Journal of Leukocyte Biology Volume 61, May 1997 583 Monocytes from mobilized stem cells inhibit T cell function Kazuhiko Ino, Rakesh K. Singh, and James E. Talmadge Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha Abstract: Granulocyte-macrophage colony-stimulating factor, mobilized peripheral blood stem cell (PSC) products, and peripheral blood leukocytes post- transplantation contain cells that cause allogeneic and autologous T cell apoptosis. Isolation and char- acterization ofthese cells demonstrated that they were low-density (Percoll fractionation) CD14’ monocytes. T cells in PSC products have a depressed phytohe- magglutinin (PHA) mitogenic response; however, pu- rifled CD4 or CD8 T cells exhibit a statistically normal mitogenic function. Furthermore, no T cell inhibitory activity was observed in CD14’, CD4, and CD8 cell-depleted fractions enriched in CD4CD8 TCRa/ T cells. Inhibition of T cell function by CD14 monocytes required cell-cell contact, and the analyses of DNA fragmentation by Southern and TUNEL analysis demonstrates an activation-induced T cell apoptosis in the presence ofCDl4’ monocytes. Reverse-transcriptase polymerase chain reaction studies suggested that high levels of interleukin-lO or tumor necrosis factor gene transcripts in the PSC prod- ucts may contribute to the inhibition of T cell func- lion. J. Leukoc. Riot. 61: 583-591; 1997. Key Words: traruplantation . peripheral blood stem cell trans- plantation . apoptosis INTRODUCTION Myeloablative, high-dose therapy (HDT) followed by autol- ogous peripheral blood stem cell transplantation (PSCT) is used for the treatment of advanced malignancies [1, 2]. Myeloid recovery is more rapid following PSCT compared with autologous bone marrow transplantation (AuBMT) when the peripheral blood stem cells (PSC) are collected after mobilization with hematopoietic growth factors, che- motherapy, or both [3]. In addition, PSCT results in an earlier reconstitution of the immune system compared with AuBMT, perhaps due to the large number of lymphocytes in the PSC product [4-7]. Although immune function re- turns to pretransplant levels, it remains signfficantly de- pressed compared with normal individuals [7]. Further- more, one retrospective study demonstrated that the failure-free survival (FF5) of lymphoma patients after PSCT using steady state PSC products was superior to that observed after AuBMT [2]. However, a high relapse rate is still observed after PSCT and only a minority of patients achieve long-term disease-free survival [1, 2]. Disease re- lapse following PSCT may be attributable not only to sub- optimal ablative chemotherapy or reinfusion of tumor cells but also to inadequate immunological restoration. Thus, strategies to enhance immune function and overcome im- mune tolerance after PSCT are needed to reduce relapse and prolong remission [8, 9]. Recently we found that granulocyte-macrophage co’ony- stimulating factor (GM-CSF) mobilized PSC products as well as peripheral blood (PB) leukocytes post-transplantation contain cells that can inhibit T cell functions [7, 10, 11]. Because immune function is a balance of positive and nega- tive regulators, this observation has potential clinical impor- tance. These cells, which can inhibit T cell function, are associated with hematopoiesis [12-22] and can be in- creased by tumor secretion or administration of hematopoi- etic growth factors [14, 15]. However, the cellular lineage and mechanism of this T cell inhibitory activity remains controversial [12-22]. These studies demonstrate that CD14 cells in PSC products can inhibit T cell function and lead to activation-induced T cell apoptosis. Further- more, when CD14 cells are depleted, isolated CD4 and CD8 T cells from PSC products demonstrate a normal proliferative response to phytohemagglutinin (PHA) com- pared with undepleted stem cell products. This provides one explanation for the immune dysfunction found post- transplantation and suggests that the manipulation of stem cell products could improve immune reconstitution after transplantation with mobilized PSC. MATERIALS AND METHODS Patients Between April and October, 1995, 21 patients with advanced malig- nancies who were candidates for HDT and autologous PSCT were Abbreviations: PSC, peripheral blood stem cells; PHA, phytohemag- glutinin; HDi high-dose therapy; PSCT, peripheral blood stem cell transplantation; AuBMT, autologous bone marrow transplantation; FF5, failure-free survival; GM-CSF, granulocyte-macrophage colony- stimulating factor; PB, peripheral blood; WBC, white blood cell; FR, fraction; PBS, phosphate-buffered saline; BSA, bovine serum albumin; Flit, fluorescein isothiocyanate; TdT, terminal deoxynucleotidyl trans- ferase; PE, phycoerythrin; APC, allophycocyanin; PFA, paraformalde- hyde; TCR, T cell receptor; IL-2, interleukin-2; IFN-y, interferon-y; TNF-a, tumor necrosis factor a; GBHD, graft versus host disease; NS, natural suppressor. Correspondence: James E. Talmadge, Ph.D., Department of Pathol- ogy and Microbiology, University of Nebraska Medical Center, 600 South 42nd Street, Omaha, NE 68198-5660. Received September 11, 1996; revised January 27, 1997; accepted January 31, 1997.

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Journal of Leukocyte Biology Volume 61, May 1997 583
Monocytes from mobilized stem cells inhibit T cell functionKazuhiko Ino, Rakesh K. Singh, and James E. Talmadge
Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha
Abstract: Granulocyte-macrophage colony-stimulating
factor, mobilized peripheral blood stem cell (PSC)
products, and peripheral blood leukocytes post-
transplantation contain cells that cause allogeneic
and autologous T cell apoptosis. Isolation and char-
acterization ofthese cells demonstrated that they were
low-density (Percoll fractionation) CD14’ monocytes.
T cells in PSC products have a depressed phytohe-
magglutinin (PHA) mitogenic response; however, pu-
rifled CD4� or CD8� T cells exhibit a statistically
normal mitogenic function. Furthermore, no T cell
inhibitory activity was observed in CD14’, CD4�, and
CD8� cell-depleted fractions enriched in CD4CD8
TCRa/� T cells. Inhibition of T cell function by
CD14 monocytes required cell-cell contact, and the
analyses of DNA fragmentation by Southern and
TUNEL analysis demonstrates an activation-induced
T cell apoptosis in the presence ofCDl4’ monocytes.
Reverse-transcriptase polymerase chain reaction
studies suggested that high levels of interleukin-lO or
tumor necrosis factor gene transcripts in the PSC prod-
ucts may contribute to the inhibition of T cell func-
lion. J. Leukoc. Riot. 61: 583-591; 1997.
Key Words: traruplantation . peripheral blood stem cell trans-
plantation . apoptosi�s
INTRODUCTION
Myeloablative, high-dose therapy (HDT) followed by autol-
ogous peripheral blood stem cell transplantation (PSCT)
is used for the treatment of advanced malignancies [1, 2].
