Novel lanthanide luminescent materials based on multifunctional complexes of 2-sulfanylpyridine-3-carboxylic acid and silica/titania hosts† Lei Guo, a Lianshe Fu, b Rute A. S. Ferreira, b Luis D. Carlos, b Qiuping Li a and Bing Yan * a Received 21st May 2011, Accepted 28th July 2011 DOI: 10.1039/c1jm12264a Three different types of organic–inorganic hybrid materials formed by trivalent lanthanide (Ln 3+ ¼ Eu 3+ , Tb 3+ ) complexes covalently grafted to silica-, titania-, or silica/titania-based hosts have been prepared and fully characterized. Since the organic ligand 2-sulfanylpyridine-3-carboxylic acid (SPC), a derivative of nicotinic acid, exhibits three potential binding sites (pyridine N, sulfhydryl S and carboxylic O), the multifunctional precursor can be prepared through the reaction of the carboxylic group with titanium alkoxide and the modification of the sulfhydryl group with silane crosslinking reagents. Thus, the organic–inorganic hybrid materials covalently grafted with Eu 3+ or Tb 3+ complexes are synthesized through coordination of the Ln 3+ ions with the heterocyclic group in the multifunctional precursor during the sol–gel process. The obtained hybrid materials were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetric analysis (TG), Fourier transform infrared (FTIR) spectroscopy, and photoluminescence (PL). The detailed PL studies showed that, compared with the titania-based hybrid materials (denoted as Ln–SPC–Ti), the silica- and silica/titania-based hybrid materials (denoted as Ln– SPCSi and Ln–SPCSi–Ti, respectively) exhibited higher luminescence intensity and emission quantum efficiency. 1. Introduction Trivalent lanthanide (Ln 3+ ) complexes are well-known molecular luminescent materials, which are characterized by long-lived excited-states and efficient narrow-width emission bands in the visible and near infrared (NIR) regions. 1 This is mainly because the effective intramolecular energy transfer from the coordinated ligands to the luminescent central Ln 3+ ions, which in turn undergoes the corresponding radiative emitting process (the so- called ‘‘antenna effect’’). 2 Therefore, they are expected to be promising luminescent dopants for the preparation of organic– inorganic hybrids with potential applications as phosphors, in solid-state lighting, in integrated optics and optical telecommu- nications, in solar cells, and in biomedicine. In recent years, there has been a strong interest in Ln 3+ -containing organic–inorganic hybrid materials. 3 In these materials, the Ln 3+ complexes are entrapped in sol–gel-derived hosts, or alternatively, an inorganic Ln 3+ compound (like a polyoxometalate complex or an Ln 3+ -doped nanoparticle) is embedded in an organic polymer matrix. In general, these hybrid materials have superior mechanical properties and have better process abilities than the pure Ln 3+ complexes. Moreover, embedding an Ln 3+ complex in a hybrid matrix is also beneficial for its thermal stability and luminescence output. 4 Among the innumerous examples reported in the literature, hybrid materials formed through the grafting of Ln 3+ complexes with b-diketones, aromatic carboxylic acids, and heterocyclic ligands to the inorganic backbone (essentially a siloxane-based skeleton) via a covalent bond have earned significant interest. 5 The extension of the concept to other metal oxides or mixed- metal oxides would then allow new interesting options for the development of innovative Ln 3+ -containing organic–inorganic hybrid materials. Titania is an essential functional material because of its peculiar and fascinating physicochemical proper- ties and a wide variety of potential use in diverse fields, including solar-cells, energy conversion, environmental purification, and photocatalysis. 6 Thus, it would be highly attractive to incorpo- rate Ln 3+ complexes into titania- or silica/titania-based hosts and to investigate their luminescence properties, comparing with those of the analogous silica-based hybrids. Although lanthanide luminescent complexes have been in situ synthesized in titania- based host or were adsorbed on silica/titania-based host, 7 only weak interactions between inorganic and organic parts exist in these hybrid materials and it is difficult to prevent clustering of emitting centers and inhomogeneous dispersion of two phases. 8 a Department of Chemistry, Tongji University, Shanghai, 200092, P. R. China. E-mail: [email protected] b Department of Physics, CICECO, University of Aveiro, 3810-193 Aveiro, Portugal † Electronic supplementary information (ESI) available: Fig. S1: XRD patterns of the (A) Eu–SPC–Ti, (B) Eu–SPCSi-1, and (C) Eu–SPCSi-1–Ti hybrid materials. Fig. S2: UV-visible diffuse reflection absorption spectra of Tb 3+ -containing hybrid materials. See DOI: 10.1039/c1jm12264a 15600 | J. Mater. Chem., 2011, 21, 15600–15607 This journal is ª The Royal Society of Chemistry 2011 Dynamic Article Links C < Journal of Materials Chemistry Cite this: J. Mater. Chem., 2011, 21, 15600 www.rsc.org/materials PAPER Downloaded by Universidade de Aveiro (UAveiro) on 31 October 2012 Published on 26 August 2011 on http://pubs.rsc.org | doi:10.1039/C1JM12264A View Online / Journal Homepage / Table of Contents for this issue

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Dynamic Article LinksC<Journal ofMaterials Chemistry
Cite this: J. Mater. Chem., 2011, 21, 15600
www.rsc.org/materials PAPER
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online / Journal Homepage / Table of Contents for this issue
Novel lanthanide luminescent materials based on multifunctional complexes of2-sulfanylpyridine-3-carboxylic acid and silica/titania hosts†
Lei Guo,a Lianshe Fu,b Rute A. S. Ferreira,b Luis D. Carlos,b Qiuping Lia and Bing Yan*a
Received 21st May 2011, Accepted 28th July 2011
DOI: 10.1039/c1jm12264a
Three different types of organic–inorganic hybrid materials formed by trivalent lanthanide (Ln3+ ¼Eu3+, Tb3+) complexes covalently grafted to silica-, titania-, or silica/titania-based hosts have been
prepared and fully characterized. Since the organic ligand 2-sulfanylpyridine-3-carboxylic acid (SPC),
a derivative of nicotinic acid, exhibits three potential binding sites (pyridine N, sulfhydryl S and
carboxylic O), the multifunctional precursor can be prepared through the reaction of the carboxylic
group with titanium alkoxide and the modification of the sulfhydryl group with silane crosslinking
reagents. Thus, the organic–inorganic hybrid materials covalently grafted with Eu3+ or Tb3+ complexes
are synthesized through coordination of the Ln3+ ions with the heterocyclic group in the
multifunctional precursor during the sol–gel process. The obtained hybrid materials were characterized
by X-ray diffraction (XRD), scanning electron microscopy (SEM), differential scanning calorimetry
(DSC), thermogravimetric analysis (TG), Fourier transform infrared (FTIR) spectroscopy, and
photoluminescence (PL). The detailed PL studies showed that, compared with the titania-based hybrid
materials (denoted as Ln–SPC–Ti), the silica- and silica/titania-based hybrid materials (denoted as Ln–
SPCSi and Ln–SPCSi–Ti, respectively) exhibited higher luminescence intensity and emission quantum
efficiency.
