Alessio DA, Verchere CB, Kahn SE, Hoagland V, Baskin DG, Palmiter RD, Ensinck JW 1994 Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes 43:1457-61
174. Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, Butler PC 1996 Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A 93:7283-8
175. Yagui K, Yamaguchi T, Kanatsuka A, Shimada F, Huang CI, Tokuyama Y, Ohsawa H, Yamamura K, Miyazaki J, Mikata A, et al. 1995 Formation of islet amyloid fibrils in beta-secretory granules of transgenic mice expressing human islet amyloid polypeptide/amylin. Eur J Endocrinol 132:487-96
176. Verchere CB, D'Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, Kahn SE 1996 Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A 93:3492-6
177. Couce M, Kane LA, O'Brien TD, Charlesworth J, Soeller W, McNeish J, Kreutter D, Roche P, Butler PC 1996 Treatment with growth hormone and dexamethasone in mice transgenic for human islet amyloid polypeptide causes islet amyloidosis and beta-cell dysfunction. Diabetes 45:1094-101
178. Soeller WC, Janson J, Hart SE, Parker JC, Carty MD, Stevenson RW, Kreutter DK, Butler PC 1998 Islet amyloid-associated diabetes in obese A(vy)/a mice expressing human islet amyloid polypeptide. Diabetes 47:743-50
179. Hoppener JW, Oosterwijk C, Nieuwenhuis MG, Posthuma G, Thijssen JH, Vroom TM, Ahren B, Lips CJ 1999 Extensive islet amyloid formation is induced by development of Type II diabetes mellitus and contributes to its

REFERENCES
- 70 -
progression: pathogenesis of diabetes in a mouse model. Diabetologia 42:427-34
180. Westermark GT, Gebre-Medhin S, Steiner DF, Westermark P 2000 Islet amyloid development in a mouse strain lacking endogenous islet amyloid polypeptide (IAPP) but expressing human IAPP. Mol Med 6:998-1007
181. Kahn SE, Andrikopoulos S, Verchere CB, Wang F, Hull RL, Vidal J 2000 Oophorectomy promotes islet amyloid formation in a transgenic mouse model of Type II diabetes. Diabetologia 43:1309-12
182. Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC 2004 Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes 53:1509-16
183. Kahn SE 2001 Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86:4047-58
184. Rorsman P, Renstrom E 2003 Insulin granule dynamics in pancreatic beta cells. Diabetologia 46:1029-45
185. Rhodes CJ, Halban PA 1987 Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol 105:145-53
186. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D, Jr. 1984 Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 74:1318-28
187. Porte D, Jr. 1991 Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes 40:166-80
188. O'Rahilly S, Turner RC, Matthews DR 1988 Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med 318:1225-30
189. Porte D, Jr., Kahn SE 1989 Hyperproinsulinemia and amyloid in NIDDM. Clues to etiology of islet beta-cell dysfunction? Diabetes 38:1333-6
190. Kahn SE, Halban PA 1997 Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes 46:1725-32
191. Alarcon C, Leahy JL, Schuppin GT, Rhodes CJ 1995 Increased secretory demand rather than a defect in the proinsulin conversion mechanism causes hyperproinsulinemia in a glucose-infusion rat model of non-insulin-dependent diabetes mellitus. J Clin Invest 95:1032-9
192. Furukawa H, Carroll RJ, Swift HH, Steiner DF 1999 Long-term elevation of free fatty acids leads to delayed processing of proinsulin and prohormone convertases 2 and 3 in the pancreatic beta-cell line MIN6. Diabetes 48:1395-401
193. Rhodes CJ 2005 Type 2 diabetes-a matter of beta-cell life and death? Science 307:380-4
194. Butler AE, Janson J, Soeller WC, Butler PC 2003 Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52:2304-14
195. Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC 1988 Islet amyloid, increased A-

