MODELING OF A HYDROGENATED VACUUM GAS OIL HYDROCRACKER by JAGANNATHAN GOVINDHAKANNAN, B.Tech., M.Tech. A DISSERTATION IN CHEMICAL ENGINEERING Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Approved Chairperson of the Commip^o^ -.3^ -—^.. ^1 «- Accepted Dean of the Graduate School May, 2003

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

MODELING OF A HYDROGENATED VACUUM
GAS OIL HYDROCRACKER
by
JAGANNATHAN GOVINDHAKANNAN, B.Tech., M.Tech.
A DISSERTATION
IN
CHEMICAL ENGINEERING
Submitted to the Graduate Faculty of Texas Tech University in
Partial Fulfillment of the Requirements for
the Degree of
DOCTOR OF PHILOSOPHY
Approved
Chairperson of the Commip^o^
- . 3 ^ -—^.. ^1 « -
Accepted
Dean of the Graduate School
May, 2003

ACKNOWLEDGMENTS
I have been blessed throughout my life having wonderful people around me
guiding and correcting my path. The acknowledgments section is a very small tribute to
all of them.
When I started working on the hydrocracker project in the fall of 1998, I really
couldn't have imagined that the research work would grow to such great dimensions.
IVlany of my fiiends in the industry commented that I am trying to do something very
ambitious and some of them cautioned me that I might not get the relevant industrial data.
As I am in the final stages of my doctoral research, it becomes very clear to me that this
project would not have been possible without the constant support and encouragement
from Professor James Riggs. I would like to thank him for the excellent financial support.
The expression of my gratitude will not be complete if I forgot to mention the memorable
trips to Sunoco Refinery at Samia, Canada (we saw the beautifiil Niagara), Baker Process
at Salt Lake City, and numerous visits to meet Professor Froment at Texas A&M
University.
Professor Froment has been kind enough to offer his valuable guidance to my
research work. The single event concept is his brainchild and I have been fortunate
enough to work with one of the greatest minds in the history of chemical engineering.
Every time I meet him at College Station, I was rejuvenated and motivated. He has
become more than a mentor to me. I would like to thank him for his guidance and also for
the post-doctoral fellowship offer. I am really excited to work with him to ftirther our
research interests in modeling, simulation and optimization of complex reactor systems.
I would like to thank Professor Karlene Hoo for teaching us the whole new way
of understanding and solving problems. After she came to Texas Tech University, the
process control education took a new turn in probing and learning fiindamental concepts
and modem control theory. Her profound knowledge and high ethical standards are the
greatest assets to any educational program. I would like to thank her for serving in my
11

doctoral committee and for the several stimulating discussion sessions on various topics
including multivariate statistical methods.
I would like to thank my other committee members, Professor Richard Tock and
Professor Surya Liman for their assistance in the project. Prof Liman will always be
remembered for his lucid teaching of linear programming, spontaneous wit, and humor.
The hydrocracker project would not have been complete without the SOL data
from Dr. Stephen Jaffe of ExxonMobil. I wish to express my sincere thanks to him for his
willingness to take the time to answer my questions and providing us the valuable help on
the data. He and his group are doing a wonderfiil job of elevating the kinetic modeling of
complex refinery reactor systems to a very advanced level.
My special thanks go to Professor M. Chidambaram of Indian Institute of
Technology, Chennai, for his guidance through the years. His enthusiasm for research has
a perpetual influence in my career. Professor Chang Bock Chung helped me a great deal
in understanding continuum modeling. I would like to thank him. Dr. In-Su Han's
resourcefulness and timely help is a wonderfiil thing to have. I would like to thank
Professor Ashok Khanna of Indian Institute of Technology, Kanpur and Professor Balu of
Aima University, Chennai for motivating me to pursue higher studies. I would like to
extend my sincere thanks to Professor Arasarethinam, an impressive teacher and a very
close family fiiend. His passionate service to the student community is highly
appreciated.
The section on acknowledgements could not be completed without mentioning the
help and advice I received from Dr. Ramesh Krishnan during the past four-and-half
year's stay at Lubbock. I would like to express my sincere thanks to him and his family.
My fellow students and fiiends helped me a lot. I want to thank Kishor, Xuan Li,
Satish, Rohit, Shihai Feng, Surjo, Matt, Dale, Eric, Daguang, Zhenhua, Rahul, Vibha, and
Vasantha. My sincere thanks to Marybeth and Jan for helping me with every problem
from parking permit to travel vouchers.
My wife Subhashini does not like me thanking her at any time. Without her help,
caring, and understanding, I would not have kept round-the-clock schedule for research. I
111

am indebted to her. Her mother's help when our son Surya was bom is highly
appreciated.
Finally, I would like to dedicate this work to my parents Jagannathan and
Amaravathi and my sister Valli whose wisdom and love kept me going all these years.
IV

TABLE OF CONTENTS
ACKNOWLEDGEMENTS ii
ABSTRACT vii
LIST OF TABLES ix
LIST OF FIGURES x
LIST OF SYMBOLS xii
1. INTRODUCTION 1
1.1. Scope of the Present Work 3
1.2. Modeling Methodology 4
1.3. Organization of the Dissertation 6
2. LITERATURE REVIEW 7
2.1. Lumped Models 7
2.2. Mechanistic Models 9
3. HYDROCRACKING PROCESS DESCRIPTION AND CHEMICAL REACTIONS 11
3.1. Hydrocracking Process Description 11
3.1.1. Process Variables 13
3.2. Hydrocracking Catalysts 14
3.3. Chemical Steps Involved in Hydrocracking 14
4. REACTION NETWORK GENERATION 28
4.1. Standardized Labeling 35
4.1.1. Standardized Labeling for Acyclic Hydrocarbon Species 3 5
4.1.2. Standardized Labeling for Cyclic Hydrocarbon Species 37
4.2. Network Generation 40
4.2.1. List of Possible Species 48
4.2.2. Network Generation Software 48
4.2.3. Network Generation Sample Results 50

5. SINGLE EVENT KINETICS AND NORMAL OCTANE HYDROISOMERIZATION AND HYDROCRACKING 52
5.1. Single Event Theory 52
5.1.1. Calculation of Number of Single Events 54
5.2. Normal Octane Hydroisomerization and Hydrocracking 57
5.2.1. Rate Expressions for Paraffins, Olefins and Carbenium Ions 58
5.2.2. NormaUzation Scheme 62
5.2.3. Reactor Model 63
5.2.4. Results and Discussion 63
6. MODEL PARAMETER ESTIMATION 71
6.1. Lumping Coefficients 72
6.1.1. Single Event Rate Parameters 77
6.2. Model Parameter Estimation 77
6.2.1. Discussion 86
7. HYDROCRACKER SIMULATIONS 88
7.1. Profiles along the Length of the Catalyst Bed 90
7.2. Optimization 99
8. SUMMARY AND CONTRIBUTIONS 103
8.1. Summary 101
8.2. Contiibutions 107
8.3. Recommendations 107
BIBLIOGRAPHY 110
VI

ABSTRACT
Hydrocracking is used in the petroleum industry to convert low-quality feedstocks
into highly-valued fransportation fuels such as gasoline, diesel, and jet fiiel.
Hydrocracking is usually carried out in two stages. The first stage decomposes sulfur-
and nifrogen-containing compounds and hydrogenates the aromatics. The liquid fraction
from the first stage is hydroisomerized and hydrocracked in the second stage. The
primary objective of the present research is to develop a very detailed, fiindamental, and
molecular-level model for the second stage hydrocracking process.
The modeling methodologies reported in the literature for the hydrocracking
process thus far, describe the feed and product compositions based on the boiling range
and the actual reaction network is reduced to a smaller number of reactions between the
lumped species. The present approach applies the concept of single event kinetics to the
hydrocracking process. In this approach, the various reactions involved in hydrocracking
are considered in terms of fiindamental elementary steps involving carbocations.
A computer algorithm, in which feed and product molecules, carbocations, and
olefinic intermediates are represented by means of Boolean relation matrices and
characterization vectors has been developed to generate the elementary reaction networks
for paraffinic, naphthenic, and aromatic feed components. The standardized labeling
algorithms for acyclic and cyclic hydrocarbon stmctures are developed. The network
generation leads to a very large network of elementary reactions (>10^). However, due to
the molecular nature of the approach, the number of rate parameters is kept within the
tractable limits (<30) and the rate parameters are independent of the feedstock
composition.
Since the number of chemical species generated in the reaction network is very
large, a certain degree of lumping is required to reduce the number of continuity
equations for the components to be integrated along the reactor. The lumps should be
chosen in terms of the present day analytical capabilities. The single event kinetic model,
in the present work, considers the pure components and lumps according to the carbon
number. Each lump is defined by its carbon number and the type of chemical structure
Vll

that represents that lump. The type of chemical stmctures considered here are n-paraffins,
iso-paraffins, mono-, di-, tri-, and tetra-naphthenes, mono-, di-, tri-, and tetra-aromatics,
and naphtheno-mono-, naphtheno-di-, and naphtheno-tri-aromatics. Some lumps are
individual molecules while most are collection of molecules. For the lump involving a
collection of molecules, the properties of the lump are determined by averaging of the
properties of each individual molecule comprising the lump.
The model parameters are estimated from the synthetic product distribution data
obtained from an industrial organization. A partially hydrogenated vacuum gas oil (VGO)
is considered as the feedstock. The single event kinetic model is inserted into a
homogeneous reactor model and the resulting continuity equations are integrated
numerically along the length of the catalyst bed. The reactor simulation results are the
temperature profile, composition profiles, and hydrogen consumption profile through the
catalyst bed. The hydrogen consumption is calculated in a very rigorous way in the single
event model, which is not possible with the lumped models. The reactor simulation
results are consistent with industrial practice and published information.
A profit optimization study is carried out to evaluate the aspects of the single
event approach for process optimization. The molecular nature of the single event
approach provides a framework to calculate important properties such as Reid vapor
pressure (RVP) and octane number that are difficult to estimate using the lumped models.
Vll l

LIST OF TABLES
3.1 Relative Stabilities of Gas-Phase Carbenium Ions 17
4.1 Network Details for Paraffins 50
4.2 Network Details for Naphthenes 51
4.3 Network Details for Aromatics 51
5.1 List of Cracked Products from n-Octane Hydrocracking 5 8
5.2 Single Event Rate Coefficients for n-Octane Hydroisomerization
and Hydrocracking 62
5.3 Operating Conditions for Model Parameter Estimation 64
5.4 Target Distribution and Predicted Distribution 65
5.5 Single Event Rate Coefficients from Parameter Estimation 65
6.1 Weight Percent of Individual Groups in HYGO Feedstock 78
6.2 Typical Operating Conditions for Product Distribution 79
6.3 Single Event Rate Coefficients 80
6.4 Single Event Rate Coefficients Sensitive to Product Distribution 80
6.5 R^ for Arrhenius' Law Plots 86
7.1 Temperature Increase in Catalyst Beds with Different Inlet Temperatures 90
7.2 Components and Lumps in Terms of Oil Fractions 92
7.3 Product Distribution for Different Inlet Temperatures 101
IX

LIST OF FIGURES
1.1 Overview of the Hydrocracker Model 5
3.1 Process Flow Diagram for Hydrocracking Process 12
3.2 Paring Reaction of a Cyclo-alkane Carbenium Ion 19
3.3 Hydrocracking Reactions for Paraffins 22
3.4 Hydrocracking Reactions for Naphthenes I 23
3.5 Hydrocracking Reactions for Naphthenes n 24
3.6 Hydrocracking Reactions for Aromatics I 25
3.7 Hydrocracking Reactions for Aromatics n 26
3.8 Hydrocracking Reactions for Aromatics DI 27
4.1 Boolean Relation Matrix and Characterization Vector 29
4.2 Dealkylation Elementary Step Using Boolean Relation Matrices 34
4.3 StandardizedLabelingof Acyclic Hydrocarbon Species 37
4.4 Standardized Labeling of Cyclic Hydrocarbon Species 39
4.5 PCP Steps for an Acyclic Carbenium Ion 44
4.6 Network Generation Algorithm for Paraffins 45
4.7 Network Generation Algorithm for Naphthenes 46
4.8 Network Generation Algorithm for Aromatics 47
4.9 Stmcture of the Network Generation Software 49
4.10 CPU Time Requirement for Paraffins Network Generation 50
5.1 Conversion into C8 Isomers 66
5.2 Distribution of C8 Fraction 66
5.3 Mono-branched Isomers 67
5.4 Di-branched Isomers I 67
5.5 Di-branched Isomers n 68
5.6 Tri-branched Isomers 68
5.7 Cracked Products 69
6.1 Lumping Coefficients for PCP (s;s) Isomerization of n-Paraffins 76
6.2 Industrial Data vs. Model Predictions at a Low Temperature 82

6.3 hidustiial Data vs. Model Predictions at a Moderate Temperature 82
6.4 Industiial Data vs. Model Predictions at a High Temperature 83
6.5 Normalized Arrhenius Law Plot for Single Event Rate Coefficient kpcp(s;s) 83
6.6 Normalized Arrhenius Law Plot for Single Event Rate Coefficient kcr(s;t) 84
6.7 Normalized Arrhenius Law Plot for Single Event Rate Coefficient kcr(t;s) 84
6.8 Normalized Arrhenius Law Plot for Single Event Rate Coefficient kcr(t;t) 85
6.9 Normalized Arrhenius Law Plot for Single Event Rate Coefficient kgn 85
7.1 Temperature Profile along the Catalyst Bed 91
7.2 Hydrogen Consumption 92
7.3 Evolution of Various Oil Fractions 94
7.4 Components in LPG Fraction 95
7.5 Paraffinic Components in Gasoline Fraction I 95
7.6 Paraffinic Components in Gasoline Fraction II 96
7.7 Iso-paraffinic Lumps in MDS Fraction 97
7.8 Di-naphthenic Lumps in Residue 97
7.9 Molar Ratio of Iso-paraffins to Normal Paraffins in Gasoline Fraction 99
7.10 Profit Optimization 101
XI