1. Introduction
Trivalent lanthanide (Ln3+) complexes are well-known molecular
luminescent materials, which are characterized by long-lived
excited-states and efficient narrow-width emission bands in the
visible and near infrared (NIR) regions.1 This is mainly because
the effective intramolecular energy transfer from the coordinated
ligands to the luminescent central Ln3+ ions, which in turn
undergoes the corresponding radiative emitting process (the so-
called ‘‘antenna effect’’).2 Therefore, they are expected to be
promising luminescent dopants for the preparation of organic–
inorganic hybrids with potential applications as phosphors, in
solid-state lighting, in integrated optics and optical telecommu-
nications, in solar cells, and in biomedicine. In recent years, there
has been a strong interest in Ln3+-containing organic–inorganic
hybrid materials.3 In these materials, the Ln3+ complexes are
entrapped in sol–gel-derived hosts, or alternatively, an
inorganic Ln3+ compound (like a polyoxometalate complex or an
aDepartment of Chemistry, Tongji University, Shanghai, 200092, P. R.China. E-mail: [email protected] of Physics, CICECO, University of Aveiro, 3810-193 Aveiro,Portugal
† Electronic supplementary information (ESI) available: Fig. S1: XRDpatterns of the (A) Eu–SPC–Ti, (B) Eu–SPCSi-1, and (C)Eu–SPCSi-1–Ti hybrid materials. Fig. S2: UV-visible diffuse reflectionabsorption spectra of Tb3+-containing hybrid materials. See DOI:10.1039/c1jm12264a
15600 | J. Mater. Chem., 2011, 21, 15600–15607
Ln3+-doped nanoparticle) is embedded in an organic polymer
matrix. In general, these hybrid materials have superior
mechanical properties and have better process abilities than the
pure Ln3+ complexes. Moreover, embedding an Ln3+ complex in
a hybrid matrix is also beneficial for its thermal stability and
luminescence output.4
Among the innumerous examples reported in the literature,
hybrid materials formed through the grafting of Ln3+ complexes
with b-diketones, aromatic carboxylic acids, and heterocyclic
ligands to the inorganic backbone (essentially a siloxane-based
skeleton) via a covalent bond have earned significant interest.5
The extension of the concept to other metal oxides or mixed-
metal oxides would then allow new interesting options for the
development of innovative Ln3+-containing organic–inorganic
hybrid materials. Titania is an essential functional material
because of its peculiar and fascinating physicochemical proper-
ties and a wide variety of potential use in diverse fields, including
solar-cells, energy conversion, environmental purification, and
photocatalysis.6 Thus, it would be highly attractive to incorpo-
rate Ln3+ complexes into titania- or silica/titania-based hosts and
to investigate their luminescence properties, comparing with
those of the analogous silica-based hybrids. Although lanthanide
luminescent complexes have been in situ synthesized in titania-
based host or were adsorbed on silica/titania-based host,7 only
weak interactions between inorganic and organic parts exist in
these hybrid materials and it is difficult to prevent clustering of
emitting centers and inhomogeneous dispersion of two phases.8
This journal is ª The Royal Society of Chemistry 2011
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
In general, the key to ‘‘design’’ the covalently bonded hybrid
materials is to tailor a functional bridge molecule (ligand) by the
alkylation reaction, which can have the double functions of
coordinating to Ln3+ ions and of allowing the sol–gel process to
build up the covalent Si–O network.9 However, Ln3+ complexes
cannot be linked to the titanium centres by a hydrocarbon spacer
like silicon due to the hydrolytic cleavage of Ti–C bonds.10
Therefore, a new strategy has to be developed for tethering Ln3+
complexes to titania materials. As we know, the reaction with
bidentate ligands is a common way for modifying the metal
alkoxides through sol–gel process, and carboxylates are mainly
used for this purpose, which can react with metal alkoxides to
form surface-functionalized metal oxo clusters.11
In the present work, the organic ligand 2-sulfanylpyridine-3-
carboxylic acid (SPC) was selected to construct the linkage
between inorganic matrix and Ln3+ ions. As we know, aromatic
carboxylic acids and heterocyclic ligands are important organic
ligands for lanthanide ions. SPC, a derivative of nicotinic acid,
exhibited three potential binding sites—pyridine N, thiol S, and
carboxylicO. It can reactwith titaniumalkoxide via the carboxylic
acid group, while the heterocyclic group can coordinate to Ln3+
ions, as well as sensitize their luminescence. Ln3+ complexes can
thus be anchored to the framework of organic–inorganic titania
materials. In addition, we can furthermodify the sulfhydryl group
of ligand SPCAwith the silane crosslinking reagents. On the basis
of the above work, we can further modify the sulfhydryl group of
ligand SPC with the two typical kinds of silane crosslinking
reagents (e.g. 3-aminopropyltrimethoxysilane, APTMS and
3-chloropropyltrimethoxysilane, CPTMS), which can introduce
the inorganic silica into hybrid materials with covalent bonding.
Then the Ln3+ luminescent organic–inorganic hybrid materials
with silica/titania-based host could be obtained, structurally
characterized and their luminescence properties studied in detail.
In addition, for comparison, the covalently bonded silica-based
hybrid materials were also synthesized.
2. Experimental section
2.1. Materials
Tetraisopropyl titanate (Ti(OCH(CH3)2)4), SPC, APTMS,
CPTMS and tetraethoxysilane (TEOS) were purchased from
Aldrich and used without further purification. Ln(NO3)3$6H2O
was obtained from their corresponding oxides in dilute nitric
acid.
2.2. Measurements
Fourier transform infrared (FTIR) spectra were recorded on
a Nicolet model 5SXC spectrometer in 4000–400 cm�1 region
using KBr disk. 29Si magic-angle spinning (MAS) NMR spectra
were obtained from a Bruker Avance 400 (9.4 T) spectrometer at
79.49 MHz. The X-ray diffraction (XRD) measurements were
carried out using powder samples in a BRUKER D8 diffrac-
tometer (40 mA/40 kV), using monochromated CuKa1 radiation
(l¼ 1.54�A) over the 2q range of 10� to 70�. Differential scanning
calorimetry (DSC) and thermogravimetric analysis (TG) were
performed on a NETZSCH STA 449C at a heating rate of 15 �Cmin�1 under a nitrogen atmosphere. Scanning electronic micro-
scope (SEM) images were obtained with a Philips XL-30. The
This journal is ª The Royal Society of Chemistry 2011
ultraviolet-visible diffuse reflection spectra of the powder
samples were recorded by a BWS003 spectrophotometer. The
photoluminescence spectra were recorded at room temperature
with a modular double grating excitation spectrofluorimeter with
a TRIAX 320 emission monochromator (Fluorolog-3�2-TRIAX, Horiba Scientific) with a reciprocal linear dispersion
density of 2.64 nm mm�1 coupled with a R928 Hamamatsu
photomultiplier, using the front face acquisition mode. The
emission slits were fixed at 0.3 mm, enabling a resolution of
0.7 nm in the emission spectra. The excitation source was
a 450 W Xe arc lamp. The emission spectra were corrected for
detection and optical spectral response of the spectrofluorimeter,
and the excitation spectra were corrected for the spectral distri-
bution of the lamp intensity using a photodiode reference
detector. The lifetime measurements were performed at room
temperature with the setup described for the luminescence
spectra using a pulsed Xe–Hg lamp (6 ms pulse at half width and
20–30 ms tail). The absolute emission quantum yields were
measured at room temperature using a quantum yield measure-
ment system C9920-02 from Hamamatsu with a 150 W Xenon
lamp coupled to a monochromator for wavelength discrimina-
tion, an integrating sphere as sample chamber and a multi
channel analyzer for signal detection. Three measurements were
made for each sample so that the average value is reported. The
method is accurate to within 10%.