Myeloid recovery is more rapid following PSCT compared
with autologous bone marrow transplantation (AuBMT)
when the peripheral blood stem cells (PSC) are collected
after mobilization with hematopoietic growth factors, che-
motherapy, or both [3]. In addition, PSCT results in an
earlier reconstitution of the immune system compared with
AuBMT, perhaps due to the large number of lymphocytes
in the PSC product [4-7]. Although immune function re-
turns to pretransplant levels, it remains signfficantly de-
pressed compared with normal individuals [7]. Further-
more, one retrospective study demonstrated that the
failure-free survival (FF5) of lymphoma patients after
PSCT using steady state PSC products was superior to that
observed after AuBMT [2]. However, a high relapse rate
is still observed after PSCT and only a minority of patients
achieve long-term disease-free survival [1, 2]. Disease re-
lapse following PSCT may be attributable not only to sub-
optimal ablative chemotherapy or reinfusion of tumor cells
but also to inadequate immunological restoration. Thus,
strategies to enhance immune function and overcome im-
mune tolerance after PSCT are needed to reduce relapse
and prolong remission [8, 9].
Recently we found that granulocyte-macrophage co’ony-
stimulating factor (GM-CSF) mobilized PSC products as well
as peripheral blood (PB) leukocytes post-transplantation
contain cells that can inhibit T cell functions [7, 10, 11].
Because immune function is a balance of positive and nega-
tive regulators, this observation has potential clinical impor-
tance. These cells, which can inhibit T cell function, are
associated with hematopoiesis [12-22] and can be in-
creased by tumor secretion or administration of hematopoi-
etic growth factors [14, 15]. However, the cellular lineage
and mechanism of this T cell inhibitory activity remains
controversial [12-22]. These studies demonstrate that
CD14� cells in PSC products can inhibit T cell function
and lead to activation-induced T cell apoptosis. Further-
more, when CD14� cells are depleted, isolated CD4� and
CD8� T cells from PSC products demonstrate a normal
proliferative response to phytohemagglutinin (PHA) com-
pared with undepleted stem cell products. This provides
one explanation for the immune dysfunction found post-
transplantation and suggests that the manipulation of stem
cell products could improve immune reconstitution after
transplantation with mobilized PSC.
MATERIALS AND METHODS
Patients
Between April and October, 1995, 21 patients with advanced malig-
nancies who were candidates for HDT and autologous PSCT were
Abbreviations: PSC, peripheral blood stem cells; PHA, phytohemag-
glutinin; HDi� high-dose therapy; PSCT, peripheral blood stem cell
transplantation; AuBMT, autologous bone marrow transplantation; FF5,
failure-free survival; GM-CSF, granulocyte-macrophage colony-
stimulating factor; PB, peripheral blood; WBC, white blood cell; FR,
fraction; PBS, phosphate-buffered saline; BSA, bovine serum albumin;
Flit, fluorescein isothiocyanate; TdT, terminal deoxynucleotidyl trans-
ferase; PE, phycoerythrin; APC, allophycocyanin; PFA, paraformalde-
hyde; TCR, T cell receptor; IL-2, interleukin-2; IFN-y, interferon-y;
TNF-a, tumor necrosis factor a; GBHD, graft versus host disease; NS,
natural suppressor.
Correspondence: James E. Talmadge, Ph.D., Department of Pathol-
ogy and Microbiology, University of Nebraska Medical Center, 600
South 42nd Street, Omaha, NE 68198-5660.
Received September 11, 1996; revised January 27, 1997; accepted
January 31, 1997.
584 Journal of Leukocyte Biology Volume 61, May 1997
studied. Twelve of these patients had intermediate grade non-Hodgkin’s
lymphoma. seven had high risk breast cancer, one had Hodgkin’s dis-
ease, and one had acute myelogenous leukemia. Written informed con-
sent was obtained from each patient. All patients received a continuous
intravenous infusion of GM-CSF (Immunex, Seattle, WA) at a dose of
250 �tg/m2 to mobilize stem/progenitor cells into the peripheral blood.
PSC apheresis was started when the white blood cell (WBC) count
reached 10,000 cells4tL (3-4 days after initiation of GM-CSF adminis-
tration). PSC were collected with a Cobe spectra (Cobe BCT, Lakewood,
CO). In this study, we used fresh PSC products from either the second
or third apheresis. All samples (both patients and normal donors) were
obtained according to the protocols approved by the Institutional Review
Board of the University of Nebraska Medical Center.
Cell isolation and separation
PSCs were separated by Ficoll-Hypaque (Organon Teknika, Durham,
NC) centrifugation and used as an unfractionated PSC population. PSCs
were layered on a discontinuous Percoll gradient Ifractions (FR) at 38.6,
47.5, 52.1, 56.5, 61.1, 65.6, and 70.1%J. After centrifugation, cells
at each interface were collected and identified as follows: fraction 1
(FRI). the band on top of the 38.6% Percoll isolation; FR2, the 38.6
to 47.5% interface; FR3-5 the 47.5 to 61.1% interface; and FR6-7, the
61.1 interface to the pellet in the 70.1% layer.
To purify CD4� and CD8� T cells from Percoll-fractionated PSCs,
immunomagnetic cell sorting was performed with the use of the Mini-
MACS separation system (Miltenyi Biotec Inc., Auburn, CA). Briefly,
cells (up to 1 x 108) were resuspended in phosphate-buffered saline
(PBS) containing 0.5% bovine serum albumin (BSA) and incubated with
the magnetic microbeads-conjugated anti-CD4 or CD8 monoclonal anti-
body for 15 mm at 6-12#{176}C. The cells bound to magnetic beads were
collected by a magnetic separation column and used as purified CD4+
or CD8+ cells.
TUNEL analysis by flow cytometry
Briefly. cell suspensions were stained with specific antibodies labeled
with either PE or biotin-APC, washed twice in PBS. and resuspended
in 100 �tL of ice-cold PBS/i% BSA. One hundred microliters of FACS
fix (40% solution in PBS of 10% buffered formalin) was added and
incubated on a horizontal shaker for 30 mm at room temperature. The
cells were permeabilized using a 0.1% Triton X-100/0.1% sodium ci-
trate solution by incubation at 0#{176}Cfor 2 mm. After washing, the cells
were resuspended in 50 �tL of TUNEL reaction mixture 10.03 �imol/
fluorescein isothiocyanate (FITC)-dUTP, 3 �imol/dATP, 2 p1 25 mM
CaCI2, and 25 U terminal deoxynucleotidyl transferase (TdT), all re-
agents purchased from Boehringer Mannheim (Indianapolis, IN)J and
incubated in a 37#{176}Cwater bath for 60 mm. The tubes were then stored
in the dark at 4#{176}Cin FACS fix before flow cytometric analysis. The fre-
quency ofspecific apoptotic cells was analyzed as follows: A forward scat-
ter by side scatter plot was used to gate all the cells while excluding
debris, dead cells, and aggregated cells. CD4 � and CD8 + cells were then
individually back-gated and plotted as TdT expression versus forward
scatter. The frequency of non-apoptotic or apoptotic CD4� or CD8�
cells after 24 h of incubation in media or PHA was then calculated.