REFERENCES
- 71 -
cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9:151-9
196. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S 2002 Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 45:85-96
197. Dunning BE, Foley JE, Ahren B 2005 Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 48:1700-13
198. Meier JJ, Kayed R, Lin CY, Gurlo T, Haataja L, Jayasinghe S, Langen R, Glabe CC, Butler PC 2006 Inhibition of hIAPP fibril formation does not prevent beta-cell death: Evidence for distinct actions of oligomers and fibrils of hIAPP. Am J Physiol Endocrinol Metab
199. Ritzel RA, Butler PC 2003 Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes 52:1701-8
200. Young ID, Ailles L, Narindrasorasak S, Tan R, Kisilevsky R 1992 Localization of the basement membrane heparan sulfate proteoglycan in islet amyloid deposits in type II diabetes mellitus. Arch Pathol Lab Med 116:951-4
201. Castillo GM, Cummings JA, Yang W, Judge ME, Sheardown MJ, Rimvall K, Hansen JB, Snow AD 1998 Sulfate content and specific glycosaminoglycan backbone of perlecan are critical for perlecan's enhancement of islet amyloid polypeptide (amylin) fibril formation. Diabetes 47:612-20
202. Park K, Verchere CB 2001 Identification of a heparin binding domain in the N-terminal cleavage site of pro-islet amyloid polypeptide. Implications for islet amyloid formation. J Biol Chem 276:16611-6
203. Abedini A, Tracz SM, Cho JH, Raleigh DP 2006 Characterization of the heparin binding site in the N-terminus of human pro-islet amyloid polypeptide: implications for amyloid formation. Biochemistry 45:9228-37
204. Furth AJ 1997 Glycated proteins in diabetes. Br J Biomed Sci 54:192-200 205. Clodi M, Thomaseth K, Pacini G, Hermann K, Kautzky-Willer A,
Waldhusl W, Prager R, Ludvik B 1998 Distribution and kinetics of amylin in humans. Am J Physiol 274:E903-8
206. Kapurniotu A, Bernhagen J, Greenfield N, Al-Abed Y, Teichberg S, Frank RW, Voelter W, Bucala R 1998 Contribution of advanced glycosylation to the amyloidogenicity of islet amyloid polypeptide. Eur J Biochem 251:208-16
207. Asa SL, Henderson J, Goltzman D, Drucker DJ 1990 Parathyroid hormone-like peptide in normal and neoplastic human endocrine tissues. J Clin Endocrinol Metab 71:1112-8
208. Karlsson E 2001 The role of pancreatic chromogranins in islet physiology. Curr Mol Med 1:727-32
209. Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE 1986 Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A 83:3500-4
210. Molinete M, Irminger JC, Tooze SA, Halban PA 2000 Trafficking/sorting and granule biogenesis in the beta-cell. Semin Cell Dev Biol 11:243-51
211. Janciauskiene S, Eriksson S, Carlemalm E, Ahren B 1997 B cell granule peptides affect human islet amyloid polypeptide (IAPP) fibril formation in vitro. Biochem Biophys Res Commun 236:580-5

REFERENCES
- 72 -
212. Gebre-Medhin S, Olofsson C, Mulder H 2000 Islet amyloid polypeptide in the islets of Langerhans: friend or foe? Diabetologia 43:687-95
213. Hou X, Ling Z, Quartier E, Foriers A, Schuit F, Pipeleers D, Van Schravendijk C 1999 Prolonged exposure of pancreatic beta cells to raised glucose concentrations results in increased cellular content of islet amyloid polypeptide precursors. Diabetologia 42:188-94
214. MacArthur DL, de Koning EJ, Verbeek JS, Morris JF, Clark A 1999 Amyloid fibril formation is progressive and correlates with beta-cell secretion in transgenic mouse isolated islets. Diabetologia 42:1219-27
215. Ma Z, Westermark GT 2002 Effects of free fatty acid on polymerization of islet amyloid polypeptide (IAPP) in vitro and on amyloid fibril formation in cultivated isolated islets of transgenic mice overexpressing human IAPP. Mol Med 8:863-8
216. Enoki S, Mitsukawa T, Takemura J, Nakazato M, Aburaya J, Toshimori H, Matsukara S 1992 Plasma islet amyloid polypeptide levels in obesity, impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 15:97-102
217. Mulder H, Martensson H, Sundler F, Ahren B 2000 Differential changes in islet amyloid polypeptide (amylin) and insulin mRNA expression after high-fat diet-induced insulin resistance in C57BL/6J mice. Metabolism 49:1518-22
218. Charles MA, Eschwege E, Thibult N, Claude JR, Warnet JM, Rosselin GE, Girard J, Balkau B 1997 The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia 40:1101-6
219. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M 2003 Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422:173-6
220. Wyne KL 2003 Free fatty acids and type 2 diabetes mellitus. Am J Med 115 Suppl 8A:29S-36S
221. Reaven GM, Chen YD 1988 Role of abnormal free fatty acid metabolism in the development of non-insulin-dependent diabetes mellitus. Am J Med 85:106-12
222. Westermark G Free fatty acids promote amyloid formation without participating in the amyloid fibril. 10th International symposium on amyloidosis, Tours, France, 2004; pp 440-442
223. Knight JD, Miranker AD 2004 Phospholipid catalysis of diabetic amyloid assembly. J Mol Biol 341:1175-87
224. Jayasinghe SA, Langen R 2005 Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry 44:12113-9
225. Vance JE, Steenbergen R 2005 Metabolism and functions of phosphatidylserine. Prog Lipid Res 44:207-34
226. Westermark GT, Steiner DF, Gebre-Medhin S, Engstrom U, Westermark P 2000 Pro islet amyloid polypeptide (ProIAPP) immunoreactivity in the islets of Langerhans. Ups J Med Sci 105:97-106
227. Westermark P, Engstrom U, Westermark GT, Johnson KH, Permerth J, Betsholtz C 1989 Islet amyloid polypeptide (IAPP) and pro-IAPP immunoreactivity in human islets of Langerhans. Diabetes Res Clin Pract 7:219-26