2.3. Synthesis of hybrid materials Ln–SPC–Ti (Ln3+ ¼ Eu3+
and Tb3+)
A sample was prepared by adding 2 mmol (0.5685 g) of Ti(OCH
(CH3)2)4 to 50 mL of ethanol containing 2 mmol (0.3105 g) of
SPC under refluxing and stirring. The mixture was refluxed at
70 �C for 3 h. 0.5 mmol of Ln(NO3)3$6H2O (Ln3+ ¼ Eu3+ and
Tb3+) dissolved in ethanol was added dropwise into the clear
solution and 0.1 mL H2O was then added. The stirring was
continued for another 24 h to yield a precipitate, which was
recovered by centrifugation and dried for 24 h at 65 �C under
vacuum. The hybrid materials were denoted as Ln–SPC–Ti.
2.4. Synthesis of hybrid materials Ln–SPCSi (Ln3+ ¼ Eu3+ and
Tb3+)
The SPC ligand was modified by two different silane crosslinking
reagents, APTMS and CPTMS. The two organosilane precur-
sors were prepared by using a modified procedure based on the
methods described in ref. 12 and 13
Modified by 3-aminopropyltrimethoxysilane. 2 mmol (0.3105 g)
of SPC was first dissolved in dehydrate tetrahydrofuran (THF)
(20 mL) and then 2 mmol (0.3585 g) of APTMS was added
dropwise to the solution with stirring at 80 �C. The whole
mixture was refluxed at 80 �C for 24 h. The solution was
condensed to evaporate the solvent and then the residue was
dried on a vacuum line under argon atmosphere. A yellow oil
product denoted as SPCSi-1 was obtained.
Modified by 3-chloropropyltrimethoxysilane. 2 mmol (0.3105 g)
of SPC was first dissolved in THF (20 mL) and then 2 mmol
(0.3974 g) of CPTMS was added dropwise to the solution. An
J. Mater. Chem., 2011, 21, 15600–15607 | 15601
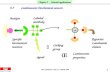
Scheme 1 Synthetic procedure and possible structure of hybrid mate-
rials Ln–SPCSi–Ti.
Fig. 1 FTIR spectra of the (A) Eu–SPC–Ti, (B) Eu–SPCSi-1, (C) Eu–
SPCSi-2, (D) Eu–SPCSi-1–Ti, and (E) Eu–SPCSi-2–Ti hybrid materials.
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
appropriate amount of K2CO3 was added as catalyst. The whole
mixture was refluxed at 80 �C for 3 h. After filtration, the solu-
tion was then condensed to evaporate the solvent. The residue
was dried on a vacuum line, and the resulting yellow oil was
denoted as SPCSi-2.
Synthesis of the hybrid materials Ln–SPCSi-1 and Ln–SPCSi-
2. The organosilane precursor, SPCSi-1 or SPCSi-2, was dis-
solved in N, N-dimethyl formamide solvent with stirring. Then,
a stoichiometric amount of the Ln(NO3)3$6H2O (Ln3+ ¼ Eu3+
and Tb3+) was added. After 3 h, TEOS and one drop of diluted
hydrochloric acid were added into the solution to promote the
hydrolysis and polycondensation reaction. The molar ratio of Ln
(NO3)3$6H2O : precursor : TEOS : H2O was 1 : 3 : 6 : 24. The
mixture was agitated magnetically to achieve a single phase
solution after two days, and then it was aged at 65 �C until the
onset of gelation in about one week. The gels were collected and
were ground as powders for the photophysical studies. The
resulting hybrid materials, denoted as Ln–SPCSi-1 and Ln–
SPCSi-2 (Ln3+ ¼ Eu3+ and Tb3+), were grounded as powders for
the photophysical studies.
2.5. Synthesis of hybrid materials Ln–SPCSi–Ti (Ln3+ ¼ Eu3+
and Tb3+)
The organosilane precursor, SPCSi-1 or SPCSi-2, was dissolved
in ethanol with stirring and an equimolar amount of Ti(OCH
(CH3)2)4 was added into the solution. The mixture was refluxed
at 70 �C for 3 h. Then, the Ln(NO3)3$6H2O (Ln3+ ¼ Eu3+ and
Tb3+) in ethanol was added dropwise into the clear solution and
0.1 mL H2O was added. The stirring was continued for another
24 h to yield a precipitate, which was recovered by centrifugation
and dried for 24 h at 65 �C under vacuum. The hybrid materials
were denoted as Ln–SPCSi-1–Ti and Ln–SPCSi-2–Ti (Ln3+ ¼Eu3+ and Tb3+). The general synthetic procedure and the possible
structures of the hybrids are displayed in Scheme 1.
3. Results and discussion
3.1. Structural characterization
Fig. 1 shows the FTIR spectra of obtained Eu3+-containing
hybrid materials in the 4000–400 cm�1 range. In the spectrum of
Eu–SPC–Ti (Fig. 1A), the typical antisymmetric and symmetric
stretching vibrations of carboxylate appear at about 1572 and
1491 cm�1. No absorption bands from the corresponding acid
can be observed, indicating that SPC was completely reacted
with Ti(OCH(CH3)2)4 and no dissolution occurred during the
sol–gel procedure. In addition, the quite broad band locating at
the range of 500 to 650 cm�1 is ascribed to the characteristic
absorption of Ti–O stretching and Ti–O–Ti bridging stretching
modes,14,15 which indicates the presence of TiO2. From the
spectra of B and C, the modifying reactions of SPC are evidenced
by the vanishing of the n(S–H) vibration at 2410 cm�1 and an
increase of n(C–S–C) vibration at about 700 cm�1. This is in good
agreement with the results reported previously.16 The bands at
1031 cm�1 and 1215 cm�1 are attributed to the stretching vibra-
tion n(Si–O) and the stretching vibration n(Si–C). The broad
absorption band n(Si–O–Si) at 1200–1100 cm�1 indicates the
formation of the Si–O–Si framework.