Flow cytometry
Three-color cytometric analysis was used for cell surface immunopheno-
typing of each fraction of the PSC product flO, 11J. After blocking with
immunoglobulin, aliquots of cells were stained with saturating levels of
monoclonal antibodies specffic for the following cell surface markers:
FITC-labeled anti-a�3TCR (T-cell Diagnostics, Cambridge, MA), phyco-
erythrin (PE)-labeled anti-CD4 or anti-CD13 (Becton-Dickinson, San
Jose, CA), and biotin-conjugated anti-CD8 (Becton-Dickinson), anti-
CD14, or anti-CD19 (Coulter, Hialeah, FL) added with streptavidin allo-
phycocyanin (APC) as the third fluorochrome. Background staining
using isotype controls was used to determine the thresholds for positive
cells. All data were acquired on a FACStar Plus (Becton-Dickinson) and
detailed data analysis was performed using CELLQUEST software
(Becton-Dickinson). Each subpopulation was gated using side scatter
(cell granularity) and fluorescence intensity for the specific markers with
the use of this technique; CD14� monocytic cells (intermediate side
scatter and high expression of CD14) were clearly distinguished from
lymphocytes (lower side scatter and negative CD14 expression) or gran-
ulocytes (higher side scatter and lower expression of CD14).
PHA mitogenesis
Mononuclear cells were cultured in the presence of PHA at 1 x i0�
cells/well in 96-well flat-bottom microtiter plates (Falcon, Becton-
Dickinson Labware, Lincoln Park, NJ). Cells were cultured for 54 h
either in the presence or absence of 5, 2.5, or 0.5 �tg/mL PHA. For
the final 18 h of culture 1 �.tCi (3H�thymidine/well (Amersham Life
Sciences, Arlington Heights, IL) was added (total 96 h). The cells were
then harvested onto fiberglass filters with the use of a 96-well harvester
(Packard Instruments, Downers Grove, IL). The filters were allowed to
air dry, scintillation cocktail was added, and the samples were counted
in a Packard multi-well beta counter. Specific incorporation of
�3HJthymidine was compared with control cells (no mitogen stimulus).
Allogeneic and autologous T cell inhibition cell assay
The methodology for the co-culture assay to measure T cell inhibitory
activity has been previously described �7, 10, 11J. Briefly, Ficoll-
Hypaque-purified normal (allogeneic) PBLS or isolated autologous lym-
phocytes (1 x 10�) as responder cells were co-cultured with varying
numbers ofirradiated (500 cGy) putative inhibitory cells (each PSC frac-
tion) at inhibitor-to-responder (I:R) ratios of 2:1, 1:1, 0.5:1, and 0.25:1
in the presence of an optimal concentration of PHA (0.5 �tg/mL) in a
96-well flat-bottom microplate. The allogeneic or autologous lympho-
cytes (isolated CD4 � and CD8 � cells at a 1 :1 ratio) were also cultured
alone with PHA as a control. Cells were cultured in complete medium
for 72 h at 37#{176}Cin a humidified 5% CO2 atmosphere. The mitogenic
response of the co-culture or responder cells was determined by �3H�thy-
midine incorporation over the final 18 h ofculture. All experiments were
performed in triplicate. The percent inhibitory activity was calculated
according to the following formula: % suppression = �1 - (mean cpm
in tested wells with co-cultured cells)/(mean cpm in control wells without
co-cultured cells)J x 100.
Paraformaldehyde treatment
The mechanism of inhibitory activity was examined using cells fixed with
a 1% paraformaldehyde (PFA) solution in RPM! 1640 medium (w/v)
for 30 mm at room temperature. After washing three times, these PFA-
fixed cells were used instead of irradiated cells in co-cultures (96-well
plates) with responder PBLs and T cell inhibitory activity was evaluated.
Transwell co-culture
The mechanism of T cell inhibition was also examined with co-culture
studies using a 96-well transwell chamber system incorporating 0.2-�tm
Anopore membrane (Nunc, Roskilde, Denmark) to prevent cell-to-cell
contact between T cell inhibitory cells and responder cells.
Measurement of apoptosis by DNA fragmentation
DNA from the co-cultured cells was extracted and analyzed using a modi-
fled procedure as described by Mollereau et al. f23J. Briefly, after ap-
propriate treatment cells were washed, pelleted, resuspended in lysis
buffer (10 mM ethylenediaminetetraacetate, 50 mM Tris-HC1 pH 7.5,
0.57% sodium dodecyl suifate, and 50 �tg/mL of proteinase K), and in-
cubated for 1 h at 50#{176}C.After addition of 50 �tg/mL RNase A, samples
were incubated for 1 h at 37#{176}Cand then 5 mm at 70#{176}C.Proteins were
salted out �y the addition of NaCI (0.5 M) and DNA precipitated with
100% ethanol. DNA samples were washed with 70% ethanol and quan-
titated. Samples of 10 �tg of DNA per lane were electrophoresed on a
1.2% agarose gel containing 0.1 pig/mL ethidium bromide, visualized
by ultraviolet light, and photographed.
mo et al. CD14� cells in mobilized PSC products 585
Semi-quantitative analysis of cytokine mRNA[reverse transcriptase-polymerasechain reaction (RT-PCR)]
Total cellular RNA from 106 cells was isolated from PSC and steady
state or PHA-activated (24 h) normal PBL products by use of Trizol#{174}
reagent (GIBCO-BRL, Gaithersburg, MD) per the manufacturer’s rec-
ommended procedures. The RNA concentration was determined by ab-
sorbance at 260 nm. Two micrograms of RNA were aliquoted and re-
precipitated in 15% 3 M sodium acetate and 2.5 vol 100% ethanol and
stored at - 70#{176}C. First-strand DNA was synthesized at 42#{176}Cfor 1 h
using 4.5 �tL of RNA (2 �tg) in dH2O. One microliter of 5 x HT buffer
(GOBCO-BRL), 1 �tL dNTP mix (10 mM each dATP, dCTP, dGTP, and
dTTP), 1 p1 oligo (dT)18 primer (GIBCO-BRL), and 1 �tL of super-
script l1T� (GIBCO-BRL). The reaction was stopped by incubating the
mix at 70#{176}Cfor 10 mm. Two microliters of first strand cDNA were
added to 5 iiL of lOx PCR buffer (GIBCO-BRL), 1 �tL of dNTP mix
(10 mM each of dTTP, dATP, dCTP, and dGTP), 1.5 iiL of 1.5 mM
MgC12 (GIBCO-BRL) and 0.25 �tL of Taq DNA polymerase (GIBCO-
BRL). Each primer was added (2.5 �tL) to give a final primer concen-
tration of 250 pmol/mL. The total volume was adjusted to 50 �tL with
sterile dH2O. The mixture was subjected to DNA amplification using a
DNA thermal cycler (Perkin Elmer, Foster City, CA) set at different
annealing temperatures (60#{176}C for !L-2 and IFN-a and 55#{176}Cfor all
other genes) for a total of 20 cycles for �3-actin and 40 cycles for the
other genes. PCR fragments were separated on 2% agarose gel in TAE
buffer and stained with ethidium bromide (0.25 �ig/mL). The gels were
visualized and photographed using ultraviolet trans-illuminators (Kodak,
Rochester, NY). For quantitative studies and to confirm the specificity
of the amplified sequences, gels were processed for Southern blot analy-
sis. Oligonucleotide primers (Table 1) were synthesized commercially
(Genosys, Houston, TX) and purified by reverse-phase high-performance
liquid chromatography. Primers were designed based on the published
sequences with 5’ primers (+ strand) and 3’ primers (- strand) chosen
from within the coding region such that they were approximately 20 base
pairs in length, had similar melting temperatures, and flanked a single
intron so that any amplification of contaminating genomic DNA would
be readily identified. Specific oligonucleotide probes for exon se-
quences were synthesized in a similar fashion for use in Southern blot
analysis. The specificity of the product was verified by blotting the frag-
ments onto nylon membranes. Briefly, gels were denatured with 0.25
N HC1, washed with dH2O, and neutralized with 0.4 N NaOH. The am-
plified, denatured sequences were passively transferred to nylon mem-
brane. After transfer, membranes were washed in 2 x saline sodium cit-
rate and prehybridized for 4-18 h in a hybridization oven (Stratagene).