LIST OF SYMBOLS
C^^ Concentration of free active acid sites on catalyst
Co. Olefin concenfration on the catalyst surface
C, Total concentration of active acid sites
C,a, Saturation concentration of physisorbed hydrocarbons
C + Concentration of a carbenium ion
Cp Specific heat capacity
fi, Fj Molar flow rate of component /
AG Free energy change at standard conditions
AH Enthalpy change at standard conditions
H^ A Proton from an acid site
^DH iu) Equilibrium constant for dehydrogenation of a paraffin P, to olefin Oy
ATpr iOy; m) Single event equilibrium constant for protonation of Olefin Oij to a
carbenium ion of type m
K isom iOyiO^) Single event equilibrium constant for isomerization of Olefin Oy to a
reference olefin Or
K^. Langmuir physisorption coefficient for hydrocarbon i
kjj^ im; Oy ) Rate coefficient for deprotonation of a carbenium ion of type m to an
olefin
k^^ im) Rate coefficient for protonation of olefin to a carbenium ion of type m
kff^ im;n) Rate coefficient for hydride shift for a carbenium ion of type m to type n
k^fg im; n) Rate coefficient for methyl shift for a carbenium ion of type m to type n
kpcp {^\«) Rate coefficient for protonated cyclopropane (PCP) branching of a
carbenium ion type m to type n
Xll

k(.^ im;n,0) Ra te coefficient for cracking of a carbeium ion of type m to carbenium ion
of type n and a olefin
k^^ Ra te coefficient for endocyclic scission of a naphthenic carbenium ion
^Q, Rate coefficient for cyclization
^Deaik ( '") Rate coefficient for dealkylation of an aromatic carbenium ion with
formation of an acycHc carbenium ion of type m
^Aik i^) Ra te coefficient for alkylation of an aromatic carbenium ion with an
acyclic carbenium ion of type m
kj^^p Ra te coefficient for disproportionation
m Mass flow rate of hydrocarbon and hydrogen feed
«g N u m b e r of single events for an elementary reaction
AS" Entropy change at standard conditions
w Catalyst weight in the reactor
jV; Mole fraction of a component in a lump
z Axial distance in the reactor
Greek Letters
a Symmetry number
\\ Number of optical isomers
Q Cross-sectional area of the reactor
p^ Bulk density of the catalyst
Xll l

CHAPTER 1
INTRODUCTION
Hydrocracking is one of the most versatile of all petroleum-refining processes. It
usually converts a heavy, low quality feedstock into lighter, valuable transportation fiiels,
contributing significantly to the overall profitability of the refinery. Any fraction from
naphtha to non-distillables can be processed to produce a desired product with a
molecular weight lower than that of the charge stock. Hydrocracking is predominantly
suited to producing middle distillates with excellent product qualities. Jet and diesel
fractions can be obtained with very low sulfur contents often below 200 ppm and very
good combustion properties with kerosene smoke points above 25 mm and diesel cetane
numbers above 55, respectively (Minderhoud, 1999). The reason is the use of high
hydrogen partial pressures, which results in high removal rates of hetero-atoms (sulfiir,
nitrogen) contained in the feedstock and deep saturation of the aromatic compounds.
Other applications of this process include the upgrading of petrochemical feed stocks,
improving the gasoline octane number, and producing high quality lubricants (Mohanty
et al. 1990). The recent interest in the hydrocracking process is due to the shift in the
demand for gasoline as compared to the middle distillates and the availability of large
amounts of low-cost, byproduct-hydrogen from catalytic reforming operations (Gary and
Handwerk, 1984). Some of the advantages of the hydrocracking process are Usted below
(Gary and Handwerk, 1984):
1. AbiUty to vary the ratio of gasoline/distillate in product streams,
2. Improved octane number for gasoline,
3. High ratio of iso-butane/n-butane in butane fraction.
Hydrocracking supplements catalytic cracking to upgrade heavy cracking stocks,
aromatics, cycle oils, and coker gas oils to gasoline, jet fuels, and light fiiel oils. In a
modem refinery, catalytic cracking and hydrocracking units work as a team. The catalytic
cracker takes the more easily cracked paraffinic atinospheric and vacuum gas oils as
charge stocks, while the hydrocrackers process more aromatic cycle oils and coker

distillates as feedstocks. The latter feed streams are very refractory and resist catalytic
cracking while higher pressures (35-100 bar) of hydrogen make them relatively easy to
hydrocrack.
The terms hydrotreating, hydroprocessing, hydrocracking, and
hydrodesulfurization are used rather loosely in the petroleum industry (Gary and
Handwerk, 1984). Hydrotreating refers to a relatively mild operation whose primary
purpose is to saturate the olefins and/or reduce the sulfur and/or nitrogen content and
leave the boiling range of the feed unchanged. Hydrocracking refers to processes whose
primary purpose is to reduce the boiling range in which the feed is converted to boiling
ranges lower than that of the feed. Hydrotreating and hydrocracking set the two ends of
the spectrum and those processes that resulting a substantial amount of sulfiir and/or
nitrogen removed and a significant change in the boiling range of the products, with
respect to the feed, are called hydroprocessing. In general, the hydrocracking operations
process distillate feeds (naphtha to gas oil) and the hydroprocessing operations usually
freat heavy residues (boiling point >475 °C).
The first modem hydrocracking operation was placed on-stream in 1959 by the
Standard Oil Company (now ExxonMobil) of Califomia. The unit was small, producing
1000 barrels per sfream day (BPSD). When a unit was installed to complement an
existing fluid catalytic cracking (FCC) unit, it was quickly recognized that the
hydrocracking process had the flexibility to produce varying ratios of gasoline and
middle distillates. Thus, the stage was set for rapid growth in US hydrocracking capacity
from about 3000 BPSD in 1961 to about 120,000 BPSD in just 5 years. From 1965 to
1983, the US capacity has grown eight-fold, to about 980,000 BPSD (Meyers, 1996). The
world hydrocracking capacity is estimated to be 200 million tones per aimum and the
highest growth rate is expected to be in the Asia-Pacific zone (Minderhoud, 1999).
Strong pressures to increase refinery margins has led to an increasing interest in
optimizing and revamping existing hydrocrackers in the last five years and this frend is
expected to continue into the next decade (Minderhoud, 1999).

Developing reliable kinetic models for hydrocracking process is an important
activity both from a commercial and a research viewpoint. The use of comprehensive
process models with an accurate representation of hydrocracking kinetics will reduce the
expensive experimentation in pilot plants. The mathematical model is a useful tool in
process design to predict detailed product distribution and optimum operating conditions
for a given range of feedstocks. The potential use of such a model can be fully realized in
optimizing (offline/online) the process for maximum profit. Further, the model can be
used for de-bottlenecking studies, planning plant strategies, and process control. In
hydrocracker-optimization projects the typical benefits are shown to be $0.30 per barrel
of the hydrocracker feed when the optimizer is implemented along with proper
multivariable advanced confroUer to implement the set points calculated by the optimizer
(Kane, 2002). The benefits include 3-15% increase in the distillate yield over the design
specifications.
1.1 Scope of the Present Work
The hydrocracking process is often carried out in two stages. In the first stage,
sulfur, nitrogen, and oxygen compounds are decomposed; aromatics and olefins are
saturated. The liquid fraction from the first stage is hydroisomerized and hydrocracked in
the second stage. The current research work primarily focuses on the reactions taking
place in the second stage.
The following are the primary objectives of the present work.
1. Develop a detailed steady state model of an industrial hydrocracker for optimization
studies and/or optimization applications;
2. Estimate the kinetic parameters from hydrocracker feed/product data obtained from
an industrial organization;
3. Carry out simulation studies to develop insights (e.g., i-paraffins/n-paraffins ratio
with inlet temperature) about the hydrocracking process;
4. Identify the limitations of the modeling approach used in this research work.

1.2 Modeling Methodology
The feedstocks processed in the petroleum industries contain a large number of
feed components in measurable quantities. Each of these innumerous components reacts
in a complicated reaction pathway leading to very large network of reactions. In
fraditional kinetic modeling, the actual reaction network is reduced to a small number of
reactions among a smaller number of lumped species. The lumps are normally defined
based on the boiling point description. It is often very difficult or almost impossible to
characterize the chemical species present in the lumps. Since the composition of the lump
is not rigorously defined, it is possible to have many different possible components with
the same boiling point representation. Another potential problem associated with the
lumps is the number of rate parameters. The total number of rate parameters is directly
proportional to the number of lumps (Vynckier and Froment, 1991). As the number of
lumps increase, the number of rate parameters also increase thus, making the parameter
estimation problem more difficult in computing perspective.
In confrast to the fraditional approach, the present research applies the novel
concept called Single Event Kinetics (SEK) to model the hydrocracking kinetics. The
single event concept was originally developed by Froment and co-workers (Vynckier and
Froment, 1991). The essential features of this approach are:
1. Single event kinetics considers individual molecules.
2. Full details of the reaction pathways are retained.
3. Reaction network is generated in terms of fimdamental elementary reactions.
4. Rate parameters are invariant with respect to the feed composition and they are
fractable (<30) in number.
The important steps involved in developing a hydrocracker model are illustrated
in Figure 1.1. The first important step is to understand the carbenium ion chemistry,
which forms the backbone of the hydrocracking kinetics. The elementary steps are
generated using a computer algorithm in which the reactants and products are represented
by Boolean relation matrices. The reaction network generation is an intensive
computational activity that requires high-speed computers and a considerable amount of

computer memory. The elementary reaction network for paraffins up to carbon number
40 takes approximately nine days of CPU time on a 600 MHz alpha machine using a
Compaq Fortran compiler. The amount of computing time involved in generating the
reaction networks need not be a concem to the end user of the hydrocracker model
because network generation is an offline activity. In other words, once the networks are
generated they can be used as look-up tables for ftirther model building. Once the
elementary step network generation is completed, the concept of single events can be
used to identify the independent set of rate coefficients. It is worth noting that unlike
other lumping approaches, in the single event approach, the number of rate parameters
does not increase with the number of components or lumps (Vynckier and Froment,
1991).
Figure 1.1 Overview ofthe Hydrocracker Model

The model building activity requires the estimation of large number of
dehydrogenation and isomerization equilibrium constants (> 10,000), which are calculated
using Benson's group contribution methods (Benson et al., 1969). The single event
kinetic parameters require the geometry of the transition states or activated complex
formed in various elementary reactions. The geometry of a transition stmcture can
reliably be calculated using any of the quantum chemical packages such as Gaussian,
MOP AC, and GAMESS (Young, 2001; Schmidt et al, 1993). The lumping coefficients
calculations are required to formulate the rate expressions and once the rate expressions
are known, they can be inserted into a suitable reactor model for parameter estimation
and further simulation studies. Finally, the model should be validated against plant data
obtained from an industrial hydrocracker facility.
1.3 Organization ofthe Dissertation
The dissertation is organized based on the major steps shown in Figure 1.1. A
review of the hydrocracker modeling is provided in the second chapter. The
hydrocracking process and the effect of critical process variables are discussed in the
third chapter. This chapter also presents a comprehensive summary of various chemical
reactions involved in hydrocracking. Chapter 4 explains how the elementary reaction
networks are generated for paraffinic, naphthenic, aromatic, and naphtheno-aromatic feed
components. The single event theory and the rigorous formulation of rate expressions for
the single event model are explained in Chapter 5. The chapter also discusses n-octane
hydroisomerization and hydrocracking as an example to explain how the single event
kinetic parameters can be estimated using a model component such as n-octane. The
calculation of lumping coefficients and subsequent formulation of rate expressions for the
relumped hydrocracker model with a partially hydrogenated vacuum gas oil (YGO) feed
is presented in the sixth chapter. This chapter also discusses model parameter estimation
resuhs from industrial data. The hydrocracker simulation results are presented in Chapter
7. Finally, the conclusions and recommendations for future work are presented in Chapter
8.

CHAPTER 2
LITERATURE REYIEW
Modeling hydrocracking kinetics has become necessary since hydrocracking has
become an important secondary process in refineries. Mathematical models are needed to
predict yields of various products for process design, optimization, and control
applications.
The feedstocks processed in the petroleum industries consist of a large number of
components. A typical feed for an industiial hydrocracker unit contains paraffins,
isoparaffins, naphthenes, aromatics, and naptheno-aromatic components. These
components follow very complicated reaction pathways with carbenium ion
intermediates. Modeling such chemical processes becomes very complex due to the
extiemely large number of reactions and difficulties in measuring feed and product
compositions. The modeling methodologies developed over the years for cracking
systems, such as catalytic cracking and hydrocracking, can be classified into two broad
categories: (1) Lumping models and (2) Mechanistic models. The past and new
developments in these two areas of modeling are reviewed briefly below.
2.1 Lumped Models
In lumped models, the actual reaction network is reduced to a small number of
reactions among the lumped species. The lumps, based on compound types present in
feedstocks and products (e.g., lumps of gas oil, Liquefied Petroleum Gases (LPG),
gasoline, diesel, coke, etc.), are often defined by boiling point ranges. This approach is
also known as discrete lumping. Weekman and Nace (1970) proposed a three-lump
model with 3 reactions for the catalytic cracking of gas oil fractions. Jacob et al. (1976)
revised this three-lump model to a ten-lump model with 17 reactions. Zhorov et al.
(1971), Tom et al. (1972), Stangeland and Kittrell (1972), and Quader et al. (1970)
proposed similar models based on the discrete lumping approach. The ability to model
the cracking process accurately, using this lumping procedure, lies in choosing as many

lumps as possible. However, this may lead to a large number of model parameters (e.g.,
rate constants). A major disadvantage of all the lumping approaches is that a change in
the product specifications (e.g., reducing the initial boiling point of gasoline) or in the
number of products (e.g., eliminating heavy naphtha cut) requires reformulating the
model and refitting the data.
Stangeland (1974) suggested a discrete lumping approach for modeling
hydrocracking kinetics based on ordinary differential equations with a suitable yield
distribution function. The model considers pseudo-components derived from the boiling
point description for the feed and products. This approach is only suitable for
accommodating first-order kinetics. Krishna and Saxena (1989) suggested a different
approach, in which, they considered hydrocracking to be analogous to axial dispersion.
This results in a simple model with a minimum number of model parameters. The major
drawback of this model is that it shows significant error in yield predictions of various
products at higher severities (Laxminarasimhan et al., 1996).
In confrast to discrete lumping approach, the continuum theory of lumping
considers the reaction mixture to form a continuous mixture with respect to its species
type, boiling point, molecular weight, etc. The notion of a continuous mixture has been
applied to distillation (Amundson and Acrivos, 1955), thermodynamics (Briano and
Glandt, 1988), polymerization (Zeeman and Amundson 1965), reactions in continuous
mixtures (Aris and Gavalas, 1966; Luss and Hutchison, 1971; Krambeck, 1984; Astarita
and Ocone, 1988; Chou and Ho, 1989), and coke formation from olefinic oligomers
(McCoy and Balasubramaniam, 1995). Chou and Ho (1988) developed an approach for
freating the continuum of nonlinear mixtures.
Cicarelli et al. (1992) have formulated a methodology for modeling a catalytic
cracking process using the continuum theory of lumping. Lasminarasimhan et al. (1996)
appUed the continuum theory of lumping to the hydrocracking of vacuum gas oil. The
model formulation in terms of integro-differential equations is based on the feedstock
tme-boiling-point (TBP) data. A skewed Gaussian-type distribution is used to describe
the yield distribution of various petroleum fractions. This function was developed from