15602 | J. Mater. Chem., 2011, 21, 15600–15607
Similar to the spectra of Fig. 1B and C, the FTIR spectra for
all silica–titania based hybrid materials (Fig. 1D and E) clearly
show the bands at 1058, 1122, 774 and 914 cm�1. The former
three bands are ascribed to the characteristic Si–O–Si vibrations
and Si–O–H stretching vibration, while the weak peak at
914 cm�1 was assigned to the asymmetric stretching vibration of
Ti–O–Si.17 Meanwhile, the peaks at around 2410 cm�1 origi-
nating from the absorption of S–H groups disappeared, which
can prove the completion of the alkylation reactions. Besides, the
n(O–H) vibration at around 3380 cm�1 can also be observed,
indicating the presence of unsaturated Si–OH groups and/or
H2O molecules.
The 29Si MAS NMR spectra of Eu–SPCSi-1 and Eu–SPCSi-2
show the various organosiloxane Tm (Tm ¼ RSi(OSi)m(OH)3�m,
m ¼ 1–3) and siloxane Qn (Qn ¼ Si(OSi)n(OH)4�n, n ¼ 2–4)
species (Fig. 2). The organosiloxane Tm species result from the
hydrolysis and condensation of SPCSi-1 and SPCSi-2, whereas
the siloxane Qm species originate from the hydrolysis and
condensation of TEOS. The T3 and Q4 environments are clearly
dominant, suggesting that there are two main types of local
structures, RSi(OSi)3 and Si(OSi)4.18 On the other hand, the 29Si
This journal is ª The Royal Society of Chemistry 2011
Fig. 2 29Si MAS NMR spectra of (A) Eu–SPCSi-1, (B) Eu–SPCSi-2, (C)
Eu–SPCSi-1–Ti and (D) Eu–SPCSi-2–Ti.
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
MAS NMR spectra of Eu–SPCSi-1–Ti and Eu–SPCSi-2–Ti
exhibit characteristic signals of T1, T2 and T3 units, showing the
presence of two (for Eu–SPCSi-1–Ti) or three (for Eu–SPCSi-2–
Ti) main types of local structures, (SiO)Si(CH2)3(OH)2, (SiO)2Si
(CH2)3OH and (SiO)3Si(CH2)3. The absence of signals due to Qm
species between �90 and �120 ppm indicates that no cleavage of
the Si–C bonds occurred and that all the silicon atoms are
covalently connected to carbon atoms. The peak positions for
Eu–SPCSi-1, Eu–SPCSi-2, Eu–SPCSi-1–Ti and Eu–SPCSi-2–Ti,
and the relative populations of the various silicon sites (T and Q)
were quantitatively estimated and are listed in Table 1.
The evaluations of the condensation degree for T units (CT),
the condensation degree for Q units (CQ) and the global
condensation degree (C) of the materials have been performed by
applying the method outlined in ref. 19. For Eu–SPCSi-1, the
condensation degree of the T units (96.2%) is larger than that of
Q units (92.0%), showing that the T units are more effective than
that of Q units for the condensation. This behaviour can be
explained by the higher reactivity of organosilane precursors
than that of TEOS.20 For Eu–SPCSi-1–Ti, due to the absence of
Q units, the value of CT (95.9%) is the same as its global
condensation degree (C). The C for Eu–SPCSi-1–Ti (95.9%) is
larger than that of Eu–SPCSi-1 (93.6%), which further elucidates
the higher reactivity of organosilane precursors than that of
TEOS. However, the situation of the Eu–SPCSi-2 and Eu–
SPCSi-2–Ti is opposite. This observation can be ascribed to the
different structure of the organosilane precursor.
The powder XRD patterns of the Eu3+-containing hybrid
materials with different inorganic matrices are shown in Fig. S1
(see the ESI†). The XRD patterns of the samples are similar,
which showed only one broad band from 20� to 30� assigned to
an amorphous substance. From Fig. S1A† (Eu–SPC–Ti), no
characteristic peaks of anatase- or rutile-phase titania were
Table 1 29Si MAS NMR spectral chemical shifts (ppm), population of the d
Samples T1 (%) T2 (%) T3 (%) CT (%)
Eu–SPCSi-1 �47.1 (1.3) �57.4 (8.9) �67.5 (89.8) 96.2Eu–SPCSi-2 �49.3 (8.1) �57.8 (11.4) �66.5 (80.5) 90.8Eu–SPCSi-1–Ti �59.2 (12.2) �68.4 (87.8) 95.9Eu–SPCSi-2–Ti �54.3 (10.2) �58.9 (17.9) �67.6 (71.9) 87.2
This journal is ª The Royal Society of Chemistry 2011
observed, which is in good agreement with the results reported
previously.26,30 The absence of any crystalline regions in these
samples correlates well with the presence of organic chains in the
host inorganic framework. These phenomena lead us to conclude
that neither free europium/terbium nitrate salt, pure crystalline
organic ligand SPC, nor crystalline Ln3+ complexes occur
throughout the range of those hybrid materials.
The thermal stabilities of obtained hybrid materials were
demonstrated by TG and DSC measurements. Fig. 3 shows the
TG curves, derivative weight loss (DTG) curve and DSC curve
for Eu–SPC–Ti and Eu–SPCSi-1–Ti. For Eu–SPC–Ti, there are
two main weight loss regions: the initial stage ranging from 55 to
110 �C is attributed to the desorption of physically absorbed
water and the residuary solvent, while the second stage of the
peak at about 266 �C is due to the thermo-decomposition of the
organic Ln3+ complex. Correspondingly, two endothermic peaks
appear in the DSC curve around 94 and 264 �C, which corre-
spond to the two main weight loss of the DTG curve. Compared
with the TG curve of the hybrid material Eu–SPC–Ti, the TG
curve of Eu–SPCSi-1–Ti is similar, which also exhibits two main
weight loss stages. The slight difference in the weight loss
between them may be due to the modification of the silane
crosslinking reagents with the sulfhydryl group of organic ligand
SPC. On comparing these results with those achieved with the
hybrid materials containing silica as inorganic matrix,21 the
thermal stabilities of the obtained Ln3+-based hybrid materials
with different inorganic matrices are similar or even better.
Therefore, the choice of the inorganic matrix other than silica
becomes more extensive and thus more multifunctional lumi-
nescent hybrid materials could be obtained.