The membranes were hybridized with 32P-labeled (by nick translation)
specific oligonucleotide internal probes for 24 h, washed under strin-
gent conditions for each cytokine, and analyzed by digital (Phosphor-
Imager. Molecular Dynamics, Sunnyvale, CA) autoradiography. Relative
mRNA transcripts were obtained by using an equal number of cells with
simultaneous amplification, blotting, and probing the samples to be corn-
pared. Results were not used if the housekeeping gene signals between
groups were undetectable in a given sample/experiment. Cytokine sig-
nals are expressed as the ratio of each cytokine signal to the signal from
the housekeeping gene �-actin.
Statistics
The unpaired Student’s t-test was used to compare control and experi-
mental groups. The correlation coefficients were obtained by the Pearson’s
method using SPSS 7.0 for Windows (SPSS, Inc., Chicago, IL). A P value
of less than 0.05 was taken as significant.
RESU LTS
The frequency of monocyte and lymphocyte phenotypes in
PSC products was determined by fluorescence cytometry.
Each subpopulation was gated using side scatter and fluo-
rescence intensity for CD14, CD4, CD8, CD19, and T cell
receptor (TCR) a/� expression. Monocytes (CD14� cells)
were found in the low-density fraction (FR2), while lympho-
cytes were in the higher-density fractions (FR3-5). The re-
Cvtokine
TABLE 1. Oligonucleotide Primers and Probes
Primers
IL-2 CAA CTC CTG TCT TGC ATT GC
GCA TCC TGG TGA GTT TGG G
IL-4 ATG TGC CCG GGA ACT TTG TC
GTC TGT TAC GGT CAA CTC G
IFN-y CAG GTC ATT CAG ATG TAG CG
GCT TTT CGA AGT CAT CTC C
�3-actin TGA ACT GTG ACG TGG ACA TC
ACT CGT CAT ACT CCT GCT TG
TNF-a GAG CTG AGA GAT AAC CAG CTG GTG
CAG ATA CAT GGG CTC ATA CCA GGG
IL-b ATG CCC CAA GCT GAG AAC CAA GAC CCA
TCT CAA GGG GCT GGG TCA GCT ATC CCA
Probes
IL-2 GCA CTT GTC ACA AAC ACT CC
IL-4 GCG ATA TCA CCT TAC AGG AG
IFN-y GCA TCC AAA AGA GTG TGG AG
�3-actin CAA CAT CAT TGC TCC ICC TG
TNF-a CCC TCC ACC CAT GTG CTC CTC
IL-b CAG GTG AAG AAT CCC TTT AAT AAG CTC CAA CAG AAA CCC
ATC TAC AAA GCC ATG ACT GAC TTT GAC ATC
Purified ci�+
Purified CD8+
CD4,8-neg. FR2
CD4,8-neg.FR3-5
Normal PBL
I#{174}
I.
1’
#1
I#{174}
- I I I I I
0 20 40 60 80 100
% T cell Inhibition
-40 -20
TABLE 2. Cellular Phenotyping in each PSC Fraction
586 Journal of Leukocyte Biology Volume 61, May 1997
a/I3TCR�Cell population CD14� monocytes CD4� T cells CD8� T cells Granulocytes CD19� B cells CD4CD8
Unfractionated 41.1 ± 2.7a 22.3 ± 2.0 19.3 ± 2.1 6.6 ± 1.8 7.0 ± 2.1 0.49 ± 0.24
FR2 71.1 ± 3.4 7.2 ± 1.9 6.3 ± 1.8 4.3 ± 0.9 NDb 0.18 ± 0.12
FR3-5 4.1 ± 1.1 38.3 ± 3.2 34.5 ± 4.3 10.5 ± 4.1 ND 0.83 ± 0.36
Purified CD4� 0.7 ± 0.2 92.7 ± 1.1 2.4 ± 0.4 0.5 ± 0.1 ND ND
Purified CD8� 0.7 ± 0.4 8.6 ± 1.7 88.8 ± 0.7 0.5 ± 0.1 ND ND
FR2 after depletion of
CD4� and CD8� 86.8 ± 1.7 0.6 ± 0.2 1.9 ± 0.5 5.9 ± 2.1 1.2 ± 0.3 0.31 ± 0.20
FR3-5 after depletion
ofCD4� and CD8� 5.1 ± 1.1 3.6 ± 1.2 9.6 ± 3.5 18.7 ± 6.6 20.8 ± 3.1 1.77 ± 0.80
#{176}Mean± SE.
6ND: not determined.
sults of the cellular phenotyping in each fraction are sum-
marized in Table 2 . In the unfractionated PSC products,
the mean frequency of CD14�, CD4�, and CD8� T cells
were 41, 22, and 19%, respectively. After Percoll separa-
tion, CD14� cells were enriched to 71% in FR2. Purified
CD4� and CD84 cells from FR3-5, by immunomagnetic
separation, showed high enrichment of each T cell popu-
lation (93 and 89%, respectively). After depletion of
CD4� and CD8� cells, the remaining FR2 population
was highly enriched for CD14� cells (87%), and the re-
maining FR3-5 population contained few CD14�, CD4�,
or CD8� T cells, resulting in a relative enrichment of B
cells (CD19� cells), granulocytes, and CD4CD8 a/�T cells.
The unfractionated PSC had a depressed PHA (0.5 �ig/
mL) response (31,365 ± 6,486 cpm) compared with nor-
mal PB mononuclear cells (101,256 ± 7,214 cpm; P �
0.001). After Percoll separation, lymphocyte-enriched
FR3-5 (which had a 4.1 ± 1.7% monocyte contamina-
tion) had an increased PHA response (50,050 ± 8,741cpm), which was still lower than that observed with normal
PBL (P � 0.001). However, the PHA response in both the
purified CD4� (87,602 ± 20,120 cpm) and CD8� cells
(78,564 ± 9,215 cpm) was increased compared with the
unfractionated PSC (P � 0.001) and no significant differ-
ence in the mitogenic response was observed compared
with normal PBL, suggesting that in the absence of CD14�
cells, T cell functionality in these populations was normal.