experimental data on the yield pattems of the hydrocracking various model compounds
reported in the literature. This formulation however is unable to account for the actual
chemistry of the hydrocracking process. Other problems associated with this approach
include, estimating the correct quantity of hydrogen consumption and obtaining a
continuous description of the feed components from an otherwise discrete feed
distribution. Govindhakannan (2001) describes a methodology to obtain such a
continuous feed disfribution using a constrained optimization approach.
The problem with the lumped schemes and lumped kinetic parameters is their
specificity: for each different hydrocarbon feed, even within the same homologous series,
different sets of lumped parameters have to be determined (Baltanas et al., 1989). This
means that in complex mixtures, as dealt with in industrial practice, the number of
parameters becomes overwhelming. Such issues have forced a more fundamental
approach that considers the chemistry ofthe hydrocracking process.
2.2 Mechanistic Models
Mechanistic models can account for the actual chemistry of the cracking process.
A new methodology known as the single event kinetics to model complex reaction
systems has been developed at the 'Laborarotium voor Petrochemische Techniek' in Gent
by Froment and co-workers (Baltanas et al., 1989; Vynckier and Froment, 1991).
Baltanas and Froment (1985) used a computer algorithm to generate the complete
reaction network for hydrocracking of paraffins, taking into account all the reactions
involving individual molecules. Yynckier and Froment (1991) extended the single event
approach to complex feedstocks and introduced the concept of lumping coefficients to
formulate rate expressions in a convenient way. Feng et al. (1993) formaUzed the single
event kinetics for catalytic cracking and estimated single event parameters for catalytic
cracking of paraffins on a RE-Y zeolite catalyst. Svoboda et al. (1995) estimated single
event parameters for hydrocracking of paraffins on a Pt/US-Y zeolite catalyst. Schweitzer
et al. (1999) estimated single event rate parameters from experiments canied out on
hexadecane and validated single event concept for the hydrocracking of paraffins in a

three-phase reactor for the Fishcher-Tropsch process. Dewachtere et al. (1999) applied a
single event kinetic model in the simulation of an industrial riser reactor for the catalytic
cracking of vacuum gas oil. Martens et al. (2000) applied a single event kinetic model for
the hydrocracking of Cg to C12 paraffins on Pt/US-Y zeolites. Martens et al. (2001)
estimated single event parameters for the hydrocracking of cyclo-alkanes on Pt/US-Y
zeolites. Due to the molecular nature of the approach, it was found that a finite and
limited number of kinetic parameters could describe the hydrocracking of heavy
feedstocks.
Liguras and Allen (1989) developed an approach based on carbon centers, in
which, the model components are developed using linear programming. This
methodology was used to describe the reactions in catalytic cracking where it was found
that there were no significant differences in the product distribution for different sets of
pseudocomponents, as long as the number of pseudocomponenets is large (>100). Klein
et al. (1991) considered thousands of model components and generated reaction networks
for asphaltene hydroprocessing using a Monte-Carlo simulation approach. Quarm and
Jaffe (1992) proposed a novel approach, which is very close to the chemistry of the
process. The lumping sfrategy is based on the molecular stmcture ofthe feed and product
components. They call this approach Stmcture Oriented Lumping (SOL).
The recent shift in research towards the mechanistic models clearly shows the
importance of incorporating the actual chemistry in building high fidelity models for the
hydrocracking process. It is sfrongly believed that the present research will contribute to
the understanding of the complexities of the hydrocracking process, provide economic
and operational benefits to the petroleum refineries, and stimulate further research both in
academia and industry.
10

CHAPTER 3
HYDROCRACKING PROCESS DESCRIPTION AND CHEMICAL REACTIONS
3.1 Hydrocracking Process Description
Many different flow schemes have been developed for the hydrocracking process
so that various feeds can be processed to produce a full range of products. All of the
processes are vendor specific with respect to reactor design and catalyst selection. As a
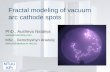
typical example, a single stage flow scheme for the hydrocracking process is presented in
Figure 3.1 (Meyers, 1996). The single-stage flow scheme is the mostly widely used
because of its efficient design, which results in minimum cost for a flill-conversion
operation. The feedstock, recycle oil, and recycle gas are exchanged against reactor
effluent to recover process heat and are then sent through a final charge heater and into
the reactor section. Yarious reactions such as, hydrogenation, dehydrogenation,
isomerization, C-C bond scission, paring reaction, alkylation, dealkylation,
disproportionations, and cyclization take place in the hydrocracking reactors.
Hydrogenation reactions are highly exothermic (42,000 kJ/kmol hydrogen consumed)
and the cracking reactions are endothermic. The amount of heat liberated in the
hydrogenation reactions is greater than the heat required for the endothermic cracking
reactions. The surplus heat released causes the reactor temperature to increase thereby
accelerating the reaction rate. Cold hydrogen is injected into the reactor as a quench to
confrol the reactor temperature profile. The reactor effluent is sent through the heat
exchangers to a hot separator, where light products are flashed overhead and heavy
components are recovered as liquid bottoms. The use of a hot separator improves the
energy efficiency of the process by allowing the hot liquid to go to the fractionation frain
and prevents poly-nuclear aromatic fouling in cold parts of the plant. The overhead
fraction from the hot separator goes to a cold separator, where recycle gas is separated
from the product. The product is then sent to fractionation and recycle gas is retumed to
the reactor via the recycle compressor.
11

Reactors 1 & 2
Charge Heater
Feed
H2
\ /
=a
Recycle
High Pressure Separator Fractionator
Flash Gases
Flash Drum
Hot Separator
PNA Control
04-
LN
IN
MD
LN: Light naphtha, IN: Intermediate naphtha, MD: Middle distillates
Figure 3.1 Process Flow Diagram for Hydrocracking Process
12

The fractionation train contains a stripper column and a main fractionator. The
stripper column removes hydrogen sulfide and this removal ensures a relatively clean
product in the main fractionator, thus reducing the column cost and metallurgy
requirements (e.g., corrosion). The main fractionator separates all valuable products. The
heavy material from the bottom fraction is recycled back to the reactor.
The severity of the hydrocracking operation is measured by the degree of
conversion ofthe feed to lighter products. Conversion is defined as the volume percent of
the feed, which disappears to form products boiling below the desired product end point.
A given percent conversion at a low product endpoint represents a more severe operation
than does the same percent conversion at a higher product endpoint.
3.1.1 Process Variables
The primary reaction variables are reactor temperature, pressure, space velocity,
hydrogen consumption, nifrogen content of the feed, and hydrogen sulfide content of the
gases.
Reactor temperature is the primary means of conversion control. As the catalyst
ages it is necessary to raise the average temperature to compensate for the loss in catalyst
activity (0.1 °F/day) (Gary and Handwerk, 1984). Temperatiire confrol is achieved by
injecting cold hydrogen between the adjacent catalyst beds. The primary effect of reactor
pressure is through the partial pressures of hydrogen and ammonia. An increase in total
pressure increases the partial pressures of both hydrogen and ammonia. Conversion
increases with increasing hydrogen partial pressure and decreases with ammonia partial
pressure. The hydrogen effect is greater, however, and the net effect of raising the total
pressure is to increase conversion.
The space velocity, generally reported as Liquid Hourly Space Velocity (LHSV),
is the ratio ofthe liquid volumetric flow rate to catalyst volume. The catalyst volume is
constant; therefore, the space velocity varies directly with feed rate. As the feed rate
increases, the time of the catalyst contact for a given volume of feed is decreased and
13

conversion is lowered. In order to maintain conversion at the proper level, when the feed
rate is increased, it is necessary to increase the temperature.
The organic nifrogen content of the feed is of great importance as the
hydrocracking catalyst is deactivated by contact with organic nitrogen compounds. An
increase in the organic nifrogen content of the feed is known to cause a decrease in the
conversion. At low concenfrations, the presence of hydrogen sulfide acts as a catalyst to
inhibit tiie saturation of the aromatic rings. This conserves hydrogen and produces a
product with a higher octane than its naphthenic counterpart. However, hydrocracking in
the presence of a small amount of hydrogen sulfide normally produces a very low smoke
point jet fuel. At high hydrogen sulfide levels, corrosion ofthe equipment is possible and
the cracking activity ofthe catalyst is also adversely affected.
3.2 Hydrocracking Catalysts
There are a number of hydrocracking catalysts available (McKetta, 1992) and the
actual composition is tailored to the process, feed material, and the products desired.
Hydrocracking catalysts are bi-functional in nature, in that, they combine cracking and
hydrogenation activity in varying proportions to obtain the desired product distribution.
Hydrogenation activity is achieved through the use of metal promoters impregnated on a
catalyst support. The hydrogenation components can be from noble metals and non-noble
metals of VIB and Vin groups. Sufficient metal loading on the catalyst ensures that the
rate determining steps are on the acidic sites. This means the hydrogenation and
dehydrogenation reactions occurring on the metal sites can be considered to reach quasi-
equilibrium. The cracking activity comes from the acidic part of the catalyst by using
Si02/Al203 support.
3.3 Chemical Steps Involved in Hydrocracking
Hydrocracking in the petroleum industry is often carried out in two stages
(Froment, 1991). In the first stage, sulfur and nitrogen containing compounds are
decomposed and aromatics are hydrogenated. The liquid fraction coming from the first
14

stage is hydroisomerized and hydrocracked in the second stage. In the present work, the
kinetic modeling focuses primarily on the various chemical reactions (Corma et al., 1992;
Vynckier and Froment, 1991; Campbell and Wojciechowski, 1971; Olah, 1973; Egan et
al., 1961; Greenfelder et al., 1949) taking place in the second stage.
The hydrocracking of petroleum fractions involves complex chemistry, which
includes hydrogenation-dehydrogenation, isomerization, carbon-carbon bond scission,
paring reaction, ring opening, alkylation, disproportionation, and cyclization reactions.
These reactions take place on a bifunctional catalyst consisting of both cracking (e.g.,
silica/alumina, etc.) and hydrogenation/dehydrogenation components (noble metal and
non-noble metals of VIB and VHI). The cracking catalysts such as SiOz-AbOs and the
zeolites have been foimd to have both Bronsted and Lewis acid sites (Gates et al., 1979).
The Bronsted sites are responsible for proton donation and the Lewis sites for accepting
election pairs.
The chemistry of hydrocracking is essentially the carbocation chemistry plus the
chemistry of hydrogenation and dehydrogenation. The concept of carbocations grew
through kinetic, stereochemical, and product studies of a wide variety of reactions,
especially uni-molecular, nucleophilic substitutions and eliminations (Olah, 1973). Direct
observation of stable, long-hved carbocations, generally in highly acidic (superacid)
solvent systems, has become possible in recent years due to improvements in analytical
techniques. The thermochemistry of gaseous cations has become experimentally
accessible by the technique of mass spectrometry. In this method, the neufral molecules
are ionized and the molecular or fragment ions or both are recognized according to their
mass/charge ratio and kinetic energy (Levsen, 1978). Although there is ample
experimental evidence of carbocations in the gas phase and in solutions, direct evidence
(e.g., half-life, energy, etc.) for the presence of carbocations on the surface of a working
catalyst has, however, never been provided (Martens, 1990).
Olah (1973) classified the carbocations into two distinct classes: (1) Trivalent
("classical") carbenium ions and (2) Penta- (or tefra) coordinated ("non-classical")
carbonium ions. The trivalent classical carbenium ions (e.g., CR3 ) containing an sp -
15

hybridized election deficient central carbon atom (with six elections in the valence shell),
which, in the absence of constraining skeletal rigidity or steric interference, tends to give
a planar (or close to planar) arrangement with the directly bonded atoms. The non-
classical carbonium ions (e.g., CRj" ) contain five or four coordinated carbon atoms with
eight elections in the valence shell. The carbon atom carrying the positive charge in a
carbonium ion is bonded by three single bonds, and a two electron, three-center bond.
Note tiiat the "carbenium ion" should be used only for the trivalent ions and not as a
generic name for all carbocations.
The carbocations, mentioned above, can form by several different paths. The
protonation of a saturated hydrocarbon in the presence of a superacid with very high
proton-donor sfrength generates a non-classical carbonium ion intermediate. The penta-
coordinated carbon atom loses a hydrogen molecule to become a carbenium ion. Hydride
absfraction (-H") from a saturated hydrocarbon yields a classical carbenium ion on a
Lewis site. Protonation of olefinic intermediates (on the Bronsted sites) formed by
dehydrogenation on the metal generates a classical carbenium ion. The reaction path by
way of the olefins is still generally accepted for reforming, hydroisomerization, and
hydrocracking.
In the primary carbenium ions, the positively charged carbon is connected to two
hydrogen atoms and one aUcyl group. The methyl carbenium ion is a primary cation with
three hydrogen atoms connected to the charged carbon. In secondary carbenium ions, the
carbon atom with the positive charge is connected to two methyl groups and a hydrogen
atom. Similarly, in tertiary ions, the charged carbon atom is connected to three alkyl
groups. The energy required for carbenium ion formation increases with an increase in
the number of hydrogen atoms attached to the carbon atom carrying the positive charge.
The stability of the carbenium ion decreases in the order of increasing ionization
energies, E+. The relative values of ionization energy for primary, secondary, and tertiary
ions are given in Table 3.1 (Gates et al., 1979).
16

Table 3.1 Relative Stability of Gas-Phase Carbenium Ions
Type of Ion Relative Value of E+, kJ/gmol
Primary ion 87.864
Secondary ion 58.576
Tertiary ion 0.0
The tertiary carbenium ion is by far the most stable species, and it is the easiest to
form and the most prevalent whenever it can be formed. The pattern observed in the gas
phase, regarding the stability of carbenium ions, is valid for the carbenium ions in
solution and on surfaces, as inferred from product distributions of many reactions (Gates,
1979).
The present work considers partially hydrogenated Vacuum Gas Oil (VGO) as the
feed to the hydrocracking imit. The VGO feed contains paraffinic, naphthenic, aromatic
and naphtheno-aromatic feed components. The reactions for paraffins are given in Figure
3.2. After physisorption (described by Langmuir isotherm) in the zeolite cages of a
hydrocracking catalyst, the paraffins are dehydrogenated on the metal component of the
catalyst. The resulting olefins are protonated in the Bronsted acid sites, yielding the
alkane carbenium ions. These cations isomerize through hydride shifts and methyl shifts.
Hydride shifts and methyl shifts preserve the degree of branching but it is hypothesized
that protonated cyclopropane branching (PCP) steps after the number of side chains
present in the reactant molecule. Cracking occurs through scission of the carbon-carbon
bond in the p-position with respect to the carbon atom carrying the positive charge.
Cracking requires that the |3-carbon with respect to the positively charged carbon be a
tertiary carbon atom. This ensures that the hydrocracking reactions avoid producing or
produce very little amount of methane and ethane in the cracked products. After
deprotonation ofthe alkane carbenium ions the resulting acyclic olefins are hydrogenated
to produce highly branched paraffin isomers with reduced chain lengths.
17