Surface morphology of obtained hybrid materials was studied
using SEM. Fig. 4 shows the SEM images of the hybrid materials
Eu–SPC–Ti (A), Eu–SPCSi-1 (B), Eu–SPCSi-1–Ti (C), and Eu–
SPCSi-2–Ti (D). From these images for the hybrid materials, the
shape of the particles shows much difference obviously. This
means that the different hybrid materials with different inorganic
matrices have an apparent influence on the morphology. The
hybrid material Eu–SPC–Ti exhibits many acicular clusters that
are composed of plentiful needlelike cuboid configurations with
widths of about 2–3 mm. The morphology of the hybrid material
with silica matrix (Fig. 4B) seems to be largely different from that
of Eu–SPC–Ti, forming the bulk materials with the columned
configurations. This can be ascribed to the different way of
coordination with the Ln3+ ions. In this kind of hybrid material,
the coordination behavior depends on the carboxylate group. As
we know, Ln3+ complexes of carboxylate derivatives readily form
the polymeric structure, and the carboxylate groups possess the
bridging coordination ability. This tendency will compete with
the construction of a polymeric network structure of Si–O–Si in
the hydrolysis and copolycondensation processes of silica. Thus,
ifferent T and Q species (%), CT, CQ and C (%)
Q2 (%) Q3 (%) Q4 (%) CQ (%) C (%)
�92.0 (3.4) �101.0 (25.4) �110.9 (71.2) 92.0 93.6�92.6 (1.6) �101.4 (24.6) �110.7 (73.8) 93.1 92.1
95.987.2
J. Mater. Chem., 2011, 21, 15600–15607 | 15603
Table 2 The I02/I01 intensity ratio and the energy (E00) and fwhm (fwhm00) values of the5D0 /
7F0 line of Eu3+-containing hybrids
Hybrids Eu–SPC–Ti Eu–SPCSi-1 Eu–SPCSi-2 Eu–SPCSi-1–Ti Eu–SPCSi-2–Ti
I02/I01 6.5 4.5 4.4 5.4 4.7E00/cm
�1 17 293.5 � 0.5 17 299.6 � 0.1 17 302.0 � 0.3 17 292.3 � 0.3 17 300.8 � 0.1fwhm00/cm
�1 26.5 � 0.3 25.1 � 0.2 27.0 � 0.6 36.3 � 0.6 30.4 � 0.2
Fig. 3 TG (TG1), DSC and DTG (----) curves of Eu–SPC–Ti and TG
(TG2) curve of Eu–SPCSi-1–Ti.
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
these hybrid materials retain the intensive tendency of growing
into a polymeric microstructure and reserve the coordinated
positions in the corresponding bulk materials.
Compared to the above-mentioned hybrid materials, the
morphologies of hybrid materials Eu–SPCSi-1–Ti and Eu-
SPCSi-2–Ti are aggregates of small particles and microspheres
with dimensions up to 0.2–0.5 mm, which can be seen from
Fig. 4C and D. It is speculated that the addition of the silica
inorganic matrix through the modified ligand SPC changes the
morphology of the titania based hybrid materials. From the
above discussion, we can see that the self-assembly process can be
influenced not only by the coordination behavior of the Ln3+
complexes but also by the different inorganic parts in the hybrid
materials. Here, it should be clarified that the hybrid materials
mentioned in our manuscript belong to the non-crystalline state
materials with amorphous nature. Therefore, there exist no exact
long-range ordered structure and microstructure like crystals or
crystalline materials. The SEM characterization only shows the
basic morphology of the materials but cannot give exact infor-
mation of the unit in the hybrid systems like crystalline materials.
Fig. 4 SEM images of (A) Eu–SPC–Ti, (B) Eu–SPCSi-1, (C) Eu–SPCSi-
1–Ti and (D) Eu–SPCSi-2–Ti hybrid materials.
15604 | J. Mater. Chem., 2011, 21, 15600–15607
We have tried to provide more information on the surface
morphology of them using TEM of SEM images with higher
resolution. But unfortunately, it cannot provide satisfactory and
clear results (see Fig. S2 and S4 in the ESI†). Besides, some
crystal-like microstructure particles may be derived from TiO2
from the sol–gel products of Ti(OCH(CH3)2)4 precursors and
excess Ln(NO3)3 salts, which are not the nanocrystals from the
covalently bonded hybrid system.
The UV-vis diffuse reflection absorption spectra were
measured on the powder materials for all the obtained hybrid
materials. The corresponding absorption spectra of Tb3+-con-
taining hybrid materials are shown in Fig. S2 (see the ESI†). All
of the spectra exhibit a broad absorption band in the range of
300–450 nm. It can be primarily predicted that in the hybrid
materials the central Ln3+
ions can be efficiently sensitized by the
ligand through the so-called ‘‘antenna effect’’. For the hybrid
materials with different inorganic matrices, an obvious red shift
and broadening of the absorption band are observed; the degree
of red shift and broadening increases in the order of silica
(329 nm) < silica/titania (389 nm) < titania (408 nm) based host
hybrid materials. This may be due to the different structures and
different ways of combination between the inorganic compo-
nents and Ln3+ complexes in the Ln3+ hybrid materials. Thus, the
different energy absorption in ultraviolet-visible, different
intramolecular energy transfer coefficients and different fluo-
rescence emission intensities can be observed. This will be further
discussed later in detail combined with the luminescence prop-
erties of these hybrid materials.
3.2. Photoluminescence
Fig. 5 shows the room temperature excitation spectra of Eu–
SPC–Ti, Eu–SPCSi-2, Eu–SPCSi-2–Ti, Tb–SPC–Ti, Tb–SPCSi-
1, and Tb–SPCSi-1–Ti, monitored within the 5D0 / 7F2
(614 nm) and 5D4 /7F5 (544 nm) transitions, respectively, for
Eu3+- and Tb3+-containing hybrids. For Eu3+-containing hybrid
materials, all the spectra are similar, showing a broad excitation
band between 300 and 350 nm, which partially overlap with the
UV-vis diffuse absorption spectra and can be assigned to the p–
p* states of the organic ligand.22,23 A peak at 393 nm is observed
due to the Eu3+ 7F0/5L6 f–f transition. This transition is weaker
than the absorption of the organic ligand, which proves that
luminescence sensitization via ligand excitation is much
more efficient than the direct excitation of the Eu3+ ion. For
Tb3+-containing hybrid materials, a similar broad band is also
detected and no apparent f–f transitions could be observed in the
spectra. For Tb–SPCSi-1 and Tb–SPCSi-1–Ti hybrid materials,
the broad band is blue-shifted compared with that of the Tb–
SPC–Ti (from 335 nm to 320 and 323 nm). Such a difference may
be attributed to the modification between the silane crosslinking
reagents and the sulfhydryl group of ligand SPC, and the change
This journal is ª The Royal Society of Chemistry 2011
Fig. 5 Excitation spectra of (A) Eu–SPC–Ti, (B) Eu–SPCSi-2, (C) Eu–
SPCSi-2–Ti, (D) Tb–SPC–Ti, (E) Tb–SPCSi-1, and (F) Tb–SPCSi-1–Ti
monitored at 614 nm for Eu and 544 nm for Tb, respectively. Fig. 7 Room temperature emission spectra of (A) Tb–SPC–Ti excited at
335 nm, (B) Tb–SPCSi-1 (solid line) and Tb–SPCSi-1–Ti (----) excited at
320 nm and 325 nm, (C) Tb–SPCSi-2 (solid line) and Tb–SPCSi-2–Ti (----)
excited at 320 nm and 325 nm. All spectra are normalized to a constant
intensity at the maximum.