Unfractionated PSC cells had significant T cell inhibitory
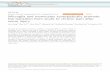
activity (71%) at an I:R ratio of 2:1 (Fig. 1), which was
dependent on the I:R ratio (results not shown). The CD14�
cell-enriched FR2 had increased inhibitory activity (87%)
when compared with unfractionated PSC. After T cell deple-
tion a further enrichment for CD14� cells was found in
FR2, which showed a significant increase in allogeneic T
cell inhibitory activity (94%) compared with unfractionated
cells (P � 0.001). In contrast, the lymphocyte-enriched
FR3-5 and purified CD4� or CD8� T cells had no inhib-
itory activity. Furthermore, the B and CD4CD8TCRa/
I�+ cell-enriched populations in the CD4- and CD8-
depleted FR3-5 cells also did not inhibit allogeneic T cell
proliferation. Similar results (Fig. 2) regarding susceptibil-
ity to CD14� cell inhibition of T cell blastogenesis were
found using autologous CD4� and CD8� cells after isola-
tion and addition to co-culture with highly purified CD14�
cells. These studies used an admixture (1:1) of isolated
(MiniMacs) autologous CD4� and CD8� cells from the
PSc products and >99% pure CD14� cells. The highly
purified monocytes were obtained by Percoll density gradi-
ent centrifugation and cell sorting by flow cytometry. This
resulted in a significant and cell ratio-dependent autolo-
gous inhibition of T cell proliferation. A similar inhibition
of T cell response to PHA was observed compared with
that of normal aliogeneic cells (Fig. 1). The isolated autol-
ogous T cells had a statistically normal response to PHA,
which was inhibited >80% by the isolated CD14� cells at
a 2:1 I:R ratio (n = 7). In all studies the inhibition of T
cell proliferation was dependent on the ratio of CD14�
cells in the co-culture. Thus, these results indicate that
CD14� cells from PSC products are the source of the in-
hibition for T cell proliferation. Further support for the
role of CD14� cells in the inhibition of T cell function is
Unfractionated
FR2
FR3-5
Fig. 1. Normal responder PBLS were co-cultured with irradiated inhib-
itory cells (PSC fractions) at an I:R ratio of 2:1 in the presence of 0.5
�.tg/mL of PHA for 4 days. The mitogenic response was determined by
�3H�thymidine incorporation and the percent inhibition calculated.Each bar represents mean ± SEM. � Significant increase compared with
normal PBL (P < 0.01). � Significant decrease compared with unfrac-
tionated PSC (P � 0.001).
100
>
c�o= U)0)1!)0!�- 0.
�U)
0.
Cl)-50
0 20 40 60 80 100
% CD14� cells
*
:�-CC
.�
U
I-.
100
80
60
40
20
0
*
2:1
*
0.5:1
lao et a!. CD14� cells in mobilized PSC producta 587
the significant correlation (Fig. 3) between CD14� cell fre-
quency and the inhibition of allogeneic T cell activity (r =
0.936, P � 0.001).
A role for cell-cell contact, compared with soluble fac-
toys, in the inhibition of T cell activity was suggested by ex-
periments using culture supernatants and transwell studies
that were negative for inhibitory activity compared with par-
allel co-culture assays (Table 3). The requirement for cell-
cell contact was directly demonstrated using PFA-treated
autologous CD14� and isolated CD4� and CD8� T cells.
PFA-fixed low density PSC cells (FR2), but not PFA-fixed
purified CD4� cells, showed significant T cell inhibitory
cell activity (75.1% at I:R = 2.1 and 14.2% at I:R =
0.5:1). In contrast, when the cells from PSC products were
co-cultured with normal PBMC in transwell chambers, T
cell inhibitory activity was completely abrogated (- 7.0%
at I:R = 2:1 and -9.3% at I:R = 0.5:1; Table 3). Studies
of T cell hypodiploidy (results not shown), DNA fragmenta-
tion (Fig. 4), and TUNEL analysis (Fig. 5) suggest that
apoptosis is involved in the mechanism of cytotoxicity. The
role of apoptosis is suggested by the studies using co-
cultures of CD14� cells and T lymphocytes with PHA,
which results in DNA fragmentation (Fig. 4, lane 5). In con-
trast, CD14� cells and lymphocytes without PHA stimula-
tion did not result in DNA fragmentation (Fig. 4, lane 4).
Similarly, lymphocytes alone, monocytes alone, and PHA
co-cultured lymphocytes did not result in DNA fragmenta-
tion (lanes 1, 2, and 3, respectively). Backgating of CD4�
or CD8� cells and TUNEL analysis revealed that, after
the co-incubation of PSC products in the presence of PHA,
41.2% of CD4� cells and 41% of CD8� cells became
1:1
l:Rratio
Fig. 2. T cell inhibitory activity for purified autologous CD4� and
CD8� cells was examined with highly purified CD14� monocytes pun-
fled to >99% purity by Percoll gradient centrifugation followed by flu-
onescent activited cell sorting. CD4� and CD8� cells were isolated to
>90% purity by Percoll gradient centrifugation followed by MiniMACS
isolation. The CD4� and CD8� cells were admixed at a 1:1 ratio and
co-cultured with the purified monocytes for 72 h with 0.5 �tg PHA
and the mitogenic response was determined by �3H�thymidine incon-
poration. Each bar represents mean ± SD (n = 7). ‘K Significant de-
crease compared with CD4 and CD8 cells without the addition of mono-
cytes (P � 0.01).
Fig. 3. Correlation between inhibitory cell function (I:R = 2:1) and
the frequency of CD14� cells within the Percoll separated fractions.
apoptotic (Fig. 5). In contrast, in the absence of PHA the
percent of apoptotic CD4� and CD8� cells were both 6.8%.
To determine the expression of potential immunoregula-
tory cytokines, we analyzed the expression of cytokine
genes in the PSC products compared with normal or PHA-
stimulated (24 h) PBL (Table 4). Significantly increased
expression of interleukin-2 (IL-2), interferon-y (IFN-’y),
TNF-a, IL-b, and IL-4 mRNA transcripts were observed
in PSC products compared with normal PBLs. Further-
more, the PSC product had significantly higher expression
of IL-b and IFN-’y compared with PHA-stimulated PBLS.
The level of IL-4 was also significantly higher in the PSC
products compared with PHA-stimulated PBLS, suggesting
a possible TH-2 phenotype. Overall, the levels of cytokine
mRNA from the PSC products more closely resembled
that of PHA-activated PBL than that of steady state PBL
cells.