The hydroisomerization and hydrocracking reactions for the naphthenic feed
components are shown in Figure 3.3. Dehydrogenation of naphthenes on the metal sites
of the catalyst leads to the formation of cyclic mono-olefins, which are protonated to
cyclic carbenium ions. The cyclic carbenium ions isomerize through hydride shift, methyl
shift, and PCP branching. Hydride and methyl shifts do not alter the branching degree in
the naphthenes. However, methyl shifts alter the relative positions of the substituents on
the ring.
Three different kinds of PCP steps are possible in naphthenes. The first one,
known as acyclic PCP branching, which is similar to the PCP step in paraffins, changes
the branching degree in the alkyl side chain attached to the ring. The second type is
known as the intra-ring alkyl shift, which leads to ring contraction or ring expansion
without altering the branching degree of the naphthenic ring. The third type is known as
the cyclic PCP step, which causes ring contraction or ring expansion while altering the
degree of branching ofthe naphthenic ring.
There are two kinds of P-scissions possible with the naphthenic carbenium ions. If
the P-scission occurs on the alkyl side chain or if the alkyl side chain is severed from the
ring, then it is known as the exocyclic P-scission (Vynckier and Froment, 1991). The
cleavage of a carbon-carbon bond that is part of the ring is known as an endocyclic P-
scission (Vynckier and Froment, 1991). Exocyclic p-scission produces an olefinic and a
cationic species of reduced carbon number whereas the endocyclic P-scission produces a
single species containing a double bond and a positively charged carbon atom. The
alkene- or cyclo-alkene carbenium ions produced by endocyclic p-scission steps may
ftirther undergo reactions similar to that of alkane- and cycloalkane carbenium ions.
Compared to p-scission in an aliphatic chain (acyclic beta scission), endocyclic P-
scission is known to proceed at a much slower rate (Brouwer, 1970). Another typical
reaction, known as the paring reaction, occurs in the hydrocracking of cycloalkanes of
carbon number 10 or above, hi this reaction, peeling or paring ofthe methyl groups from
the cycloalkane ring occurs with essentially no loss of ring stmctiire (Egan et al.,1961).
18

This paring reaction can be seen as the sequence of hydride shift, methyl shift, and ring
confraction followed by an (t, t) exocyclic P-scission as shown in Figure 3.2.
Figure 3.2 Paring Reaction of a Cyclo-alkane Carbenium Ion
Additionally, the paring reaction produces lower molecular weight cycloalkanes and
branched alkanes, principally isobutene with a high conservation of ring stmcture (Egan
et al.,1961).
The typical reactions involved in hydrocracking of aromatic feed components are
shown in Figure 3.4. Alkyl-substituted aromatic hydrocarbons are highly reactive in
catalytic cracking systems when the alkyl groups are C3 or larger. Therefore, the
characteristics of both the aromatic ring and the alkyl side chain are responsible for the
case of cracking of such compounds, although the aromatic ring remains essentially intact
(Greensfelder, 1949). Since the aromatic ring is not cleaved in the hydrocracking
reactions, the carbon-carbon bond breaking occurs mostly in substituted alkyl and
cycloaUcyl groups and saturated rings condensed (e.g., naphtheno-aromatic components)
with the aromatic rings (Greensfelder, 1949). The alkyl chain attached to the aromatic
ring undergoes dehydrogenation on the metal sites of the hydrocracking catalyst to
produce an aromatic olefin. This aromatic olefin can imdergo protonation on the acid
sites to produce an aromatic carbenium ion. Aromatic carbenium ions can also form
through the direct protonation ofthe aromatic molecules as experienced from cracking of
model components such as cumene (Campbell, 1971). hi the case of direct protonation,
the positive charge lies on the aromatic ring. Aromatic carbenium ions with the positive
charge on the alkyl group carbon atom may isomerize through hydride shift, methyl shift
and PCP branching, ft is believed that the isomerization steps are similar to those
19

observed in paraffinic and naphthenic carbenium ions. Alkyl side chains attached to the
aromatic ring can also undergo P-scission to produce an acyclic (aromatic) olefin and an
aromatic (alkane) carbenium ion.
The major reaction of olefins with added aromatics over acidic zeolites at
temperatures 300 °C or less is alkylation (Olah, 1964). Carbon-carbon bond formation
occurs in alkylation. The electrophilic attack of an alkane carbenium ion on the n-
elections of the aromatic ring system forms an aromatic carbenium ion with positive
charge on the ring. The product cation can be thought of as a a-complex, which can re-
aromatize to an alkylated aromatic component by giving up the proton from the
tetiahedral carbon by deprotonation. Kinetic studies of aromatic alkylation indicate the
rate-determining step in this process is the formation ofthe sigma complex (Olah, 1964).
On a solid catalyst, the alkylation reaction takes place by way of a Friedel-Crafts
mechanism. This mechanism presupposes the adsorption of the alkylating agent on an
active site on the catalyst surface. In the case of a solid-acid catalyst, the active site is a
Bronstead acid site and the adsorbed product is the alkane carbenium ion (Corma, 1992).
Alkyl aromatic molecules undergo disproportionation reaction also known as
frans-alkylation, with an aromatic carbenium ion having the positive charge on its ring. In
this reaction, the alkyl group from the aromatic carbenium ion (with positive charge on
the ring) breaks away from the ring and joins with the aromatic ring of an aromatic
component. The disproportionation reaction produces an aromatic component and an
aromatic carbenium ion with the positive charge located on its ring. Another important
reaction that occurs in alkyl aromatics is cyclization. This leads to the formation of
naptheno-aromatic components. For this reaction to occur, the reacting aromatic
carbenium ion must have five or more carbon atoms in its alkyl chain with the positive
charge suitably located on one ofthe carbons in the alkyl chain. The resulting naphtheno-
aromatic carbenium ion has the positive charge on the aromatic ring. Naphtheno-aromatic
components and their corresponding carbenium ions undergo similar reactions as that of
aromatics and naphthenes.
20

The aromatic carbenium ions with positive charge on the ring deprotonate to
produce aromatic components. The aromatic carbenium ions with positive charge on the
alkyl group and the naphtheno-aromatic carbenium ions with the positive charge either on
the alkyl chain or on the naphthenic ring deprotonate to yield the corresponding olefins
which are hydrogenated rapidly on the metal sites ofthe catalyst.
The reactions listed in Figures 3.2-3.4 are considered as elementary steps, except
the hydrogenation and dehydrogenation reactions occurring on the metal sites of the
hydrocracking catalyst.
21

DEHYDROGENATION
PROTONATION
HYDRIDE SHIFT
METHYL SHIFT
PCP BRANCHING
ACYCLIC p-SCISSION
DEPROTONATION
HYDROGENATION
Figure 3.3 Hydrocracking Reactions for Paraffins
22

DEH'.'DROGENATION
PROTONATION
HYDRIDE SHIFT
METHYL SHIFT
P A «
PCP BRANCHING (IN ALKYL GROUP )
PCP BRANCHING (RING CONTRACTION /EXPANSION WTTHOUT ALTERING THE BRANCHING DEGREE )
Figure 3.4 Hydrocracking Reactions for Naphthenes I
23

PCP BRANCHING (RING CONTRACTION / EXPANSION ALTERING THE BRANCHING DEGREE)
EXOCYCLIC P-SCISSION (IN ALKYL GROUP )
EXOCYCLIC P-SCISSION
ENDOCYCLIC P -SCISSION
DEPROTONATION
. H,
HYDROGENATION
Figure 3.5 Hydrocracking Reactions for Naphthenes n
24

DEHYDROGENATION
PROTONATION ON THE SIDE CHAIN
PROTONATION ON THE RING
HYDRIDE SfflFT
METHYL SHIFT
PCP BRANCHING
Figure 3.6 Hydrocracking Reactions for Aromatics I
25

-^^
EXOCYCLIC P-SCISSION
ALKYLATION
DEALKYLATION
H
DISPROPORTIONATION
CYCLIZATION
Figure 3.7 Hydrocracking Reactions for Aromatics n
26

H
DEPROTONATION (CHARGE ON RING)
H
DEPROTONATION (CHARGE ON ALKYL GROUP)
H,
HYDROGENATION
Figure 3.8 Hydrocracking Reactions for Aromatics HI
27

CHAPTER 4
REACTION NETWORK GENERATION
The yarious elementary reactions involved in hydrocracking of paraffinic,
naphthenic, aromatic and naphtheno-aromatic components are generated using a
computer algorithm. This chapter describes the methodology used for reaction network
generation.
Clymans and Froment (1984) used Boolean relation matrices to represent
reactants and products in generating reaction networks for thermal cracking of normal
and branched paraffins. BaUanas et al. (1985) used a Boolean relation matrix along with a
characterization vector to represent a hydrocarbon stmcture and they generated the
reaction networks for the hydrocracking of paraffins. Figure 4.1 shows such a Boolean
relation matrix and a characterization vector for a naphthenic carbenium ion with the
positive charge on the alkyl carbon atom. If a hydrocarbon has n carbon atoms, the order
ofthe Boolean relation matrix will be n and the size ofthe characterization vector will be
2n+I. The Boolean relation matrix element ii,j) will have the status "TRUE" if there is a
bond between carbon atoms i and j . If there is no bond between carbons i and j , the
element (y) will be assigned a status "FALSE". In Figure 4.1, the Boolean relation
matrix has a "TRUE" status in elements (1,7) and (7,1) because carbon atoms 1 and 7 are
bonded and vice versa. The statuses ofthe location (2,6) and (6,2) are "FALSE" because
carbon atoms 2 and 6 are not bonded.
The first element in the characterization vector (shown below the Boolean relation
matrix in Figure 4.1) represents the location ofthe positive charge, the next n elements
represent the nature of the carbon atoms and the last n elements indicate the type of the
carbon atoms. The indicators 1, 2, 3, and 4 represent the primary, secondary, tertiary, and
quaternary carbon atoms, respectively. An arbitrary set of numbers is used to designate
the type of the individual carbon atoms in a hydrocarbon species (Froment, 1999). The
type indicators are: (1) bridge-head aromatic carbon; (2) bridge-head cyclo-olefinic
carbon; (3) bridge-head naphthenic carbon; (4) aromatic carbon; (5) cyclo-olefinic
carbon; (6) naphthenic carbon; (7) acyclic olefinic carbon; (8) acyclic paraffinic carbon.
28

hi Figure 4.1, the value ofthe first element in the characterization vector is 7 because the
charge is located on the carbon atom labeled as 7. The next eight elements describe the
nature of the eight carbon atoms. For example, the carbon atom labeled as 1 in the
hydrocarbon stiiicture is a tertiary carbon connected to three different carbon atoms 2, 6,
and 7. The nature of this tertiary carbon atom is indicated in element 2 of the
characterization vector with a value 3. The value 3 is assigned to note that the particular
carbon atom with the label 1 is a tertiary carbon atom. Similarly, the 9" element in the
characterization vector represents the carbon atom labeled as 8 in the hydrocarbon
stiiicture. This location has a value of 1 meaning that the carbon atom labeled as 8 in the
hydrocarbon stiiicture is a primary carbon. The last eight elements are reserved for the
type of carbon atoms. The carbon atoms labeled as 1 through 6 in the hydrocarbon
stioicture are all naphthenic carbons, which are represented by the type indicator 6 and the
carbon atoms labeled as 7 and 8 are paraffinic atoms, which are represented by the type
indicator 8.
1 2
1 ^ I T
2 1 T
3 1 T
4 :
5
6 T
7 i T
8
3
T
T
4
T
: T
5
T
T
6
T
T
7
T
T
8
T
7 3 2 i 2 2 2 1 2 2 1 6 6 6 6 6 6 8 j 8
Figure 4.1 Boolean Relation Matrix and Characterization Vector
29

The concept of a Boolean relation matrix is as follows. If the carbon atoms (y) in
a hydrocarbon species are bonded, then the mafrix element will have a status "TRUE"
otherwise it is "FALSE". However, constmcting a characterization vector for a
hydrocarbon species is more involved. The following examples will show how the
characterization vector is consttiicted for a given hydrocarbon species.
Example 1: An acyclic carbenium ion
The above carbenium ion has 8 carbon atoms. The positive charge is located at the carbon
atom labeled as 3. Its characterization vector of size 17, which comes from the formula
2n+I, is given below.
3 1 4 2 3 1 1 7 1 8 8 8 8 8 8 8 8
The first element in the characterization vector specifies the location of the
positive charge. The next 8 elements are for the nature of the carbon atoms. The carbon
atom, labeled as 2, in the hydrocarbon stmcture is a quaternary carbon. Hence, element 3
will have a value 4. The carbon atom, labeled as 4 in the hydrocarbon stmcture is a
tertiary atom, whose corresponding location in the characterization vector 5 will have a
value of 3. Since all the carbon atoms are of paraffinic type, the locations from 10 to 17
in the characterization vector are assigned a value 8.
30

Example 2: A mono-aromatic olefin
The above aromatic component has 9 carbon atoms. This species does not carry any
positive charge. Its characterization vector of length 19 is given below.
0 3 3 2 2 2 2 2 1 1 4 4 4 4 4 4 7 7 8
The first element in the characterization vector is zero because this species does not carry
any positive charge. The second element corresponds to the carbon atom labeled as 1,
which is a tertiary carbon atom. The carbon atoms labeled as 8 and 9 in the hydrocarbon
stmcture are primary ones and their corresponding locations in the characterization vector
9 and 10 have the value 1 and 1 respectively. The type indicator for a double bonded
paraffinic carbon is 7, thus elements 17 and 18 have the value 7.
Example 3: A di-naphthenic carbeneium ion
31

The above naphthenic carbenium ion has 10 carbon atoms. This species carries a positive
charge on the carbon labeled as 3. fts characterization vector of length 20 is given below.
3 3 2 2 2 2 3 2 2 2 2 3 6 6 6 6 3 6 6 6 6
The carbon atoms labeled as 1 and 6 in the hydrocarbon stmcture are called bridge-head
naphthenic carbons. Since these two carbons are tertiary, elements 2 and 7 hold the value
of 3. The type indicator for a napthenic carbon is 6. If a napthenic carbon is the part of
the bridge-head, then it will have a value of 3. Elements 12 and 17 show that the carbon
atoms 1 and 6 are bridge-head napthenic carbons.
The following properties of Boolean relation matrices are very useflil in
developing an algorithm for reaction network generation.
Property 1: Boolean relation matrices are symmetric in nature. Only the upper diagonal
matrix or the lower diagonal matrix is needed to describe the hydrocarbon species. The
order ofthe matrix is equal to the number of carbon atoms in the hydrocarbon stmcture.
Property 2: The total number of "TRUE" entries in a row or a column determines
whether that the carbon atom is primary, secondary, tertiary or quaternary.
Property 3: The beta neighbors of each carbon atom in a hydrocarbon can be identified by
multiplying its Boolean relation matrix by itself and setting the status of all diagonal
elements as "FALSE". This property is very usefial when programming beta scission
reactions.
The Boolean relation matrices become very sparse for species with a large
number of carbon atoms, but they still occupy considerable amount of computer memory
space. Therefore, the Boolean relation matrices are used in generating the elementary
reactions, whereas the reactant and products are stored by their corresponding
characterization vectors.
An example of how the Boolean relation matiix is used in generating an
elementary reaction is shown in Figure 4.2. hi this dealkylation reaction, the side chain
from the aromatic ring is separated; an aromatic component and an acyclic carbenium ion
32

are formed. To simulate this reaction, the carbon-carbon bond (1,7) is broken by turning
off the status in the matrix elements (1,7) and (7,1) as 'FALSE". From this matrix, the
aromatic component's Boolean relation matrix can be recovered by tracing all the carbon
atoms starting from 1. Similarly, the acyclic carbenium ion's Boolean relation matrix can
be recovered by going through all the carbon atoms starting from 7. This procedure yields
two new Boolean relation matrices corresponding to the two products ofthe reaction. The
corresponding characterization vectors for both products can be generated using their
Boolean relation matrices and type of the individual carbon atoms. The newly formed
aromatic component, benzene, will not have any positive charge on the ring; accordingly
the first element in its characterization vector has a value zero indicating that the species
does not carry a positive charge. But, the other product, the propyl ' t carbenium ion will
have a positive charge on its second carbon atom. The location ofthe positive charge for
this secondary carbenium ion is stored in the first element of its characterization vector.
33

l / ^ ^ \ . 3
1
2
3
4
5
6
7
8
9
1 _ :
j'<
T
T
1
21 T;
T
1
I
3
T
T
4
T
T
5 i j
T;
T:
6
T
T
7
T
T
8
T
\ T |
9
T
2 ^ 3 2 2
i 1
2 j 2 ; 2 - 3 1 1 1 4 4 4 1 4 4 4 8 8 8
1
3
4
5
6
1
T
T
_2 : 3 j 4
TI
T | = T
i Ti
i T
5
T
T
6
T
T
1 2 3
1 T
2 T T
3 T
0 2 2 2 2 2 2 4I 4. 4 1
1 :
4 4 4 2 : 1 2 1 8 8
Figure 4.2 Dealkylation Elementrary Step Using Boolean Relation Matrices
34