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
in the polarity of the environment surrounding the Ln3+ complex
in the inorganic matrix.
Fig. 6 presents the normalized emission spectra at room
temperature for the obtained Eu3+-containing hybrid materials.
All the spectra exhibit the characteristic intra-4f 6 sharp lines
ascribed to the 5D0 /7F0–4 transitions. From the spectra of the
hybrids with the silica–titania based host, there is no broad band
emission observed in the blue and green spectral regions.
However, a broad emission is observed for the titania based
hybrid material (Fig. 6A). It is assumed that the difference in the
inorganic parts of the hybrid materials and the environment of
Ln3+ complexes can affect the energy transfer. Fig. 7 illustrates
the typical photoluminescence spectra of the Tb3+-containing
hybrid materials. The emission bands at 488, 543, 583, and
620 nm are recorded. These bands are attributed to the 5D4 /7F6,
5D4 /7F5,
5D4 /7F4, and
5D4 /7F3 transitions of Tb
3+
ions, respectively (Table 2).
Furthermore, the lifetime values of 5D0 (Eu3+) and 5D4 (Tb
3+)
excited states are estimated on the basis of the emission decay
curves monitored within the more intense Eu3+ (5D0 /7F2) and
Tb3+ (5D4 /7F5) transitions, respectively. All the curves reveal
Fig. 6 Room temperature emission spectra of (A) Eu–SPC–Ti excited at
325 nm, (B) Eu–SPCSi-1 (solid line) and Eu–SPCSi-1–Ti (----) excited at
328 nm, (C) Eu–SPCSi-2 (solid line) and Eu–SPCSi-2–Ti (----) excited
at 330 nm.
This journal is ª The Royal Society of Chemistry 2011
a single exponential behavior, which corroborates that all the
Ln3+ ions occupy the same average local environment within each
hybrid material. For the Eu3+-containing and Tb3+-containing
hybrid materials, the obtained lifetime values are given in Table 3
and Table 4, respectively. On the basis of the emission spectra
and 5D0 lifetimes, the emission quantum efficiency (h) of the 5D0
emitting level can be determined.24–27 Assuming that only non-
radiative and radiative processes are essentially involved in the
depopulation of the 5D0 state, h can be expressed as:
h ¼ Ar/(Ar + Anr) (1)
The radiative processes (Ar) are calculated from the relative
intensities of the 5D0 / 7F0–4 transitions (the 5D0 / 7F5,6
branching ratios are neglected because of their poor relative
intensity with respect to that of the remaining 5D0 /7F0–4 lines).
The 5D0 / 7F1 transition does not depend on the local ligand
field and thus may be used as a reference for the whole spectrum.
An effective refractive index of 1.5 was used leading to A01 z50 s�1,28 where A01 stands for Einstein’s coefficient of sponta-
neous emission between the 5D0 and the 7F1 Stark levels. Life-
time, radiative (Ar), and nonradiative (Anr) transition rates are
related through the following equation:
sexp ¼ (Ar + Anr)�1 (2)
On the basis of the above discussion, the parameters Ar, Anr
and the quantum efficiency values, h, for the 5D0 Eu3+ ion excited
state in the five hybrids can be obtained, as shown in Table 3.
Comparing the h values of the Eu–SPC–Ti hybrid materials with
those previously reported for the organic–inorganic titania
hybrid materials, the values in Table 3 are smaller. In spite of
higher Ar values, Eu–SPC–Ti displays much higher Anr values
relative to those known for the titania materials, namely, Ar and
Anr values of 0.336 and 2.68 for Ti–nit–Eu material.26 Comparing
the h values of the obtained hybrids with different inorganic
matrix, an increase in the h values is observed. For the case of
silica based hybrid materials Eu–SPCSi-1 and Eu–SPCSi-2, such
J. Mater. Chem., 2011, 21, 15600–15607 | 15605
Table 3 Lifetime (s), radiative (Ar) and nonradiative (Anr) transition probabilities, quantum efficiency of the 5D0 level (h), quantum yield (f), U2,4
intensity parameters (� 10�20 cm2) and number of coordinated water molecules (nw) for Eu3+-containing hybrid materials
Hybrids Eu–SPC–Ti Eu–SPCSi-1 Eu–SPCSi-2 Eu–SPCSi-1–Ti Eu–SPCSi-2–Ti
s/ms 0.202 � 0.003 0.489 � 0.003 0.367 � 0.007 0.283 � 0.002 0.358 � 0.003Ar/ms�1 0.483 0.329 0.304 0.350 0.381Anr/ms�1 4.467 1.716 2.421 3.184 2.412h (%) 9.8 16.1 11.2 9.9 13.7f (%) <1 2 1 <1 <1nw 4.6 1.6 2.3 3.2 2.3U2 9.8 6.7 6.6 7.1 8.0U4 5.0 2.5 1.1 2.9 2.4
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
an increase is due to both an increase in the lifetime value and
a decrease in the Anr value. For Eu–SPCSi-1–Ti and Eu–SPCSi-
2–Ti, after incorporation of the titania inorganic host, although
there is an increase in the radiative transition probability Ar,
there is a decrease in the lifetime value. In addition, the hybrid
materials modified with the different silane crosslinking reagents
have the different quantum efficiency values in different inor-
ganic hosts. In silica host hybrid materials, the quantum effi-
ciency of Eu–SPCSi-1 is higher than that of Eu–SPCSi-2.
However, the opposite result can be observed in the silica/titania-
based hybrid system. On the basis of these results, we may
presume that the modification with the titania matrix hybrids is
very helpful for improving the quantum efficiency. At the same
time, the different composition of the hybrid SPC materials may
have an influence on the luminescent lifetimes and quantum
efficiency. Moreover, the variations in h and Anr values may be
rationalized in terms of the number of water molecules coordi-
nated to the Eu3+ ions (nw) based on the empirical formula
reported by Supkowski and Horrocks:29
nw ¼ 1.11�(s�1 � Ar � 0.31) (3)
The results for obtained Eu3+-containing hybrid materials are
shown in Table 3. On the basis of the results, the different nwvalues of hybrid materials are possibly due to the fact that the
ways of the coordination between the Eu3+ ion and ligand SPC
are different. In addition, the decrease in the nw value is in good
agreement with the increase in the value of quantum efficiency.
The coordination water molecules produce the severe vibration
of the hydroxyl group, resulting in the large nonradiative tran-
sition and decreasing the luminescent efficiency.
In order to further quantify the different photoluminescence
features of the different hybrid materials, the absolute emission
quantum yields (f) were measured for all the materials (Tables 3
and 4). The maximum quantum yield values are found for the
Eu– and Tb–SPCSi-1 hybrids, whereas for some of the remaining
hybrids the values are lower than the detection limits of our
equipment (<0.01). According to ref. 1, the overall sensitization
efficiency, hsens, of the Eu3+-containing hybrids is defined as the
ratio between the quantum efficiency of the 5D0 level (h) and the
Table 4 Lifetime (s) and quantum yield (4) of Tb3+-containing hybrid mate
Hybrid materials Tb–SPC–Ti Tb–SPCSi-1
s/ms 0.364 � 0.003 0.516 � 0.0064 (%) <1 1
15606 | J. Mater. Chem., 2011, 21, 15600–15607
emission quantum yield f. A maximum value of hsens ¼ 0.12 is
estimated for Eu–SPCSi-1, indicating that the relatively low
quantum yield values of these hybrid materials could be
explained by an inefficient ligand sensitization, besides the
presence of a large number of coordinated water molecules.