REFERENCES
- 73 -
228. Schagger H, von Jagow G 1987 Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368-79
229. Kohler G, Milstein C 1975 Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-7
230. Paddock SW 2000 Principles and practices of laser scanning confocal microscopy. Mol Biotechnol 16:127-49
231. Puchtler H, Sweat F 1965 Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem 13:693-4
232. Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB 2006 Impaired NH2-Terminal Processing of Human Proislet Amyloid Polypeptide by the Prohormone Convertase PC2 Leads to Amyloid Formation and Cell Death. Diabetes 55:2192-201
233. Beinfeld MC, Wang W 2002 CCK processing by pituitary GH3 cells, human teratocarcinoma cells NT2 and hNT differentiated human neuronal cells evidence for a differentiation-induced change in enzyme expression and pro CCK processing. Life Sci 70:1251-8
234. Kaufmann JE, Irminger JC, Mungall J, Halban PA 1997 Proinsulin conversion in GH3 cells after coexpression of human proinsulin with the endoproteases PC2 and/or PC3. Diabetes 46:978-82
235. Pucci A, Wharton J, Arbustini E, Grasso M, Diegoli M, Needleman P, Vigano M, Polak JM 1991 Atrial amyloid deposits in the failing human heart display both atrial and brain natriuretic peptide-like immunoreactivity. J Pathol 165:235-41
236. Sletten K, Westermark P, Natvig JB 1976 Characterization of amyloid fibril proteins from medullary carcinoma of the thyroid. J Exp Med 143:993-8
237. Badman MK, Pryce RA, Charge SB, Morris JF, Clark A 1998 Fibrillar islet amyloid polypeptide (amylin) is internalised by macrophages but resists proteolytic degradation. Cell Tissue Res 291:285-94
238. de Koning EJ, van den Brand JJ, Mott VL, Charge SB, Hansen BC, Bodkin NL, Morris JF, Clark A 1998 Macrophages and pancreatic islet amyloidosis. Amyloid 5:247-54
239. Jayasinghe SA, Langen R 2004 Identifying structural features of fibrillar islet amyloid polypeptide using site-directed spin labeling. J Biol Chem 279:48420-5
240. Jaikaran ET, Clark A 2001 Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta 1537:179-203
241. Burke MJ, Rougvie MA 1972 Cross- protein structures. I. Insulin fibrils. Biochemistry 11:2435-9
242. Dische FE, Wernstedt C, Westermark GT, Westermark P, Pepys MB, Rennie JA, Gilbey SG, Watkins PJ 1988 Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia 31:158-61
243. Bergstrom J, Murphy C, Eulitz M, Weiss DT, Westermark GT, Solomon A, Westermark P 2001 Codeposition of apolipoprotein A-IV and transthyretin in senile systemic (ATTR) amyloidosis. Biochem Biophys Res Commun 285:903-8
244. Bergstrom J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U, Westermark P 2004 Two different types of amyloid deposits--apolipoprotein

REFERENCES
- 74 -
A-IV and transthyretin--in a patient with systemic amyloidosis. Lab Invest 84:981-8
245. Krampert M, Bernhagen J, Schmucker J, Horn A, Schmauder A, Brunner H, Voelter W, Kapurniotu A 2000 Amyloidogenicity of recombinant human pro-islet amyloid polypeptide (ProIAPP). Chem Biol 7:855-71
246. Hoppener JW, Lips CJ 2006 Role of islet amyloid in type 2 diabetes mellitus. Int J Biochem Cell Biol 38:726-36
247. Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE 2005 Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes 54:2235-44
Related Documents






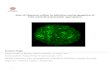

![November 2016 AHS Scottsdale Amylin...11/10/2016 1 Amylin (other names –islet amyloid polypeptide [IAPP], diabetes-associated peptide) Professor Debbie L Hay School of Biological](https://static.cupdf.com/doc/110x72/5fa080ae893c796f1318561d/november-2016-ahs-scottsdale-amylin-11102016-1-amylin-other-names-aislet.jpg)