DISCUSSION
The inhibition of T cell function is measured as the ability
of irradiated cells to depress the response of allogeneic or
autologous lymphocytes to PHA mitogenesis. This activity
is found with human [12, 19] and rodent BM cells [13, 16],
and spleen cells from mice after total lymphoid irradiation
[18], undergoing chronic graft-versus-host disease (GVHD)
[20], or after cyclophosphamide treatment [21]. In addi-
tion, Young et al. have reported that the induction of myelo-
poiesis-associated immune suppressor cells is stimulated
by GM-CSF and IL-3 [14, 15]. Thus, the observation of
T cell inhibitory activity in GM-CSF-mobilized PSC prod-
uct is not unanticipated. We recently reported that inhibi-
tory activity for T cell function is present in both GM-CSF-
mobilized PSC products from cancer patients [11] and in
the PB following autologous PSCT [7, 10]. We also re-
ported that non-mobilized PSC had little ability to inhibit
T cell mitogenesis [11] nor did cells from the patient’s PB
prior to mobilization [10]. We suggest, therefore, that the
inhibitory activity in PSC products (or their frequency) is
associated with mobilization and leukapheresis and may
TABLE 3. Role of Cell-to-Cell Contact for T Cell Inhibitory Cell Activity
588 Journal of Leukocyte Biology Volume 61, May 1997
Inhibitory cells Treatment
% Inhibition’�
I:Rd 2.1 I:Rd 0.5:1
PSC Normal coculture 82.7 ± 4.2 25.7 ± 6.9
PSC Transwell culture�’ -7.0 ± 2.6e �9.3 ± 1.8e
Low-density PSC No PFA treatment 91.6 ± 6.0 25.0 ± 9.1
Low-density PSC With PFA treatmentb 75.1 ± 4.4 14.2 ± 2.6
Purified CD4� cells With PFA treatment -1.5 ± 8.1� -8.7 ± 2.8�
(1 Responder PBMCs were cocultured with irradiated inhibitory cells for 72 h in culture plates with (or
without) inserting transwell chamber.
b Non-irradiated inhibitory cells were fixed with 1% PFA for 30 mm and similar T cell inhibitory cell assay
was performed.
( Values are mean ± SEM from three independent experiments.
�1l:R. inhibitor to responder ratio.
�Significant decrease compared with normal coculture (P � 0.05).
1Significant decrease compared with no PFA treatment (P � 0.05).
contribute to the immunosuppression observed in the pe-
ripheral blood post-transplantation.
To determine the phenotype of the apoptosis-inducing
cells, we fractionated PSC products by Percoll density gra-
dient centrifugation and immunomagnetic bead cell isola-
tion. The unfractionated PSC had a significantly decreased
proliferative response to PHA compared with normal PBL
consistent with our previous report [11J. In addition, the
inhibition of allogeneic and autologous PHA mitogenesis
by cells from the PSC products would suggest that post-
transplant T cell function is depressed (at least in part)
I 2 3 4 5
Fig. 4. CD14� cells induce activation-induced T cell apoptosis. CD14�
cells were coincubated with lymphocytes with or without PHA for 24 h,
washed, and analyzed for DNA fragmentation. Lane 1, lymphocytes
alone; lane 2, CD14� monocytes alone; lane 3, PHA-activated lympho-
cytes; lane 4, monocytes and lymphocytes without PHA after 24 h
co-culture; lane 5, monocytes and lymphocytes with PHA after 24 h
co-culture.
by the infused CD14� cells. However, the immune re-
sponse to PHA of purified CD4� or CD8� cells from the
PSc products was normal, suggesting that unstimulated T
cells remain functional. Furthermore, the CD14� cell fre-
quency correlated directly with the inhibition of T cell ac-
tivity. These results demonstrate that the primary pheno-
type of cells with T cell inhibitory activity in mobilized PSC
products is a CD14� cell that may be either a mature
monocyte or a monocytic-dendritic cell precursor [24].
This is consistent with our previous observation that non-
mobilized PSC cells from healthy donors with a low fre-
quency of CD14� cells (10%) had significantly lower levels
of inhibitory activity compared with GM-CSF-mobilized
PSc [11].The cellular origin of cells with inhibitory activity for T
cells has been controversial, with few studies that have fo-
cused on human cells. Strober et al. demonstrated that one
phenotype with this activity in both human and murine BM
is a CD4CD8c43TCR� T cell, which they termed a nat-
ural suppressor (NS) cell [12, 16]. Other studies have vari-
ously characterized NS cells as a member of the large gran-
ular lymphocyte family [17], null cells [22], macrophage/
monocyte lineage [13], or hematopoietic progenitor cells
[14]. However, in the present study, CD4�- and CD8�-
depleted cells from FR3-5 that are enriched in CD4
CD8TCRa/I� cells (1.8% on average) had no suppres-
sor cell activity. Several reports have demonstrated the role
of mature monocytes in the immunosuppression of cancer
patients [25, 26]. Unpublished studies from our laboratory
have shown that the depletion of phagocytic cells from PSC
products by carbonyl-iron phagocytosis abrogates T cell in-
hibitor activity ( - 26 ± 8%) compared with untreated
PSCs (61 ± 10% at a 2:1 I:R ratio) and partially restores
T cell function (59,905 ± 14,073 cpm) compared with
untreated PSCs (26,905 ± 5,128 cpm) in a PHA mito-
genic assay. This observation supports the suggestion that
CD14� monocytes are associated with the loss of T cell
function in human PSC products. We believe that this
CD14� cell activity differs from conventional NS cells [12]
based on the cellular phenotype and phagocytic nature.
Several investigators have reported that suppressor cells
CD4+ cells no PHA CD4+ cells PHA
IL8(SI
#{149}.‘i#{149}_6.8#{176}h
P.U.
iiA
� 10 1o2A�B
CD8+ cells no PHA
100 �;1 �;2 i?
A�B
F
CD8+ cells PHA
100 �;iAp0HB
1o3 100 �I #{243}’ �ApoHB
. .
mo et a!. CD14� cells in mobilized PSC products 589
§
§
h.
0.
Fig. 5. PSC products were co-cultured with or without PHA (0.5 �.tg4tL) for 24 h and stained with CD4. CD8. and TUNEL. The cells were backgated
on CD4 or CD8 and the frequency of apoptotic cells determined.
in rodents or rabbits can produce soluble factors that in-
hibit T cell functions [27, 28]. For example, prostaglandins
that are released by monocytes can mediate the suppres-
sion of T cell function in cancer patients [25]. The involve-
ment of nitric oxide and membrane-associated TNF in
macrophage-derived T cell inhibitory activity has also
been suggested [29-31]; although recently apoptosis has
also been proposed as a mechanism [32-34]. Wu et al.
[33, 34] demonstrated that human monocyte-induced
apoptosis of T cell lines required cell-cell contact. In our
studies, no role for a soluble factor was observed and a re-
quirement for cell-cell contact that results in T cell apopto-
sis is reported. Speculatively, a lack ofco-stimulation or up-
regulation of fas ligand rather than soluble factors could
be involved in the induction of T cell apoptosis by CD14�
cells in human PSC products. Preliminary studies (Table 2)
using HTPCR demonstrated higher levels of mRNA expres-
sion for TNF-a, IL-4, and IL-b in human PSC products
(with apoptosis-inducing activity) compared with normal
PBLS. However, this does not correlate with the inhibition
of T cell function and would be expected to result in a sol-
uble inhibitory activity.