In addition, we synthesized the pure Ln3+ (Ln ¼ Eu, Tb)
complexes and compared their luminescence property with
obtained hybrid materials. The results show that the obtained
hybrids possess longer luminescence lifetimes and higher
quantum efficiencies than that of a pure complex except for the
Eu–SPC–Ti hybrids which are slightly less than the pure
complex. But it is worthwhile pointing out that the effective
content of lanthanide (Eu, Tb) species in the hybrid materials is
much lower than the pure lanthanide complex, so in fact, the
luminescence of the hybrids are enhanced compared to the pure
complex (see Tables S1 and S2†).
The experimental intensity parameters (U2 and U4) were
determined from the emission spectra of Eu3+-containing hybrids
based on the 5D0 /7F2 and
5D0 /7F4 transitions and the 5D0
/ 7F1 magnetic dipole transition as the reference, and they are
estimated according to the following equation,23,24,27
Ul ¼ [3h/64p4 e2g3][9/n(n2 + 2)2][1/|h5D0||U(l)||7FJi|2] A0J (4)
where h is Planck’s constant, e is the electronic charge, g is the
angular frequency of the transition, the refraction index n ¼1.5, and h5D0||U
(l)||7FJih5D0||U(l)||7FJi values are the square
reduced matrix elements whose values are 0.0032 and 0.0023
for J ¼ 2 and 4, respectively.30,31 The U6 parameter was not
determined since the 5D0 / 7F6 transition could not be
experimentally detected.
The U2, U4 intensity parameters for the five hybrid materials
are shown in Table 3. A point to be noted in these results is the
relatively high value of the U2 intensity parameter for Eu–SPC–
Ti. This might be interpreted as a consequence of the hypersen-
sitive behavior of the 5D0 /7F2 transition, suggesting that the
dynamic coupling mechanism is quite operative and that the
chemical environment is highly polarizable. In addition,
the similar value of theU2 intensity parameter can be observed in
the hybrid materials with the same inorganic matrix.
rials
Tb–SPCSi-2 Tb–SPCSi-1–Ti Tb–SPCSi-2–Ti
0.715 � 0.008 0.417 � 0.008 0.447 � 0.0062 <1 <1
This journal is ª The Royal Society of Chemistry 2011
Dow
nloa
ded
by U
nive
rsid
ade
de A
veir
o (U
Ave
iro)
on
31 O
ctob
er 2
012
Publ
ishe
d on
26
Aug
ust 2
011
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C1J
M12
264A
View Online
4. Conclusions
In this work we have synthesized a series of new Ln3+ organic–
inorganic hybrid materials with different inorganic hosts. The
organic ligand SPC exhibited three potential binding sites—
pyridine N, sulfhydryl S and carboxylic O to construct the hybrid
materials. The structural characterizations, physical properties
and the photoluminescence properties were studied in detail. The
differences in the profiles of emission features, in lifetime values
and in quantum efficiency among all the synthesized materials
confirm that the different inorganic matrices of the hybrid
materials have an influence on the photoluminescence properties.
Moreover, compared with titania matrix hybrid materials, the
introduction of silica inorganic matrix through the modification
of the organic ligand results in a much higher quantum efficiency.
Therefore, this facile strategy to tether Ln3+ complexes to
organic–inorganic silica/titania hybrid materials can be conve-
niently applied to other hybrid material systems and the desired
properties can be tailored by an appropriate choice of the
precursors. In this way, numerous organic ligands are expected
to be introduced into the hybrid materials and more multifunc-
tional luminescent materials could be obtained.
Acknowledgements
This work was supported by the National Natural Science
Foundation of China (20971100), Program for New Century
Excellent Talents in University (NCET 2008-08-0398) and FCT
Project of Portugal (PTDC/CTM/108975/2008).
References
1 J.-C. G. B€unzli, Chem. Rev., 2010, 110, 2729; A. P. Bassett,S. W. Magennis, P. B. Glover, D. J. Lewis, N. Spencer, S. Parsons,R. M. Williams, L. De Cola and Z. Pikramenou, J. Am. Chem.Soc., 2004, 126, 9413; S. M. Bruno, R. A. S. Ferreira,F. A. Almeida Paz, L. D. Carlos, M. Pillinger, P. Ribeiro-Claroand I. S. Goncalves, Inorg. Chem., 2009, 48, 4882.
2 S. Sato and M. Wada, Bull. Chem. Soc. Jpn., 1970, 43, 1955;J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1990, 29, 1304.
3 C. Sanchez, G. J. D. A. Sloer-Illia, F. Ribot, T. Lalot, C. R. Mayerand V. Cabuil, Chem. Mater., 2001, 13, 3061; P. Escribano,B. Juli�an-L�opez, J. Planelles-Arag�o, E. Cordoncillo, B. Viana andC. Sanchez, J. Mater. Chem., 2008, 18, 23; L. D. Carlos,R. A. S. Ferreira, V. D. Bermudez and S. J. L. Ribeiro, Adv. Funct.Mater., 2001, 11, 111; L. D. Carlos, R. A. S. Ferreira,R. N. Pereira, M. Assuncao and V. D. Bermudez, J. Phys. Chem.B, 2004, 108, 14924; L. S. Fu, R. A. S. Ferreira, S. S. Nobre,L. D. Carlos and J. Rocha, J. Lumin., 2007, 122, 265;K. Binnemans, Chem. Rev., 2009, 109, 4283; L. D. Carlos,R. A. S. Ferreira, V. D. Bermudez and S. J. L. Ribeiro, Adv.Mater., 2009, 21, 509; B. Yan and Q. M. Wang, Cryst. GrowthDes., 2008, 8, 1484.
4 M. Fernandes, M. C. Goncalves, V. D. Bermudeza, R. A. S. Ferreira,L. D. Carlos, A. Charas and J. Morgado, J. Alloys Compd., 2008, 451,201.
5 J. L. Liu and B. Yan, J. Phys. Chem. C, 2008, 112, 14168; X. M. Guo,H. D. Guo, L. S. Fu, L. D. Carlos, R. A. S. Ferreira, L. N. Sun,R. P. Deng and H. J. Zhang, J. Phys. Chem. C, 2009, 113, 12538;L. Guo and B. Yan, Eur. J. Inorg. Chem., 2010, 1267.
6 C. Sanchez, B. Julian, P. Belleville and M. Popall, J. Mater. Chem.,2005, 15, 3559; P. Wang, C. Klein, R. Humphry, S. Zakeeruddinand M. Gr€atzel, J. Am. Chem. Soc., 2005, 127, 808.