4.1 Standardized Labeling
The numbering of the carbon atoms of a hydrocarbon stmcture in a standard
fashion is known as the standardized labeling. Although the numbering technique can be
arbifrary, it is very important to be consistent to avoid confusion. The need for a uniform
style of labeling is very important because for the same hydrocarbon stmctures, a
different style of labeling will lead to different Boolean relation matrices. The carbon
atoms are numbered in an arbifrary sequence, it is reasonable to standardize the labeling
of carbon atoms. For example, paraffins are numbered in increasing order along the main
chain, then the second largest chain, and so on. In case of ring stmctures, labeling can be
started from the carbon atom, which is attached to the longest chain and continued along
the ring in a clockwise direction to reach all the side chains attached to the ring stmcture.
Whenever an elementary reaction is generated, the reactant and the resulting product
species should be labeled in a standardized way. The following section discusses
standardized labeling procedures developed in the present work. Hillewaert et al. (1989)
mention labeling procedures but information regarding labeling algorithms are not
available in the open literature.
4.1.1 Standardized Labeling for Acychc Hydrocarbon Species
Acyclic species are numbered from left to right on the main chain and then
continued on the side chains. The general algorithm to label the acyclic hydrocarbon
stmctures is illusfrated in Figure 4.3 using as an example, an alkene carbenium ion
having a positive charge and a double bond. Assume that this species is numbered in an
arbitrary way and we want to label it in a standardized way. The algorithm goes through
each ofthe carbon atom of this species and generates a look-up Table as shown in Figure
4.3. The columns in the Table are arranged as per the pre-set priorities. The first column
registers the starting location. The second colunm is for the quaternary carbon atoms. The
third, fourth, and fifth columns are for tertiary carbon atoms, positive charge, and double
bonds, respectively. The starting carbon atom must be a primary carbon. Assume the
starting location is 1. The second carbon is a tertiary carbon atom because it is connected
35

to three other carbon atoms 1, 3, and 8. The physical location of this carbon, which is 2,
is registered in the column marked for the tertiary carbon. When we come to the carbon
atom 4, which carries a positive charge, the location of the charge, which is 4, is
registered in the column corresponding to the positive charge. A double bond connects
carbon atoms 5 and 6, and the sum of these two carbon locations is noted in the column
for the double bonds. The sixth carbon is once again a tertiary carbon; its location, which
is 6, is added to the entiy already present in the column for the tertiary carbon. The same
exercise can be done starting from the primary carbon labeled as 7 or 9 in the right hand
side. Since, this species does not have any quaternary carbon atoms there are entries of
zero in the column corresponding to the quaternary carbons. Once the search from both
sides is completed, the tabulated values can be used to label the species as per our pre-set
priorities. The entries in a column must be examined to determine the smallest non-zero
number starting from the second column. If all the entries in a column are zeros, then the
next column should be considered.
The starting point corresponding to the smallest non-zero entry in a column is the
direction for the standardized labeling. If all the entries in a column are the same, the
same procedure must be continued in the next column until the smallest non-zero entry is
located. In the present example, all the entries in the second column are zero since there
are no quaternary atoms present in the species. The two entries in the third column and
the two other entries in the fourth column are also the same. But, in the fifth column, the
entry corresponding to the starting point 7 has the smallest value and this decides the
labehng direction. Based on this information, the numbering must start either from the
primary carbon, currently labeled as 7 or 9, and proceed along the main chain and then to
the side chains. The labeled species is shown below in the tabular section of Figure 4.3.
In this example, the entire species is flipped from the right side to the left side. The
location ofthe positive charge remains the same but the location ofthe double bond has
changed due to the standardized labeling.
36

Starting
Location
1
7
8 9
1 3 5 7
Quaternary
Carbon
0
0
Tertiary Carbon Positive Charge
2+6 4
2+6 4
Double bond
5+6
2+3
Figure 4.3 Standardized Labeling of Acyclic Hydrocarbon Species
4.1.2 Standardized Labeling for Cyclic Hydrocarbon Species
The algorithm for standardized labeling of cyclic hydrocarbon species follows a
similar procedure as explained for the acyclic hydrocarbon stmctures. hi case of the
cyclic hydrocarbon stmctiires, the ring carbon atoms are labeled first and then the side
chain carbon atoms are numbered. The pre-set priorities are in the following order:
quaternary ring carbon attached to the longest side chain (QL), quatemary ring carbon
(Q), tertiary ring carbon with the longest side chain (TL), tertiary ring carbon (T),
positive charge on the ring (PS), and double bonds on the ring (DB). The acychc species
have only two starting points whereas the ring stmctures have multiple starting points,
37

depending on the number of side chains attached to the ring, location of positive charge,
and presence of double bonds on the ring. Figure 4.4 illusfrates how standardized labeling
is done for a cyclic hydrocarbon stmcture. Let us assume that the species shown at the
top ofthe tabular section in Figure 4.4 is labeled in an arbitrary way and we want to label
it in a standardized fashion. Every starting point on the ring has two directions: clockwise
and counter-clockwise. All ring carbons attached with side chains, and carbon atoms
having a positive charge or double bonded carbon atoms are the suitable candidates for
starting locations. To illusfrate the procedure, start from the carbon atom labeled as 1 and
go clockwise on the ring. The starting carbon atom is a tertiary carbon with a methyl side
chain and this location is registered in the column for tertiary carbon atoms designated as
T in the Table. The next location, labeled as 3, is also a tertiary carbon attached to a
methyl side chain. The physical location of this carbon from the starting point is 2, which
is added with the entry already present in the column for tertiary carbon atoms designated
as T in the Table. The next carbon, labeled as 5, is a quatemary carbon atom attached to
the longest side chain and also to a methyl side chain. The location of this carbon atom
from starting point 1 is 3. This location, 3, is registered in the column for quatemary
carbon with the longest side chain designated as QL in the Table. When the search comes
to the carbon atom labeled as 4, the location of the positive charge, 4, is registered in the
corresponding column for positive charge designated as PS. The carbon atoms labeled as
2 and 6 are connected with a double bond. The location of the carbon atom labeled as 6
from the starting point 1 is 5. The location of the carbon atom labeled as 2 from the
starting point 2 is 6. Remember that we are traversing on the ring in the clockwise
direction starting from the carbon atom labeled as 1. The sum ofthe locations ofthe
double bonded carbons 5+6 is registered in the column for double bonds designated as
DB in the tabular section. With this, the search in the clockwise direction is completed. A
similar search can be carried out from the carbon labeled as 1 in the counter-clockwise
direction. Once all the starting locations for both directions are covered, the location of
the smallest non-zero entry in a column can be determined by the same procedure
explained in the previous section.
38

SL QL TL PS DB
1(C)
1(A)
3(C)
3(A)
5(C)
5(A)
4(C)
4(A)
6(C)
6(A)
2(C)
2(A)
3
5
2
6
1
1
6
2
5
3
4
4
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
0
1+2
1+6
1+6
1+2
5+6
2+3
4+5
3+4
3+4
4+5
2+3
5+6
4
4
3
5
2
6
1
1
6
2
5
3
5+6
2+3
4+5
3+4
3+4
4+5
2+3
5+6
1+2
1+6
1+6
1+2
(C): Clockwise Direction, (A): Anti-clockwise Direction, SL: Starting Location, QL: Quatemary Carbon with Long Chain, Q: Quatemary Carbon, TL: Tertiary Carbon with Long Chain, T: Tertiary Carbon, PS: Positive Charge, DB: Double Bond
Figure 4.4 Standardized Labeling of Cyclic Hydrocarbon Species
39

For the present case, as per the table, the smallest entry is located in the colunm
for the quatemary carbon with long chain (QL). But the two entries are of the same
magnitude. On the same rows for the starting locations 5(C) and 5(A), non-zero entries
are found at the column corresponding to the tertiary carbon (T). The sum ofthe locations
ofthe tertiary carbon atoms is 11, which corresponds to the starting point 5(C). But the
sum of the locations of the tertiary carbons corresponding to the starting point 5(A) is
only 5. The row corresponding to 5(A) has the smallest entry, which decides the starting
point and the direction of the labeling. The hydrocarbon species is numbered starting
from the carbon labeled as 5 and continues along the ring in the counter-clockwise
direction. Once the numbering on the ring is completed, the algorithm goes to the longest
side chain connected to ring and so on. The labeled species is given below the Table in
Figure 4.4. By the standardized labeling procedure, the quatemary carbon with the
longest side chain receives the label 1 and the positive charge receives the label 6. By
adopting this procedure, the hydrocarbon stmctures can be labeled in an unambiguous
way so that the same species will not have different Boolean relation matrices.
4.2 Network Generation
The generation of an elementary step must obey all the mles of carbenium ion
chemistry. The following mles are set to guide the network generation for a given feed
component.
1. Elementary steps are generated using Boolean relation matrices. The reactants and
products are stored by their characterization vectors. The Boolean relation matrices
can be reconstmcted from the corresponding characterization vectors.
2. Carbenium ion chemistry does not allow the formation of methyl and ethyl carbenium
ions. The beta carbon with respect to the positive charge must be a tertiary or a
quatemary carbon atom for all carbon-carbon bond-breaking reactions.
3. Primary carbenium ions are discarded in the network generation. In other words,
positive charge cannot be assigned to a primary carbon atom.
40

4. Alkene carbenium ions are formed in endocyclic beta scission of mono-naphthenes.
Alkene carbenium ions with a positive charge on the double bonded carbon atom
(vinyl cations) are less stable (Zabicky, 1970) than the corresponding saturated
carbenium ions. Primary and vinyl cations with a double bond should not be allowed
to form (they are unstable). The formation of conjugated (neighbor carbon of a
charged carbon is double bonded) and non-conjugated carbenium ions is allowed in
the network generation.
5. The alkene carbenium ion as per the mles in (4) undergoes deprotonation reaction to
form a di-olefin.
6. The alkene carbenium ion undergoes a hydride and methyl shift elementary steps.
7. The double bond between two carbon atoms in an alkene carbenium ion will not
cleave in the PCP and beta scission reactions.
8. Protonation of a di-olefin leads to the formation of an alkene carbenium ion.
9. Di-, tri-, tefra-ring naphthenes will not undergo intra-ring alkyl shift and ring
confraction reactions.
10. Exocyclic beta scission of an alkyl naphthene produces a cyclic olefin and an alkane
carbenium ion.
11. Endo-cyclic beta scission will not occur in the bridge locations of the poly-
naphthenes.
12. Paring (pealing of alkyl groups) reaction consists of a sequence of elementary steps
(hydride shift, methyl shift, ring confraction, and exocyclic (t,t) beta scission). During
the reaction network generation, as the paring reaction is automatically taken care of,
it is not necessary to simulate this step separately.
13. Paring reaction will not happen in di-, tri-, and tetra-ring naphthenes.
14. Five-member ring naphthenes (alkyl cyclopentanes) will not undergo intra-ring alkyl
shift and ring contraction reactions.
15. Deprotonation of an aromatic carbenium ion must reproduce the aromatic ring.
16. The positive charge cannot move to a double bonded carbon atom in the hydride shift
on an aromatic ring.
41

17. Methyl shifts producing an aromatic carbenium ion with a charge on the double
bonded carbon atoms are not allowed.
18. Aromatic rings will not participate in infra-ring alkyl shift and ring contraction steps.
However, the alkyl chain attached to the aromatic ring will undergo the PCP reaction.
19. Naphtheno-aromatics will not undergo intra-ring alkyl shift, ring contraction
reactions. However, the alkyl chains attached either to the aromatic ring or to the
naphthenic ring will undergo the PCP reaction.
20. The naphthenic side chain can participate in exocyclic and endocyclic beta scissions.
Acyclic beta scission can occur in alkyl side chains. Dealkylation of an alkyl chain
attached to an aromatic ring is a possible reaction.
21. Cyclization will not occur in naphtheno-aromatic components.
The network generation considers all possible (as allowed by generation mles)
reactant and product species for a given feed component as given in the mles discussed
above. Assume the following acyclic carbenium ion, which carries a positive charge on
the carbon atom 3, undergoes a methyl shift reaction.
In a methyl shift reaction, a methyl group from a tertiary (or quatemary) carbon atom
moves to an adjacent carbon atom carrying the positive charge. The tertiary (or
quatemary) carbon atom receives the positive charge and becomes a secondary (or
tertiary) carbon atom. In the above carbenium ion, the methyl groups labeled as 1, 7, and
8 can migrate to the carbon atom carrying the positive charge. Since the movement of
methyl groups 1 and 7 produces the same product, there are two possible methyl shift
reactions, which are given below.
42

Figure 4.5 shows a complete hst of products from a PCP step for an acyclic
carbenium ion. In the first reaction, a new bond forms between the fourth and second
carbon and then alpha cleavage occurs between the third and fourth carbon to create the
product. The positive charge for the product can be assigned either on the third or fourth
carbon. If the charge is assigned on the fourth carbon, the product is same as the reactant
and this reaction need not be considered in the network generation. If the charge is
assigned on the third carbon, then it can be counted as a new product and this reaction
must be stored.
The reaction network for a feed component is generated in a particular sequence.
The paraffinic components first undergo a dehydrogenation reaction to generate several
olefins. The olefins are protonated to become carbenium ions. The carbenium ions
undergo isomerization and cracking reactions. The olefins are saturated by hydrogenation
reactions. The network generation is completed if all the possible species have undergone
all possible elementary steps. The network generation algorithms for paraffinic,
naphthenic, and aromatic feed components are shown in Figures 4.6-4.8, respectively.
43

a
CHARGE : 4" CARBON
BETA CARBONS : 2,8,6
NEW BONDS : (4,2),(4,6),(4,8) %y
a
a
Figure 4.5 PCP Steps for an Acyclic Carbenium Ion
44

