PARTICLES PARTICLES Authors: Dr. Bajnóczy Gábor Kiss Bernadett Tonkó Csilla BUDAPEST UNIVERSITY OF TECHNOLOGY AND ECONOMICS DEPARTMENT OF CHEMICAL AND ENVIRONMENTAL PROCESS ENGINEERING FACULTY OF CHEMICAL AND BIOCHEMICAL ENGINEERING

PARTICLES
Mar 18, 2016
BUDAPEST UNIVERSITY OF TECHNOLOGY AND ECONOMICS. DEPARTMENT OF CHEMICAL AND ENVIRONMENTAL PROCESS ENGINEERING. FACULTY OF CHEMICAL AND BIOCHEMICAL ENGINEERING. PARTICLES. Authors : Dr. Bajnóczy Gábor Kiss Bernadett Tonkó Csilla. - PowerPoint PPT Presentation
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

PARTICLPARTICLESES
Authors: Dr. Bajnóczy GáborKiss BernadettTonkó Csilla
BUDAPEST UNIVERSITY OF TECHNOLOGY AND ECONOMICS
DEPARTMENT OF CHEMICAL AND ENVIRONMENTAL PROCESS ENGINEERING
FACULTY OF CHEMICAL AND BIOCHEMICAL ENGINEERING

The pictures and drawings of this presentation can be used only for education !
Any commercial use is prohibited !

ParticlesParticlesProperties: solid and/or liquid state small size varied composition can cause significant environmental damage
Aerosol: solid and/or liquid particles, dispersed in gasSmoke: solid particles, dispersed in gas Fog: liquid drops dispersed in gas
Classification: Alive or lifeless material
alive: bacterium, fungi, spores lifeless: soot/smut, dust, sea salt, etc. (deal with them)
particles origin: Primary: direct in the course of some process, e.g. condensation, erosion Secondary: from gaseous material

Harmful effects of particlesHarmful effects of particles Particles penetrate deeply into the respiratory
system: Bigger than molecule, but not in settling size
range Get deeper than molecule (slow Brownian motion)
Increase the effect of toxic gaseous air pollutants: SO2, NO2 etc. absorb at the particles surface - get deeper
Increasing turbidity of atmosphere Visibility decreasing Warming effect of solar radiation is restricted
Gaseous material → solid particles. NH3 + SO2 → ammonium sulfate

Primary emission (the particles get direct into the atmosphere)
Secondary emission (the particles formed from gaseous state in the atmosphere)
• (1.) The maximum quantity is natural origin from seawater
• seawater surface → bubble → burst → → wind → salt crystals after evaporation
• background aerosol.Bubble burst → salt crystals
Natural source of Natural source of particlesparticles

Natural source of particlesNatural source of particles (2) Volcanic eruption:
variable quantity – sometimes significant amount is emitted into the atmosphere
Volcanic ash: due to small size, long residence time in the atmosphere
(3) Natural source of hydrogen sulphide → sulfuric acid and ammonium → ammonium sulphate particles
(4) soil erosion dust (5) forest fire.
wind

Particles from human Particles from human activitiesactivities
Different industrial processes (stone crushers, grinding, metallurgy, cement and lime production, etc.) and the result of energy generation from coal.
Less, but not negligible: waste and agricultural burning and transportation.

Size of particlesSize of particles
Size range: 0,0002 – 5000 μm Atmosphere pollution: 0,001 – 100 μm.
Size depends on source: >10 μm: usually from mechanical processes (grinding, breaking,
erosion etc.) 10 – 0,1 μm: result of burning process
In atmosphere:two main group1. 10 μm ≥ particles PM10
2. 2,5 μm ≥ particles PM2,5
Another classification:1. Huge particles: diameter > 1 μm2. Large particles: 0,1 = < diameter >
= 1 μm3. Aitken particles: diameter < 0,1 μm

Dangerous range for the lungs
Particle size in micrometer
Particle size in micrometer

Formation of particlesFormation of particles
Soot Carbon-rich (55-80 %), agglomerate of 10-80 nm particles Size: may be higher than 10 μm
Compound: carbon, hydrocarbon, S and N content compounds, microelement
Carbon content compound → soot

Simplified process of soot Simplified process of soot formationformation
Initial process is similar to PAH formation, thus the soot always contains carcinogenic PAH substances.
Soot in flameSoot-C + O → COSoot-C + OH* → CO + HSoot-C + NO → CO + NSoot-C + H2O → CO + H2

Formation of particlesFormation of particles
Ash Origin: coal and biomass firing The fuel does not contain ash. It forms from the ash forming
compounds during the combustion. Coal firing: a./ acidic ash forming: SiO2, Al2O3
b./ basic ash forming: CaO, Fe2O3, MgO
Biomass firing The ash forming materials mainly SiO2 and KCl
The ash forming materials may imbedded in the solid fuel or may be individual, separated particles.