Cells that depress T cell function also have clinical po-
tential to reduce the GVHD associated with allogeneic
transplantation. Sykes et al. and Palathumpat et al. re-
ported that NS cells could prevent GVHD in murine models
of allogeneic BMT [22, 35] and we suggest that the levels
of T cell inhibitory cell activity in PSC products may be
the reason for the decreased GVHD seen in patients trans-
planted using allogeneic PSC products [36, 37]. However,
TABLE 4. Transcriptional Expression of Cytokines by Stem Cell Products
590 Journal of Leukocyte Biology Volume 61, May 1997
PSC N PBL� PHA PBLI
n 6 10 9%a CD14�’ 35.36 ± 3.93-i 2.30 ± 0.74
NSC 4:lb 40.8 ± 7.1k 10.4 ± 15.6
PHA 0.5 �tg/mLb 18,975 ± 8,440k 154,873 ± 12,592 #{149}
El. TNFbd 0.409 ± 0.161k 0.016 ± 0.006 1.135 ± O.272�
E.l. IL-2’� 0.068 ± 0.040� 0.001 ± 0.001 0.698 ± 0.215�
E.I. !L-10�’ 1.302 ± 0.493� 0.034 ± 0.016 5.983 ± 4#{149}4#{216}3e
El. wNy&d 0.509 ± 0.143k #{216}#{216}#{216}4± 0.002 0.571 ± 0.16ME.I. IL�4b,�� 0.146 ± 0.035k 0.002 ± 0.001 0.005 ± 0.001
apercent CD14+ cells found by fluorescence cytometry.
bAverage value ± SE.
Clnhibitory cell activity (I:R = 4:1).
d Ratio of each cytokine signal to the signal from the housekeeping gene 3 actin. Units were analyzed b�
digital autoradiographs of the Southern blots in the linear range using image quart software.
�PBL from normal donors.
‘Twenty-four-hour PHA-stimulated normal PBL.
�Significantly different from normal PBL.
such cellular inhibitory activities are disadvantageous in
autologous PSCT recipients based on their ability to de-
crease T cell functions. We suggest, therefore, that the re-
moval of CD14� cells from autologous PSC products
before infusion may be a reasonable therapeutic strategy
to facilitate immune recovery and reduce treatment failure
following autologous PSCT. Indeed, the inability of mobi-
lized PSCT [38] to replicate the retrospective observation
of superior failure-free survival of steady state PSCT com-
pared with BMT reported by Vose et al. [2] may be due
to the difference in the frequency of CD14� cells.
ACKNOWLEDGM ENTS
This research was supported in part by National Institutes
of Health Grant RO1-CA61593 and Nebraska Cancer and
Smoking Disease Research Program Grant 97-71.
We thank the individuals involved in the harvesting and
processing of the stem cell products. Our thanks also to
Drs. Howard Gendelman, Michael Hoffingsworth, John
Sharp, and Rita Young for their review of the manuscript
and helpful suggestions.
REFERENCES
1. Kessinger, A., Bierman, P. J., Vose, J. M.. Armitage, J. 0. (1991) High-dosecyclophosphamide, carmustine, and etoposide followed by autologous pe-
ripheral stem cell transplantation for patients with relapsed Hodgkin’s dis-
ease. Blood 77, 2322-2325.2. Vose, J. M.. Anderson, J. R., Kessinger, A., Bierman, P. J., Coccia, P. Reed,
E. C., Gordon, B., Armitage. J. 0. (1993) High-dose chemotherapy andautologous hematopoietic stem-cell transplantation for aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 11, 1846-1851.
3. To, L. B.. Roberts, M., Haylock, D. (1992) Comparison of haematologicalrecovery times and supportive care requirements of autologous recoveryphase peripheral blood stem cell transplants, autologous bone marrow trans-
plants and allogeneic bone marrow transplants. Bone Marrow Traruplant.
9, 277-284.
4. Henon, P. R.. Liang, H., Beck-Wirth. C., Eisenmann, J., Lepers, M.,
Wunder, E., Kandel, C. (1992) Comparison of hematopoietic and immunerecovery after autologous bone marrow or blood stem cell transplants. Bone
Marrow Tmnsplanz. 9, 285-291.5. Roberts, M. M., To, L. B, Gillis, D.. Mundy. J.. Rawling, C.. Ng, K.. Juttner.
C. (1993) Immune reconstitution following peripheral blood stem cell trans-plantation, autologous bone marrow transplantation and allogeneic bone
marrow transplantation. Bone Marrow Transplant. 12. 469-475.6. Kiesel, S., Pezzutto, A., Korbling, M., Haas, R., Schulz, R., Hunstein, W..
Dorken, B. (1989) Autologous peripheral blood stem cell transplantation:
Analysis of autografted cells and lymphocyte recovery. Tmnsplant. Proc.
21, 3084-3088.7. Talmadge. J. E.. Reed, E., mo, K.. Kessinger, A.. Kuszynski. C.. Heimann.
D.. Varney, M., Jackson. J., Vose. J. M., Bierman. P. J. (1996) Rapid im-
munologic reconstitution following transplantation with mobilized peniph-
eral blood stem cells as compared to bone marrow. Bone Marrow Tmns-plant. in press.
8. Klingemann, H. C. (1995) Introducing graft-versus-leukemia into autolo-gous stem cell transplantation. .L Henzatother. 4, 261-267.
9. Verma. U. N.. Areman, E., Dickerson, S. A., Kotula. P. L., Sacher, R.,
Mazumder, A. (1995) Intenleukin-2 activation of chemotherapy and growthfactor-mobilized peripheral blood stem cells for generation ofcytotoxic effec-
tors. Bone Marrow Tran.splaru. 15. 199-206.10. Talmadge. J. E., Reed, E. C., Kessinger. A., Kuszynski, C. A., Perry.
C. A.. Gordy, C. L., Mills, K. C., Thomas, M. L., Pirruccello, S. J., Letheby.B. A.. Arneson, M. A., Jackson, J. D. (1996) Immunologic attributes of cyto-kine mobilized peripheral blood stem cells and recovery following transplan-
tation. Bone Marrow Tran.splani. 17, 101-109.1 1 . Mills. K. C., Gross, 1’. C., Varney, M. L.. Heimann. D. C.. Reed, E. C..
Kessinger, A., Talmadge, J. E. (1996) Immunologic phenotype and functionin human bone marrow, blood stem cells and umbilical cord blood. Bone
Marrow Transplant. 18, 53-61.12. Schmitz-Wolf, I. C., Dejbakhsh-Jones, S., Ginzton, N., Greenberg, P.,
Strober, S. (1992) T-cell subsets and suppressor cells in human bone mar-
row. Blood 80, 3242-3250.13. Noga, S. J., Wagner, J. E.. Horwitz. R. L.. Donnenberg, A. D.. Santos, C. W..