Dehydrogenation of a paraffin
Protonation of olefins
Hydride shift, Methyl shift, and
PCP
i Beta scission
Next carbenium ion
Deprotonation of carbenium ions
Next olefin
Hydrogenation
Figure 4.6 Network Generation Algorithm for Paraffins
45

Dehydrogenation of a naphthene
Protonation of olefins
Hydride shift, Methyl shift,
PCP, and Ring contraction
1 Exocyclic beta scission and
Endocyclic beta scission
Next carbenium ion
Deprotonation of carbenium ions
Next olefin
Hydrogenation
Figure 4.7 Network Generation Algorithm for Naphthenes
46

Aromatic component
i Protonation on the ring
i Dehydrogenation on the side chain
>
Next aromatic component
Alkylation with paraffinic ions
Next carbenium ion
Deprotonation
i Hydride shift,
Methyl shift, and PCP
i Cyclization
i Exocychc beta scission and
Endocyclic beta scission Disproportionation
Protonation Next olefin
Hydrogenation
Figure 4.8 Network Generation Algorithm for Aromatics
47

4.2.1 List of Possible Species
The present research considers up to four rings in cyclic hydrocarbon stmctures.
The yarious types ofthe species encountered in the network generation for hydrocracking
are Usted below.
1. Molecules: Paraffins, mono-, di-, tri-, tetra-naphthenes, mono-,di-, tri-, tetra-
aromatics, naphtheno-mono-, naphtheno-di-, naphtheno-tri-aromatics,Acyclic olefins,
mono-, di-, tij-, tetia-cyclic olefins, mono-, di-, tri-, tetra-aromatic olefins, and mono-,
di-, tri-, tetra-aromatic-cyclic olefins.
2. Carbenium ions: Alkane, alkene, mono-, di-, tri-, tetra-cyclic, mono-, di-, tri-, tetra-
cyclic-olefinic, mono-, di-, tri-, tetra-aromatic, mono-, di-, tri-, tetra-aromatic-
olefinic, mono-, di-, tri-aromatic-cyclic-olefinic carbenium ions
4.2.2 Network Generation Software
The network generation algorithms along with the mles of carbenium ion
chemistry have been used to develop a collection of FORTRAN subroutines. The
software has been developed in a very flexible way so that new labeling routines and new
elementary steps can be added easily. The stmcture ofthe software is given in Figure 4.9.
There are separate main programs for paraffinic, naphthenic, aromatic, and naphtheno-
aromatic feed components. The main programs coordinate the control between the
labeling routines and reaction routines. A reactant before participating in an elementary
reaction is labeled and validated against the relevant mles. The reaction products are also
vahdated against the network generation mles and labeled. If the generated product is a
carbenium ion, its carbon number and location of the positive charge is determined.
Assume that the particular carbenium ion has 28 carbon atoms with a positive charge at
the 11* carbon atom. The carbenium ion library files are named as per the carbon number
and the location of the charge. The corresponding library file will have all previously
generated carbenium ions with carbon number 28 and with the positive charge on the 11'
carbon atom. The program checks against all the species that are previously stored to
make sure that the newly generated carbenium ion is not already present in the library
48

file. If the program fails to locate an identical species, the newly generated carbenium ion
is stored in the library file, hi the case of olefins, the library files are named with respect
to the carbon number and the location ofthe double bonds.
Separate main programs for paraffinic, naphthenic, aromatic, and naphtheno-aromatic feed components
Subroutines for elementary step network generation
Labeling subroutines for paraffinic, naphthenic, aromatic and naphtheno-aromatic olefins and carbenium ions
Carbenium ion library files (based on carbon number and location ofthe positive charge)
Olefin library files (based on carbon number and location ofthe double bonds)
Elementary step library files (based on carbon number of the feed component)
Figure 4.9 Stmcture ofthe Network Generation Software
This kind of file handling helps to reduce the searching time for carbenium ions
and olefins. Finally, the elementary reactions are stored in the reaction library files, which
are named as per the carbon number of the feed component. This procedure continues
until all the possible species undergo all possible elementary reactions. The CPU time
requirements for generating reaction networks for paraffins up to carbon number 40 are
given in Figure 4.10.
49

3000
Carbon Number (C8 - C40)
Figure 4.10 CPU Time Requirement for Paraffins Network Generation
4.2.3 Network Generation Sample Results
The details of the generated networks are given in Table 4.1, 4.2, and 4.3 for
paraffins, naphthenes, and aromatics, respectively.
Table 4.1 Network Details for Paraffins
Elementary Steps
Protonation
Deprotonation
Hydride Shift
Methyl shift
PCP
Beta Scission
Total Reactions
Carbenium Ions
Olefins
Cl l
549
549
420
140
464
117
2239
268
339
C15
3021
3021
2512
592
1870
533
11549
1444
1765
C18
7647
7659
6584
1268
3923
1163
28244
3653
4361
C20
12820
12838
11226
1913
5891
1777
46465
6129
7216
C33
130206
130206
120430
11734
35719
11315
439610
62836
69991
50

Table 4.2 Network Details for Naphthenes
Elementary Steps
Protonation
Deprotonation
Hydride shift
Methyl shift
PCP
Exocyclic scission
Endocyclic scission
Total Reactions
Carbenium Ions
Olefins
Mono-ring Cl l Di-ringC15 Tri-ringC18 Tetra-ring C19
3892
3890
2532
798
2957
549
79
14697
1875
1575
100320
100254
76796
17606
44625
11638
203
351442
47758
32493
265565
265565
219864
42158
34428
17142
296
845018
121591
79643
195174
195150
168066
20482
4203
5597
42
588714
86632
56216
Table 4.3 Network Details for Aromatics
Elementary Steps
Protonation
Deprotonation
Hydride shift
Methyl shift
PCP
Cyclization
Exocyclic scission
Alkylation
Disproportionation
Total Reactions
Components
Carbenium Ions
Olefins
Mono-ring C7
6785
7055
3496
1174
1993
268
891
33
300
21995
356
5163
2697
Di-ringCll
17625
17963
8802
3472
3533
166
1188
39
3406
56194
820
13402
6747
Tri-ringC15
6560
6672
3092
796
2538
42
1031
39
4561
25331
247
4892
2384
Tetra-ring C19
6560
6672
3092
796
2538
0
1031
39
4597
25325
247
4850
2384
51

CHAPTER 5
SINGLE EVENT KINETICS AND NORMAL OCTANE HYDROISOMERIZATION
AND HYDROCRACKING
The objective of this chapter is to explain the concept of single event kinetics and
describe how the single event rate parameters can be estimated from the hydrocracking
studies carried out on a model component, such as normal octane, from the data available
in the open literature.
5.1 Single Event Theory
The single event theory (Vynckier and Froment, 1991) says that the rate
coefficient for a given elementary reaction can be factored into two parts.
k = n^k' (5.1)
where k is the rate coefficient for the elementary reaction, k is the single event rate
coefficient, and n^ is the ratio of the global symmetry number of the reactant to the
global symmetry number of the activated complex formed in that particular elementary
reaction. Equation (5.1) is derived from transition state theory using thermodynamic
arguments (Vynckier and Froment, 1991).
Benson's group contribution theory can be used to explain single event rate
coefficient without resorting to transition state theory. Consider the elementary reaction
A<^ B dX quasi-equilibrium. To calculate the equilibrium constant for this reaction,
AG = A / / - J M = - i ? n n ^
_J_(AA/-rA5) ( -- ^
K^e ^^
where AG is the standard free energy change. A// is the standard enthalpy change, AS*
is the standard entropy change, T is the temperature, R is the ideal gas constant, and K is
the equilibrium constant for the above-mentioned elementary reaction. The calculation of
enthalpy change of the reaction is obtained using the enthalpy of formation data for both
the product and reactant. The enfropy change calculation is given by
52

AS^S,-S,
S^ ^SyR\na^+R\nT]^ =5^*-i?ln-V,
(5.3)
(5.4)
SA is the enfropy due to group contribution and the second and third terms are
corrections to the entropy due to molecular symmetry and optical isomerism (Benson et
al., 1969). These two corrections are necessary to arrive at the correct value of the
entiopy of any molecule. A similar equation can be written for the entropy ofthe product
molecule B.
Substituting the enfropy contributions from Equations (5.3) and (5.4) gives
AG^AH-TlsyRln^-SyRln^ (5.5)
Define the ratio of symmetry number to the number of optical isomers as a single number
called the global symmetiy number. The global symmetry numbers cr\ and o-\ are for
the reactant and product, respectively, and Equation (5.5) can be written as,
AG = A// - T\ SS* -SyPln^ (5.6)
The free energy relation from Equation (5.6) can be substitiited into the equilibrium
constant K in Equation (5.2), which gives
K = exp RT
AH -TiSs' -Sy-RT\n B J
^ = exp|--^(A//-rA5*) |exp< 1 '^A
In-4
(5.7)
(5.8)
The first term in the right hand side of Equation (5.8) is a standard definition for
equilibrium constant without the correction terms for the enfropy. The second term
becomes a factor known as the number of single events. Equation (5.8) can be written as,
K=K\y (5.9)
53

K = n,K. (5 10)
The equilibrium constant K for tiie elementary reaction is the product ofthe single event
equilibrium constant K and the number of single events n^. The number of single events
is tile ratio of global symmetry number ofthe reactant to the global symmetry number of
the product.
The above resuft can be derived for a reaction, which is not at equilibrium using
fransition state theory. The difference is that the single event equilibrium constant is
replaced by the single event rate coefficient and the equilibrium constant for the
elementary step is replaced by the rate coefficient for the elementary reaction. The
number of single events should be defined by the ratio ofthe global symmetry number of
the reactant to the global symmetry number ofthe transition state.
The effect of the stmcture for the reactant molecule (reflected via enfropy from
rotational contribution) and fransition state is factored out from an elementary rate
coefficient, which leads to a new rate coefficient called the "single event rate
coefficient." By doing this, the number of independent rate coefficients is reduced for a
given elementary step such as hydride and methyl shifts (Froment, 1999). The
appropriate number-of-single-events factor, «e, is multiplied with a single event rate
coefficient to obtain the elementary rate coefficient for that particular elementary
reaction.
5.1.1 Calculation of Number of Single Events
The calculation of number of single events for an elementary reaction requires
knowledge of the chemical stmcture of the reactant and the activated complex formed in
that elementary reaction. Baltanas et al. (1989) have presented a table which calculates
the number of single events for yarious elementary steps in hydroisomerization from the
number of hydrogen atoms or methyl groups in the alpha or beta position, with respect to
the charge bearing carbon atom, in a carbenium ion. They also observed that these simple
mles might deviate in a few cases.
54

How to determine the stmcture of activated complexes and reaction pathways is
an interesting area of considerable importance and growing research. The reactants and
products in a reaction correspond to certain finite energy points in a multi-dimensional
energy surface. This multi-dimensional energy surface may have many minimas,
maximas and meta-stable points. The minimum points on the energy surface may be
reactants and products of a chemical reaction, two confirmations of a molecule, or two
molecules that associate to form a non-covalently bound bi-molecular complex (Leach,
1996). As the system moves from one minimum to another, the energy increases to a
maximum at the fransition stmcture and then falls. It is important to distinguish the
fransition stmcture from the fransition state. The transition stmcture is the point of
highest potential energy along the pathway, whereas the transition state is the peak in the
free energy profile (Leach, 1996). The potential energy is due to the spatial arrangement
of the particles in an atom, whereas the free energy takes enthalpy and enfropy changes
into account. In many cases, the geometry at the transition state is very similar to that of
the fransition stmcture. If the transition state geometry is temperature dependent, then the
enfropy factors may be important. Thus, the transition stmcture and transition state will
be different. Traditionally, the reactants and products are represented as the minimum
energy points and the fransition stmcture sits at the top of the energy hill connecting the
reactants and products. Actually, the reaction surface is a multidimensional energy
surface where a transition stmcture corresponds to a meta-stable or saddle point. At a
saddle point, the first derivatives of the potential energy function with respect x, y, and z
coordinates are all zero as in the case of a minimum point. The matrix of the second
derivatives or the Hessian becomes indefinite in case of a saddle point, which means the
eigenvalues ofthe Hessian matrix have one or more negative real parts (Leach, 1996). A
fransition stmctiu-e corresponds to a stationary point in a multi-dimensional energy
surface with its Hessian matrix having one negative value. The negative eigenvalue is
often referred to as the imaginary frequency (in quantum chemistry literature) for the
motion ofthe system over the saddle point, which means that the transition stiiicture has
a unique nuclear displacement toward which the minimum points (reactants and
55

products) it connects may be reached. The molecular displacement in any other direction
leads to an increase in the energy.
The geometiy of the transition stmcture can be reliably predicted using quantum
chemical software such as Gaussian, General Atomic and Molecular Electronic Stmcture
System (GAMESS), Molecular Orbital PACkage (MOPAC) (Young, 2001), etc.
GAMESS (Schmidt, 1993) has been used to locate a fransition in the following hydride
shift reaction,
In this reaction, a hydride ion (H") moves from the tertiary carbon to the secondary
carbon. Subsequently, the tertiary carbon acquires the positive charge. The forward
reaction produces a more a stable carbenium ion than the reverse reaction. The hydride
shift, one of the important isomerization reactions occurring in hydrocracking, is
responsible for propagating the positive charge to different carbon atoms in a carbenium
ion.
The starting point for locating a fransition stmcture for an elementary reaction is
to guess a geometry, whose Hessian matrix has only one eigenvalue with a negative real
part and all other eigenvalues have non-negative real parts (Leach, 1996). Selecting the
proper geometry requires practice and a certain amount of chemical intuition with regard
to the reaction in question. The cartesian coordinates for the assumed stmcture can be
arrived at by trial and error using a software package such as Chem3D. The calculation of
the eigenvalues of the Hessian matrix is performed to check if the Hessian matrix for the
assumed stmcture has only one eigenvalue with a negative real part. GAMESS (Schmidt
et al., 1993) can search for a saddle point in the energy surface using the assumed
geometry as the starting point to locate a transition stmcture. The transition stmcture
obtained in the saddle point search for the above-mentioned hydride shift reaction is
given as.
56

di-2.08414 A° d2 = 1.09754 A°
hi die above fransition stiiicture, the migrating hydride ion is 2.08 angstroms (10"'° m)
away from the tertiary carbon, where it was originally attached, and 1.09 angstroms away
from tiie secondary carbon towards which it is moving.
If die geometry of the stiiicture is known, it is then easy to calculate the number
of single events for the forward and reverse reactions. The asterisk in the above transition
stmcture indicates a chiral' carbon atom. The global symmetry numbers for the reactant,
fransition stiiicture, and the product are in Equation 5.11.
3' 3 3\2 ^^ ' .^=Y '^^ ' • -"T ^./,. = — (5-11)
The number of single events for the forward and reverse reactions is given by
( " , ) , = 3 ~ = 2 M'-y^ (5.12)
These results are in agreement with the formula published by Baftanas et al. (1989).
5.2 Normal Octane Hydroisomerization and Hydrocracking
The purpose of this section is to describe how the single event rate parameters can
be estimated from the data obtained from hydrocracking studies ofthe model components
such as normal octane. A thorough investigation in the understanding of the reaction
networks that lead to the formation of isomers, and cracked products, from the normal
and branched paraffins was provided by Steijns et al. (1981). With certain types of
catalysts, normal paraffins have been shown to isomerize to a considerable extent before
undergoing cracking, but with highly branched alkanes hydrocracking predominates even
at low conversion (Weitkamp and Jacobs, 1981).
' A carbon atom connected to four different atoms (or groups). Mirror image non-superimposability is a constraint.
57