7 Y. G. Wang, L. Wang, H. R. Li, P. Liu, D. S. Qin, B. Y. Liu,W. J. Zhang, R. P. Deng and H. J. Zhang, J. Solid State Chem.,2008, 181, 562; R. F. de Farias, S. Alves, M. F. Belian and G. F. deS�a, J. Colloid Interface Sci., 2001, 243, 523.
This journal is ª The Royal Society of Chemistry 2011
8 C. Sanchez and F. Ribot, New J. Chem., 1994, 18, 1007.9 C. Y. Peng, H. J. Zhang, J. B. Yu, Q. G. Meng, L. S. Fu, H. R. Li,L. N. Sun and X. M. Guo, J. Phys. Chem. B, 2005, 109, 15278;X. F. Qiao and B. Yan, J. Phys. Chem. B, 2008, 112, 14742;J. L. Liu and B. Yan, J. Phys. Chem. B, 2008, 112, 10898;N. N. Lin, H. R. Li, Y. G. Wang, Y. Feng, D. S. Qin, Q. Y. Ganand S. D. Chen, Eur. J. Inorg. Chem., 2008, 4781; H. F. Lu, B. Yanand J. L. Liu, Inorg. Chem., 2009, 48, 3966.
10 P. Liu, H. R. Li, Y. G. Wang, B. Y. Liu, W. J. Zhang, Y. J. Wang,W. D. Yan, H. J. Zhang and U. Schubert, J. Mater. Chem., 2008,18, 735.
11 K. L. Frindell, M. H. Bartl, A. Popitsch and G. D. Stucky, Angew.Chem., Int. Ed., 2002, 41, 960; U. Schubert, J. Sol-Gel Sci. Technol.,2003, 26, 47; M. Puchberger, W. Rupp, U. Bauer and U. Schubert,New J. Chem., 2004, 28, 1289; H. R. Li, P. Liu, Y. G. Wang,L. Zhang, J. B. Yu, H. J. Zhang, B. Y. Liu and U. Schubert, J.Phys. Chem. C, 2009, 113, 3945.
12 B. Yan and H. F. Lu, Inorg. Chem., 2008, 47, 5601.13 B. Yan, K. Qian and H. F. Lu, J. Organomet. Chem., 2009, 694,
3160.14 M. Schraml-Marth, K. L. Walther, A. Wokaum, B. E. Handy and
A. Baiker, J. Non-Cryst. Solids, 1992, 143, 93.15 L. T�ellez, J. Rubio, F. Rubio, E. Morales and J. L. Oteo, Spectrosc.
Lett., 2004, 37, 11.16 S. P. Chen, Q. Yang and S. Gao, J. Therm. Anal. Calorim., 2009, 95,
685.17 D. C. M. Dutoit, M. Schneider and A. Baiker, J. Catal., 1995, 153,
165; R. Ryoo, C. H. Ko and R. F. Howe, Chem. Mater., 1997, 9,1607; N. Yamada, I. Yoshinaga and S. Katayama, J. Sol-Gel Sci.Technol., 2000, 17, 123.
18 M. C. Goncalves, V. D. Bermudez, R. A. S. Ferreira, L. D. Carlos,D. Ostrovskii and J. Rocha, Chem. Mater., 2004, 16, 2530;L. S. Fu, R. A. S. Ferreira, N. J. O. Silva, L. D. Carlos,V. D. Bermudez and J. Rocha, Chem. Mater., 2004, 16, 1507;S. S. Nobre, P. P. Lima, L. Mafra, R. A. S. Ferreira, R. O. Freire,L. S. Fu, U. Pischel, V. D. Bermudez, O. L. Malta andL. D. Carlos, J. Phys. Chem. C, 2007, 111, 3275.
19 S. Ungureanu, M. Birot, G. Laurent, H. Deleuze, O. Babot, B. Juli�an-L�opez, M. F. Achard, M. Ionel Popa, C. Sanchez and R. Backov,Chem. Mater., 2007, 19, 5786.
20 A. Van Blaaderen and A. P. M. Kentgens, J. Non-Cryst. Solids, 1992,149, 161.
21 B. Yan and X. F. Qiao, J. Phys. Chem. B, 2007, 111, 12362; K. Sheng,B. Yan, H. F. Lu and L. Guo, Eur. J. Inorg. Chem., 2010,3498.
22 H. R. Li, J. Lin, H. J. Zhang, L. S. Fu, Q. G. Meng and S. B. Wang,Chem. Mater., 2002, 14, 3651.
23 P. C. R. Soares-Santos, H. I. S. Nogueira, V. F�elix, M. G. B. Drew,R. A. S. Ferreira, L. D. Carlos and T. Trindade, Chem. Mater.,2003, 15, 100.
24 L. D. Carlos, R. A. S. Ferreira, V. D. Bermudez, B. Juli�an-L�opez andP. Escribano, Chem. Soc. Rev., 2011, 40, 536.
25 O. L. Malta, H. F. Brito, J. F. S. Menezes, F. R. G. E. Silva, S. Alves,F. S. Farias and A. V. M. deAndrade, J. Lumin., 1997, 75, 255.
26 O. L. Malta, H. J. Batista and L. D. Carlos, Chem. Phys., 2002, 282,21; L. D. Carlos, O. L. Malta and R. Q. Albuquerque, Chem. Phys.Lett., 2005, 416, 238; S. S. Nobre, X. Catto€en, R. A. S. Ferreira,C. Carcel, V. D. Bermudez, M. W. C. Man and L. D. Carlos,Chem. Mater., 2010, 22, 3599.
27 L. D. Carlos, Y. Messaddeq, H. F. Brito, R. A. S. Ferreira,V. D. Bermudez and S. J. L. Ribeiro, Adv. Mater., 2000, 12, 594;M. Fernandes, V. D. Bermudez, R. A. S. Ferreira, L. D. Carlos,A. Charas, J. Morgado, M. M. Silva and M. J. Smith, Chem.Mater., 2007, 19, 3892; S. S. Nobre, C. D. S. Brites,R. A. S. Ferreira, V. D. Bermudez, C. Carcel, J. J. E. Moreau,J. Rocha, M. Wong Chi Man and L. D. Carlos, J. Mater. Chem.,2008, 18, 4172.
28 J. C. Boyer, F. Vetrone, J. A. Capobianco, A. Speghini andM. Bettinelli, J. Phys. Chem. B, 2004, 108, 20137.
29 R. M. Supkowski and W. D. Horrocks, Inorg. Chim. Acta, 2002, 340,44.
30 W. D. Horrocks and D. R. Sudnick, J. Am. Chem. Soc., 1979, 101,334.
31 K. Binnemans, K. Van Herck and C. G€orller-Walrand, Chem. Phys.Lett., 1997, 266, 297.
J. Mater. Chem., 2011, 21, 15600–15607 | 15607
Related Documents