Behavior of ash forming particlesBehavior of ash forming particles Individual inorganic particles quickly melt
and evaporate and will condensate in amorf or crystal form on the cooler part of the boiler (heat exchanger)
Similar, but delayed process takes place with the imbedded inorganic particles.
Metal evaporation from the carbon
containing fuel, due to the metal oxide reduction by carbon at high temperature.
The metal steam is oxidized by the oxygen
content of flue gas forming metal oxide particles mainly in PM2.5 range
The non volatile inorganic matters form
PM10 particles.
potassium chloride crust in heat exchanger – frequent by burning of herbaceous plants

FATE OF PARTICLESFATE OF PARTICLES FROM THE FROM THE ATMOSATMOSPHERPHEREE
All particles deposit on the soil surface. Differences are only in the residence time
in the atmosphere The residence time depends on the particle
size Two possibilities to deposit on the soil
surface Dry deposition Wet precipitation

Dry sedimentation
particle sizeμm
Sedimentation velocitycm/sec
< 0.1 negligible
0,1 8x10-5
1,0 8x10-3
10 0,3
100 25
FATE OF PARTICLES FATE OF PARTICLES FROM THE FROM THE ATMOSATMOSPHERPHEREE
DRY DEPOSITIONDRY DEPOSITION
larger particles will have the opportunity to reach the soil by dry sedimentation
upward air currents counteract the deposition
20 % of the atmospheric particles leave the atmosphere by dry deposition

FATE OF PARTICLES FATE OF PARTICLES FROM THE FROM THE ATMOSATMOSPHERPHEREE
WET PRECIPITATIONWET PRECIPITATION
RAIN OUTWASH OUT
Falling rain or snow collects particles from the atmosphere and carries them to the earth‘s surface
Particles serve as site(nuclei) on which watercondenses or ice form
Particle size > 0.1 μm Particle size < 0.1 μm
These small particles,Aitken particles are sosmall that due to the impact pressure in frontof the falling raindrop,the Aitken particles bypass the drop.
RAIN DROP

EFFECT OF PARTICLES EFFECT OF PARTICLES ON ON PLANTSPLANTS
Reduction of photosynthesis: particles on the leaf surface reduce the irradiation. This effect was significant in the vicinity of former
cement factories. The dust settled on the surface of leaves or fruits
may contain toxic matters. The consumption of these plants by humans or animals might be unhealthy

HEALTH EFFECT OF PARTICLESHEALTH EFFECT OF PARTICLES
Particles, which penetrate the human body: themselves toxic content adsorbed toxic material
Harmful effect – size-dependent
Most of them fix in nose
or deposit on upper trachea Cilia movement→ deposited particles travels up →
pharynx
particle size > 7-10 μm

HEALTH EFFECT OF PARTICLESHEALTH EFFECT OF PARTICLESparticle size in range 0,1- (7-10) μm
Most dangerous particle size range:
Gets down into the alveoli
Settles on the gas exchange surfacedecreasing the oxygen – carbon dioxideexchange
There is no cleaning mechanismin the alveoli
mining disease: silicosisparticle size < 0.1 μm
There is no sedimentation during inhalation and exhalation.

Particle elimination techniquesParticle elimination techniques Taking into account
1. Gas flow parameters: limited opportunities at high temperature and pressure than for atmospheric pressure and below 200 °C
2. Particle parameters: size and material quality
BASED ON EXTERNAL FORCE
BASED ON BARRIERS
cyclon wet dust separator
electrostatic precipitator
decreasing particle size bag filter
gravitational settling chamber
filter-bed with granulates

Efficiency of particle Efficiency of particle separatorsseparatorsEfficiency of elimination
< 1μm 1-3 μm 3-10 μm > 10 μm
Electrostatic precipitator 96 98 99 99,5
Bag dust filter ~100 ~100 ~100 ~100
Venturi-washer >70 99 >99 >99
Multi cyclone 11 54 85 95

Gravitational settling chamberGravitational settling chamber The simplest, but least effective
effective removal: particle size >50 μm used in water purification

CYCLONECYCLONE ~15-30 m/sec gas velocity
centrifugal force is applied
several g/m3 particle content can be decreased under 0,1 g/m3
Advantage: Wide temperature range, even T >1000 °C. High efficiency (η=95%) and cheap
Disadvantage: particle size < 10 μm, efficiency decreases drastically
Better efficiency: more cyclone serial or parallel connection (multi cyclone)
particles
purified gas
dusty gas

ELECTROSTATIC PRECIPITATORELECTROSTATIC PRECIPITATOR Flue gas cleaning method of plants using solid fossil fuels.
Advantage: simple structure without moving parts good efficiency low electric energy demand efficiency: 0,2-0,5 μm particles : 97-98% particles size >2 μm : >98% removal efficiency is influenced by temperature (preferred
interval: 120 – 200 °C) and the specific resistance of particle

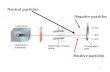
Theory of electrostatic Theory of electrostatic precipitatorprecipitator
Ionization voltage < voltage gradient < breakdown voltage

ELECTROSTATIC PRECIPITATORELECTROSTATIC PRECIPITATOR
spec
ific
elec
tric
resi
stan
ce [o
hm*c
m]
105 -105 -
105 -
1010 -
no charge upexcess of
CaO, MgO, SiO2, Al2O3
quick charge lossexcess of Fe2O3, Na2O, H2O
BEST RANGE

Bag dust filterBag dust filter The best efficiency dust separator
(> 1μm particles: ~99%) Application: 120°C – 200°C Filter cake thickening results in better efficiency
Advantage: Efficiency of elimination doesn’t
depend on the electrical properties of particles.
Due to the adsorption on filter cake elimination of gaseous pollutants: e.g. dioxins.
Disadvantage: Filter cake thickening → filter
resistance increases → batch filtration technology
elimination of filter cake with shake or back pressure
Related Documents