The reaction network for n-octane hydroisomerization and hydrocracking contains
75 olefins, 57 carbenium ions, and a total of 430 elementary reactions. The network
generation software described in the previous chapter was used to generate the reaction
network for n-octane. The cracked product has 22 different paraffins, which are listed in
Table 5.1.
5.2.1 Rate Expressions for Paraffins. Olefins and Carbenium Ions
After physisorption in the pores ofthe zeolite catalyst, n-octane is dehydrogenated
on the metal site. The resufting olefin is protonated on the acid sites into a carbenium ion,
which then isomerizes through hydride and alkyl shifts along the chain. Branched
carbenium ions are formed through a protonated cyclopropane (PCP) intermediate.
Cracking mainly occurs with the branched carbenium ions through scission of a C-C
bond in the beta position, with respect to the carbon bearing the positive charge.
Table 5.1 List of Cracked Products from n-Octane Hydrocracking
Sfraight chain
Propane
n-Butane
n-Pentane
n-Octane
Mono-branch
2-Methyl propane
2-Methyl butane
3-Ethyl hexane
2-Methyl heptane
3-Methyl heptane
4-Methyl heptane
Di-branch
2,2-Dimethyl hexane
3,3-Dimethyl hexane
2,4-Dimethyl hexane
2,3-Dimethyl hexane
3,4-Dimethyl hexane
2,5-Dimethyl hexane
3-Ethyl 3-methyl pentane
3-Ethyl 4-methyl pentane
Tri-branch
2,2,4-Trimethyl pentane
2,2,3-Trimethyl pentane
2,3,4-Trimethyl pentane
3,3,4-Trimethyl pentane
Deprotonation of the carbenium ions regenerates the acid sites and yields olefins. The
olefins are hydrogenated on the metal sites to produce paraffins and iso-paraffins as
isomeric and cracked products.
58

Paraffins disappear by dehydrogenation, but are formed by hydrogenation of
olefins on the metal sites,
i ^ . « 0 , + / / , . (5.1)
The subscript y in the product olefin denotes the production of several olefins by single
paraffin. The distinction among these olefins (double-bond isomers) is essential, because
tiie position of tiie double bond determines which carbenium ions can be formed from the
olefin. In other words, different elementary reactions are associated with different double
bond isomers. It is not necessary to distinguish between cis and trans isomers as they are
explicitly taken into account through calculations of the number of single events and
equilibrium constants for olefin isomerization and paraffin dehydrogenation.
The net rate of formation of a paraffin is then represented by
K. = E [ ^HiJi)Co,PH, -ka„(u)Cp^ ], (5.2)
where A: iji) is the rate coefficient for hydrogenation, C^ is the olefin concentration,
Pff^ is the partial pressure of hydrogen, kj^^iij) is the rate coefficient for
dehydrogenation, and Cp is the paraffin concentration. The rate coefficients of the
hydrogenation and dehydrogenation reactions are not used in the model. Since
equilibrium is assumed in this step, dehydrogenation equilibrium constants are used to
calculate the olefin concentration.
The olefins formed on the metal fimction are protonated on the acid sites. Per
olefin, two carbenium ions can be formed (sometimes three depending on the location of
the positive charge),
Oy+H^ <^R;,R,\ (5.3)
Only protonation reactions producing the more stable secondary and tertiary carbenium
ions are considered. If the olefin has a terminal double bond, one ofthe carbenium ions is
primary and the corresponding protonation reaction is excluded from the reaction
network.
59

Olefins participate in hydrogenation/dehydrogenation, protonation/deprotonation,
and cracking reactions. The general expression for the net rate of generation of an olefin
is given by
\ =kDHW)Cp, -KiJi)c„„PH, +k^XyAj)C,,^^ +k^XfAj)c,.^^^ -
{k,Xs) + k,Xt))CoC^.+k,Xv;w,Oy)C^^. ^ -" ^
where kj^^ is the rate coefficient for deprotonation, k^^ is the rate coefficient for
protonation, k^.^ is the rate coefficient for beta scission, C„, is the concentration of a
carbenium ion, C + is the concenfration of free active acid sites on the catalyst, 5 is a
secondary carbenium ion, and t is a tertiary carbenium ion. The pseudo-steady-state
approximation, applied to the olefin intermediates, sets the net rate of formation of the
olefins is zero,
Ro,=0. (5.5)
If the rate-determining step is on the acid fimction, a combination of rate expressions for
paraffins and olefins with the above mentioned pseudo-steady-state approximation yields
the following expression for the net rate of formation of a paraffin,
Rp, - Z { kDe(^-Aj)c,.^^ +k^,ifAj)c -[Ky)^k,xt)Y:oC,. + (5.6)
kcM^w,Oy)C^^, }.
This equation contains the unknown concenfration of carbenium ions. The general
expression for the net rate of formation of a carbenium is given by
^R^={Y KM)CoC^. + 1 ; k„,iq;m)C^.^ +Y.^Msir\m)C^.^ +
X kp,piu;m)C^.^ + X k,X^;m,6)C^.^ }-{ ^ KeimO)+ (5.7) u " V O
^ kj,s{m;q) + Y. ^MsC'";0 + Z ^pcpirn:u) + Y, ^cr(^'^^'O") }C^ . q r u I
where A: ^ is the rate coefficient for hydride shift, k^^ is the rate coefficient for methyl
shift, kpcp is the rate coefficient for protonated cyclopropane (PCP) step, and k^^ is the
rate coefficient for cracking reaction. Since the above rate expression requires
60

summations, the type ofthe carbenium ions is represented by 'm' instead of '5' or 'f. To
calculate the concentrations ofthe carbenium ions, the pseudo-steady-state approximation
is given by
/?,.,„ = 0 . (5.8)
The rate expression applied for the carbenium ion still contains the unknown
concentiation of free active sites and olefin concentrations. Since the active sites are
either free or occupied by carbenium ions, the following equation for the total number of
acidic sites has to be satisfied,
where C, and CH* are the total concentration of active acid sites and free acid sites,
respectively. The unknown olefin concentrations are calculated by assuming quasi-
equilibrium step for hydrogenation-dehydrogenation step.
^C^K^^ ^^^^^
PH,
The equilibrium constants for dehydrogenation, KoH.ip are calculated by means of the
thermodynamic state ftmctions (Benson et al., 1969).
Physical adsorption is also assumed to be in quasi-equilibrium (Baftanas et al.
1989). ft is described using a Langmuir-type isotherm given by
C ^sa,^L,iPi (5.11)
m
where Csat is the concentration of adsorption sites on the catalyst and KL^I are the
Langmuir adsorption coefficients. Equations (5.9) and (5.10) for olefin and paraffin
concentrations can be combined to give the following,
^ CsatKLyDH,ijPi (5.12)
'' py^+TKL,.p. y m
The olefin concentrations are now firnctions ofthe partial pressures of hydrocarbons and
hydrogen, which are known for a particular feed. This substitution in the rate expressions
61

for the paraffins finally leads to expressions for the net rates of formation ofthe paraffins
in terms of measurable quantities.
The single rate coefficients required for n-octane hydroisomerization and
hydrocracking are listed in Table 5.2. There are about 20 rate coefficients. Protonation
rate coefficients depend only on the type of the reacting carbenium ions, i.e., secondary
(s) or tertairy (t). Deprotonation rate coefficients depend not only on the product
carbenium ions but also the product olefin (O). The rate coefficients for the isomerization
and cracking reactions depend on the type of reactant and product carbenium ions
(Balatanas et al., 1989).
Table 5.2 Single Event Rate Coefficients for n-Octane Hydroisomerization and Hydrocracking
Reaction Type Single Event Rate Coefficients
Protonation (Pr) kpr(s), kpr(t)
Deprotonation (De) kDe(s;0), kDe(t;0)
Hydride shift (HS) kHs(s;s), kHs(s;t), kHs(t;s), kHs(t;t)
Methyl shift (MS) kMs(s;s), kMs(s;t), kMs(t;s), kMs(t:t)
PCP branching (PCP) kpcp(s:s), kpcp(s;t), kpcp(t;s), kpcp(t;t)
Beta scission (Cr) kcr(s;s), kcr(s;t), kcr(t;s), kcr(t;t)
5.2.2 Normalization Scheme
The following relative concentrations are introduced to avoid estimating the total
acid site concentration ( Q and total adsorption site concentration (QaO-
C V „ = % - C V = % ^ (5.13)
C, C,
The protonation rate coefficients in Equation (5.7) become
r . = Q C . „ A . . (5-14)
The rest ofthe rate coefficients take the following form, k'=C,k. (5-15)
62

All the rate coefficients used in the above equations correspond to elementary rate
coefficients, which are obtained by multiplying the single event rate coefficient with the
corresponding number of single events.
5.2.3 Reactor Model
The net rate of formation equations for 57 carbenium ions plus the site balance
equation forms a linear system of equations. The solution to this square linear system
yields the concenfrations of normalized carbenium ions and free acid site concentration.
The net rate of formation of 22 paraffins can be calculated using these concentrations.
Then the rates are substituted in the following continuity equations to solve for the molar
flow rate of 22 paraffins along the length ofthe reactor,
^ = Rp i = 1,2,..., 22, (5.16)
dw '
where f is the molar flow rate of paraffin /, w is the weight ofthe catalyst, and Rpi is the
net rate of formation of a paraffin /. At every catalyst segment w, the system of linear
equations is solved to obtain new carbenium ion concenfrations and the above set of
differential equations are numerically integrated to obtain the product profiles.
5.2.4 Results and Discussion
Table 5.3 lists the operating conditions at which the model parameters are
estimated. The product distribution from the literature (Vansina et al., 1983; BaUanas et
al., 1983) and the model-predicted product distribution are given in Table 5.4.
A sequential quadratic programming (SQP) software (Gill et al., 1986) is used to
estimate the rate constants by minimizing the weighted-squared-error between the
predicted and the target distribution. The n-octane hydrocracking parameter estimation
problem has 20 decision variables (rate coefficients) and 26 nonlinear inequality
constraints (product flows, mole fractions, etc.). The estimated rate coefficients are listed
in Table 5.5.
63

Figure 5.1 shows the isomerization conversion with respect to n-octane total
conversion. The isomerization conversion is a unique fimction ofthe total conversion of
n-octane. The isomerization selectivity is close to 100% at low conversion levels (<15%).
This eliminates the possibility of a direct cracking of the n-octane without stmctural
rearrangement via isomerization reactions (Vansina et al., 1983). The isomerization
conversion reaches a maximum of 46%, which is typical of n-octane hydroisomerization
and hydrocracking (Vansina et al., 1983). The peak conversion value is very sensitive to
rate of formation and rate of depletion of various species present in the reactor.
Figure 5.2 shows the mole percent of n-octane, mono-, di-, and tri-branched
isomers with respect to n-octane total conversion. These profiles confirm that the
dibranched isomers are formed from the monobranched isomers and not from the start of
the reaction (Vansina et al., 1983). Tribranched isomers are found to be less than 1% of
the total number of moles of n-octane isomers.
Figures 5.3-5.6 show how the molar flows of all 22 paraffins vary with respect to
catalyst weight in the reactor. The profiles of individual components indicate that they are
the products of isomerization reactions and these components undergo cracking reactions
to produce components in C3-C5 range. Figure 5.7 shows the profiles for cracked
products with respect to the catalyst weight along the reactor. The cracked products (C3,
C4, and C5) can not undergo cracking reactions and they reach equilibrium concentrations
at the end ofthe catalyst bed.
Table 5.3. Operating Conditions for Model Parameter Estimation Quantity Numerical Value Normal octane feed 1.0 kmol/hr Hydrogen to hydrocarbon molar ratio 30.0 Total pressure 10.0 bar Reactor temperature 220 °C n-Octane total conversion 67.0 %>
64

Table 5.4 Target Distribution and Predicted Distribution Pure Components/Lumps Target Predicted Absolute Error in
(kmol/hr) (kmol/hr) Prediction n-Octane Propane n-Butane n-Pentane Mono-branch Di-branch Tri-branch n-Octane total conversion
0.3299 0.0853 0.2494 0.0853 0.3081 0.1501 0.0018 67.0 %
0.3404 0.0776 0.2866 0.0776 0.3019 0.1570 0.0018 65.958 %
0.0105 0.0077 0.0372 0.0077 0.0062 0.0069 0.0 1.042%
Table 5.5. Single Event Rate Coefficients from Parameter Estimation Single Event Rate Coefficient Numerical Value (kmol/kg cat, hr) kpr(s) 5732.2171 kpr(t) 141898.5020 " - 122636.4840
18327.1752 2.8649E13 8.1437E12 383183498.0
kDe(s;0) kDe(t;0) kHs(s;s) kHs(s;t) kHs(t;s) kHs(t;t) 4.9997E09
kMs(s;s) ^ " ^S^ f kMs(s;t) kMs(t;s) kMs(t;t) kpcp(s;s) 274849.081 kpcp(s;t) 4034177.64 kpcp(t;s) 421.6748 kpcp(t;t) 997.6296 kcr(s;s) 215775.517 kcr(s;t) 39743423.5 kcr(t:s) 1342084.05 kcr(t;t) 5593137.13
151709614.0 70400.8646 134372.709
65

U.D -
f 0.5 -
i 0.4 -
!2 0.3 o E ° 0 2 00
O 0.1 -
0 -
^^-"^ " X,
/ ^ \
20 40 60
nC8 Total Conversion (%)
80 100
Figure 5.1 Conversion into C8 Isomers
52 1 . ^ -0)
i 1 -0 10
S 0-8 1 ^
° 0.6-0 + « 0.4-u. « 0.2 -o
0 -'
. .. . ^ n-Octane
Mono-branch^^_^
1 1
Di-bran£ii-
1 1
Tri-branch
20 40 60
nC8 Total Conversion (%)
80 100
Figure 5.2 Distiibution of C8 Fraction
66