Hess, A. D. (1988) Characterization of natural suppressor cell populations
in adult rat bone marrow. J. Leukoc. Biol. 43, 279-287.14. Schmidt-Pak, A.. Wright, M. A., Matthews, J. R, Collins, S. L., Petruzzelli,
C., Young, M. R. I. (1995) Mechanisms of immune suppression in patientswith head and neck cancer: Presence of immune suppressive CD34+ cells
in cancers that secrete granulocyte/macrophage colony-stimulating factor.
Clin. Cancer Re.s. 1, 95-103.15. Young, M. R. I.. Young, M. E.. Wright, M. A. (1990) Stimulation of immune-
suppressive bone marrow cells by colony-stimulating factors. Exp. Hema�l.
18, 806-811.16. Palathumpat, V., Dejbakhsh-Jones, S., HoIm, B., Wang. H.. Liang, 0..
Strober, S. (1992) Studies of CD4CD8a�3� bone marrow T cells withsuppressor activity. J. ImmunoL 148, 373-380.
17. Maier, T., Holda, J. H., Claman, H. N. (1986) Natural suppressor (NS)cells: Members ofthe LGL regulatory family. immunol. Today 7. 312-315.
18. Oseroff, A., Okada, S., Strober, S. (1984) Natural suppressor (NS) cellsfound in the spleen of neonatal mice and adult mice given total lymphoidirradiation (TLI) express the null surface phenotype. J. ImmunoL 132,101-110.
mw ci a!. CD14� cells in mobilized PSC products 591
19. Mortani, F., Singhal, S. K. (1988) Production of human bone marrow-
derived suppressor factor. Effect on antibody synthesis and lectin-activatedcell proliferation. J. Immunol. 141. 3037-3042.
20. Maier, T., Holda, J. H., Claman. H. N. (1985) Graft-vs-host reactions(GVHR) across minor munine histocompatibility barriers. II. Developmentof natural suppressor cell activity. J. Immunol. 135, 1644-1651.
21. Maier, T., Holda, J. H., Claman, H. N. (1989) Munine natural suppressorcells in the newborn, in bone marrow, and after cyclophosphamide: Geneticvariations and dependence on IFN�gammat. J Immunol. 143. 491 -498.
22. Sykes. M.. Eisenthal, A., Sachs, D. H. (1988) Mechanism of protectionfrom graft-vs-host disease in munine mixed allogeneic chimeras. I. Devel-
opment of a null cell population suppressive of cell-mediated lympholysisresponses and derived from the syngeneic bone marrow component. J.Immunol. 140. 2903-2911.
23. Mollereau, B.. Deckert, M., Deas, 0.. Rieux-Laucat. F., Hirsch, F..Bernard. A.. Fischer. A., Lynch, H., Charpentier, R. LeDeist. F.. Senik.A. (1996) CD2-induced apoptosis in activated human peripheral T cells.
J. Immunol. 156. 3184-3190.24. Szabolcs, P.. Avigan. D., Gezelter. S.. Ciocon. D. H.. Moore, M. A. S.. Stein-
man, R. M., Young, J. W. (1996) Dendnitic cells and macrophages can ma-tune independently from a human bone marrow-derived. post-colony-forming unit intermediate. Blood 87, 4520-4530.
25. Wanebo. H. J.. Riley. T.. Katz. D.. Pace. R. C.. Johns. M. E.. Cantrell, R. W.(1988) Indomethacin sensitive suppressor-cell activity in head and neckcancer patients. The role of the adherent mononuclear cell. Cancer 61,462-474.
26. Farmnas, M. C., Rodniguez-Valverde� V.. Zarrabeitia, M. T., Parra-Blanco,
J. A., Sanz-Ortiz, J. (1991) Contribution of monocytes to the decreasedlymphoproliferative response to phytohemagglutinin in patients with lung
cancer. Cancer 68, 1279-1284.27. Hertel-Wulif. B.. Strober, S. (1988) Immunosuppressive lymphokine de-
nived from natural suppressor cells. J. Immunol. 140, 2633-2638.28. Vlasselaer, P. V., Niki, T., Strober, S. (1991) Identification of a factor(s) from
cloned murine natural suppressor cells that inhibits IL-2 secretion duringantigen-driven T cell activation. Cell. ImmunoL 138. 326-340.
29. Mills, C. D. (1991) Molecular basis of “suppressor” macrophages. Argininemetabolism via the nitric oxide synthetase pathway. J. ImmunoL 146.
2719-2723.
30. Schleifer, K. W., Mansfield, J. M. (1993) Suppressor macrophages in Afri-can trypanosomiasis inhibit T cell proliferative responses by nitric oxideand prostaglandins. J. ImmwsoL 151, 5492-5503.
31 . Tomioka, H.. Sato, K., Maw, W. W.. Saito, H. (1995) The role of tumor ne-crosis factor, interferon-y, transforming growth factor-a. and nitric oxide in
the expression of immunosuppressive functions of splenic macrophages in-duced by Mycobacterium avium complex infection. J. Leukoc. BiOL 58.704-712.
32. Munn, D. H.. Pressey, J., Beall. A. C.. Hudes. R.. Alderson, M. R. (1996)Selective activation-induced apoptosis of peripheral T cells imposed by
macrophages. 1 Immunol. 156, 523-532.33. Wu, M. X.. Daley, J. F., Rasmussen, R. A., Schlossman, S. F. (1995) Mono-
cytes are required to prime peripheral blood I cells to undergo apoptosis.
Proc. Nazi. Acad. Sci. USA 92, 1525-1529.34. Wu. M. X., Ao� Z., Hegen. M.. Monimoto, C., Schlosaman. S. F. (1996) Re-
quirement of Fas(CD9S), CD4S, and CD11a/CD18 in monocyte-dependentapoptosis of human T Cells�. J. Immunol. 157, 707-713.
35. Palathumpat, V., Dejbakhsh-Jones. S., HoIm. B.. Strober, S. (1992) Differ-ent subsets of T cells in the adult mouse bone marrow and spleen induceor suppress acute graft-versus-host disease. I ImmunoL 149, 808-817.
36. Korbling, M.. Przepiorka, D., Huh, Y. 0., Engel. H., Van Besien. K., Giralt,S.. Anderson. B.. Kleine, H. D.. Seong, D., Deisseroth, A. R (1995) ADo-
geneic blood stem cell transplantation for refractory leukemia and lym-phoma: Potential advantage of blood over marrow allografts. Blood 85,1659-1665.
37. Azevedo, W. M. (1995) Allogeneic transplantation with blood stem cells
mobilized by rhG-CSF for hematological malignancies. Bone Marrow Traas-plans. 16. 647-653.
38. Liberti. C., Pearce, R., Taghipour. C.. Majolino, I., Goldsione, A. H. (1994)
Comparison of peripheral blood stem-cell and autologous bone marrowtransplantation for lymphoma patients: A case-controlled analysis of the
EBMT Registry data. Ann. OncoL 5, 5151-5153.
Related Documents