200 400 600
Catalyst Weight (i<g)
800 1000
1: 3-Methyl heptane 2: 4-Methyl heptane, 3: 2-Methyl heptane, 4: 3-Ethyl hexane
Figure 5.3 Mono-branched Isomers
0.001
i 0.0008 o E i 0.0006 «
^ 0.0004 u
0 0.0002 1
200 400 600
Catalyst Weight (kg)
800 1000
1: 2,4-Dimethyl hexane, 2: 3,3-Dimethyl hexane, 3; 2,2-Dimethyl hexane, 4: 3-Ethyl 3-
methyl pentane
Figure 5.4 Di-branched Isomers I
67

0.12
200 400 600
Catalyst Weight (kg)
800 1000
1: 3,4-Methyl hexane, 2: 2-Ethyl 3-methyl pentane, 3: 2,3-Dimethyl hexane, 4: 2,5-Dimethyl hexane
Figure 5.5 Di-branched Isomers H
0.002
I 0.0015
200 400 600
Catalyst Weight (kg)
800 1000
1: 2,2,3-Trimethyl pentane, 2: 2,3,4-Trimethyl pentane, 3: 3,3,4-Trimethyl pentane, 4: 2,2,4- Trimethyl pentane
Figure 5.6 Tri-branched Isomers
68

200 400 600
Catalyst Weight (kg)
800 1000
1: n-Butane and i-Butane, 2: Propane and n-Pentane, 3: i-Pentane
Figure 5.7 Cracked Products
There are 17 beta scission reactions in n-octane hydrocracking (generated by
reaction network program). The information about the single event numbers of the
cracking reactions are not available in the open literature. From the cracking products'
pattern (buteme fraction is always more than propane or pentane fractions), it can be
deduced that the magnitude of the single event for the reactions in which the butane
fraction is produced, is greater as compared to the rest of the cracking reactions. Further,
the butane fraction is approximately three times more than the C3 or C5 fraction in moles
(Vansina et al., 1983). However, the information about the number of single events for
the cracking reactions is important to match the experimental results exactly.
An interesting phenomenon in the simulation results is related to the mole fraction
of di-branched components with respect to C8 moles. Experimental resuhs show that the
mole fraction ofthe di-branched components will decrease after 95% conversion. But the
simulation results show that there is an increase in this mole fraction at very high
conversions though n-C8 and C8 isomers are converted into cracked products. There is
69

only one component, 3-ethyl 3-methyl pentane, in the set of 22 paraffins, which will not
participate in beta scission due to its stmcture. This component decreases very slowly
even at very high conversion. Because of this, even the presence of trace amounts of 3-
ethyl 3-methyl pentane increases the mole fraction ofthe di-branched isomers.
70

CHAPTER 6
MODEL PARAMETER ESTIMATION
The hydrocracker model developed in the present work is based on single event
kinetics. As the chemical steps considered in the reaction networks are fundamental
elementary reactions, it is possible to identify the single event rate parameters from the
model component studies. However, the data available in the literature are too scarce to
estimate all tiie rate parameters for paraffinic, naphthenic, and aromatic feed components
in a typical feedstock to a hydrocracker. histead of estimating the rate parameters from
tiie model components, they can be estimated from synthetic data obtained from an
industrial source. The industrial product distribution was generated using the stmcture
oriented lumping (SOL) approach developed by Quann and Jaffe (1992). The SOL
approach represents the individual hydrocarbon molecules as a vector of incremental
stmctural features such as CH2 and CH3 groups. The vector representation provides the
framework for constmcting the reaction networks and for developing molecular-based
property correlations. The feedstock used to generate the SOL product distribution was
published by Froment (1999). The purpose of this chapter is to describe how the single
event rate parameters are estimated from these data.
The parameter estimation problem discussed in the previous chapter for the
hydrocracking of n-octane follows a very rigorous methodology. The continuity
equations for the reactor model consider all possible but finite number of satiu-ated
species in the cracked products. The n-octane product distribution has 22 paraffins. The
inaccessible carbenium ion concentrations are calculated by solving a system of linear
equations at every reactor increment during the numerical integration. The same approach
as followed in the case of n-octane may not be feasible if the total number of components
in the product distiibution is very large. For paraffins with carbon numbers up to 40,
there are about 41,828 saturated components that are present in the cracked products. The
total number of species in a complex feed such as vacuum gas oil (VGO) is a very large
number. In addition, there are constraints on the present-day analytical capabilities, which
71

provide only limited information on the composition of such an oil fraction (Dewachtere
et al., 1999). To accommodate these constraints, the detailed model is reduced to a
partially lumped model, ft is possible to identify a normal-paraffin in the feed and
product distiibution, whereas it may be very difficult to identify all the isomers belonging
to an iso-paraffin. The partially lumped model will have both pure components and
lumps. For example, the component Cio in normal-paraffins will correspond only to pure
n-decane, whereas the lump Cio in iso-paraffins will correspond to all possible iso-
paraffins having carbon number 10. Similarly, the lump C15 in mono-napthenes
correspond to all possible mono-naphthenic isomers having carbon number 15. The basis
for such lumping is due to the limitation on analytical capabilities. If the information
about all possible isomers belonging to a particular carbon number, then the model can be
expanded to include the corresponding continuity equations. This is one ofthe significant
advantages of the single event approach compared to the lumped models. The complete
reaction network is reduced to a network containing the individual n-paraffms, i-
paraffins, mono-, di-, tri-, tefra-naphthenes, mono-, di-, tri-, tetra-aromatics, and
naphtheno-mono, naphtheno-di-, naphtheno-tri-aromatics as per the carbon number. It is
important to realize that the "lumps" are not equivalent to those defined by boiling points
as used in fraditional lumping approaches. The molecular lumps do not contain any key
component. Each possible molecule with respect to the definition of that particular lump,
is considered in the reaction network. However, care has to be taken in the selection of
the molecular lumps, since their composition is only rigorously defined when their
components are at equilibrium (Dewachtere, 1999). Selection ofthe lumps is primarily
dictated by the analytical information available. The formulation of the rate expressions
for the partially lumped model involves the calculation of lumping coefficients. The
following section describes the methodology to calculate the lumping coefficients.
6.1 Lumping Coefficients
The hydrogenation-dehydrogenation reactions occurring on the metal function of
the hydrocracking catalysts are assumed to be in quasi-equilibrium. This assumption is
72

guaranteed if the metal loading on the catalyst is between 0.1-0.5%) wt./wt. (Weitkamp,
1978). Svobada et al. (1995) observed that the protonation and deprotonation rate
coefficients were sfrongly correlated with the isomerization and cracking coefficients,
indicating that the protonation-deprotonation step was in quasi-equilibrium. Additionally,
the value calculated for the concentration of free active acid sites, CH^, revealed that the
site balance in Equation (5.8) could be neglected (i.e., the concentration of free active
acid sites is equal to the concentration of total active acid sites, Q . Thus, the carbenium
ion concenfration can be calculated from the thermodynamic equilibrium of protonation-
deprotonation reaction given by Svobada et al. (1995),
C,^.=K,XOy;Ryc,C,. (6.1)
The equilibrium constant for the protonation-deprotonation equilibrium can be changed
to the single event equilibrium constant,
( ^ \ C „ . =
^o,,.
V^ i^ik* J
K..iOy;RyCoC,. (6.2)
The single event equilibrium constant for the protonation-deprotonation reaction can be
related to the equilibrium constant for the protonation-deprotonation equilibrium constant
ofthe reference olefin using the following thermodynamic consfraint (Vynckier, 1991),
K'^riOy,m.,) = K\.iO/,m,,)K'iso.iOy;0,). (6.3)
In this equation, the equilibrium constant for the isomerization reaction between the
concemed olefin and the reference olefin can be estimated using the thermodynamic state
fimctions. Substituting Equation (6.3) into Equation (6.2) and dividing by the equilibrium
constant for the protonation-deprotonation reaction of the reference olefin gives the
following:
c\,: ^ c„. ^ '
Kiso.iOy;0,)Co,^, (6.4)
K,.iO/,myCj ^cr^,J
where C*y?,/ is known as the composite concentration of the carbenium ion Rtk^. The
quantities on the right-hand side can be calculated if the corresponding olefin
73

concenfration is known. The olefin concentration can be calculated from the
corresponding concenfration ofthe saturated species that is present in the feed.
To illusfrate the above calculations, consider the following alkane carbenium ion
Rj\
The n-pentyl 2^ carbenium ion must have been created in the following protonation
reactions ofthe two olefins Oj and O2 shown below.
H^
H^
The inaccessible concentration of the above-mentioned carbenium ion Rf can be
calculated using Equation (6.4),
C'R: 1 f^ ^ / - \
IV ^ ^ y K)son.iO,;0,)Ca +
v^«.y K isomi02;0^)Co (6.5)
The olefin concenfrations in the above equation can be calculated from the corresponding
saturated component's concentrations while accounting for physisorption.
C R* y, f - \
y^.j
t ^ \ K isom (O, ; O^ )K^iji +
y^,j K isom (O2; O^ )Ki
K,API]
[HA\ + Y,KyPj (6.6)
where yi is the mole fraction of a component in a lump. If Pj is a pure component, yi is
unity; and if Pj is a lump, then yi is approximated by the reciprocal of the number of
components in that particular lump. From Equation (6.6), the following quantity is
defined as the lumping coefficient, iLC),
74

Lc=y (^ \
v^«.y ri isomiO^',Of)Kj^fj^ +
^-o.^
v^«,y K:isomio^,o,)Ky. ie.i)
To calculate LC, following are needed:
1. Knowledge of tiie protonation reactions responsible for generating the particular
carbenium ion.
2. Knowledge of the global symmetry numbers for the carbenium ion, olefins, and the
saturated species.
3. The number of saturated components in a lump is known.
4. The equilibrium constants for isomerization and hydrogenation-dehydrogenation
reactions are known.
The olefins that generate the carbenium ion are identified from the reaction network. The
global symmetry number for the various species is calculated from their chemical
stmctures. The number of saturated components in a lump is calculated from the
carbenium ion and olefin storage files in the reaction network generation. The
equilibrium constants for the isomerization and hydrogenation-dehydrogenation reactions
are calculated using group contribution methods (Benson et al., 1968). Further, the
lumping coefficients are grouped into two different sets: in one set, lumping coefficients,
LCC corresponds to the consumption of a component or a lump and the other set of
lumping coefficients, LCF corresponds to the formation of a component or a lump. The
computer programs calculate the lumping coefficients by going through each elementary
reaction in the network to estimate the required equilibrium constants along with the
symmetry numbers for the various species, and to classify carefiiUy the carbenium ions,
olefins, and saturated components as per the definitions of the components and lumps
chosen according to the analytical constraints for the partially lumped model. Figure 6.1
shows the lumping coefficient LCC for PCP(s;s) isomerization of normal paraffins as a
function of the carbon number. An increase in the value of the lumping coefficient, with
respect to the carbon number, was observed by Froment (1999) for the PCP (s;s)
isomerization.
75

13 18 23
Carbon Nunnber
28 33
Figure 6.1 Lumping Coefficient for PCP (s;s) Isomerization of n-Paraffins
If the lumping coefficients are calculated, then it becomes fairly easy to formulate
the rate expressions. The net rate of formation of a component or a lump P, can be
calculated by subfracting its rate of consumption from the rate of formation. The rate of
formation and the rate of consumption are calculated by summing up the corresponding
lumping coefficients for formation and consumption along with the correct single event
rate coefficients.
rp,=-Y,LCCii,j)kiJ) [Hi\ + YKdm)[P„]
\ m )
Y^Y.^cFii,j,k)k\k) I^LAPJ]
(6.8)
\ m
By calculating lumping coefficients with the assumptions mentioned in the beginning of
this section, we have avoided solving the system of linear equations to calculate the
concentration of the carbenium ions and also reduced the number of continuity equations
for the reactor model.
76

6.1.1 Single Event Rate Parameters
The assumption of quasi-equilibrium in protonation-deprotonation reaction leads
to the following modified definition of the single event rate parameters. The single event
rate coefficients become the composite single event rate coefficients. Equation (6.9)
defines the composite rate coefficient for the methyl shift reaction between a secondary
and a tertiary carbenium ion,
k'us is;t) = C^„,C,K^^ iO,; s)k'Ms is; t) (6.9)
where, k'Msis;t) is the composite single event rate coefficient and k'Msis;t) is the single
event rate coefficient for the methyl shift is;t). The unknown concnetrations Csat and C,
are lumped in the composite rate coefficient. The composite single event frequency factor
is given by
k\,Msis;t) = Q,c/ , ,^"f'l \oMsis;t), (6.10) \^ko,Deis;0^)J
where k'o,?ris) and k'oMsiO^) are the frequency factors corresponding to the
protonation-deprotonation reaction, and k\j^si^;t) is the frequency factor for the single
event step. The composite activation energy is the sum ofthe activation energies for the
protonation-deprotonation step and the single event step. The composite activation
energy is given by
E\Msis\t) = AH^XOr-,s) + E^„sis;t). (6-11)
The composite frequency factor in Equation (6.10) and the composite activation energy
in Equation (6.11) are defined for a methyl shift (s;t) reaction, hi the same way, the
composite Arrhenius' law parameters are defined for all other elementary steps.
6.2 Model Parameter Estimation
Over the years the chemical analysis of feedstocks has improved fremendously.
Gas chromatography is one technique that permfts a component analysis of a gasoline.
The combined GC-MS technique allows rapid analysis of VGO (Froment, 1999).
Froment (1999) presented the detailed characterization of a partially hydrogenated VGO
77

by means of a combined GC-MS technique, hi this feedstock, there are nine different
major groups: (1) Normal paaffins, (2) Iso-paraffins, (3) Mono-naphthenes, (4) Di-
naphthenes, (5) Tri-naphthenes, (6) Quater-naphthenes, (7) Mono-aromatics, (8) Di-
aromatics, and (9) Tri-aromatics. The composition in weight percent of the individual
components and lumps are given as per the carbon number. There are 19 pure
components in normal paraffins, 18 lumps in iso-paraffins, 18 lumps in mono-
naphtiienes, 17 lumps in di-naphthenes, 16 lumps in tri-naphthenes, 15 lumps in quarter-
naphthenes, 9 lumps in mono-aromatics, 9 lumps in di-aromatics, 6 lumps in tri-
aromatics. The sums of the individual components/lumps' weight fractions are given in
terms of nine groups in Table 6.1.
Table 6.1 Weight Percent of Individual Groups in HVGO Feedstock
Name ofthe Group Carbon Number Range Weight (%) _
12.2
21.8
19.8
8.52
5.65
11.3
5.06
1.40
100.0
Normal paraffins
Iso-paraffins
Mono-naphthenes
Di-naphthenes
Tri-naphthenes
Quarter-naphthenes
Mono-aromatics
Di-aromatics
Tri-aromatics
Sum
15-33
16-33
16-33
15-33
14-33
13-33
14-22
14-22
17-22
The above-mentioned feedstock is used to generate the synthetic product
composition (Jaffe, 2002) at typical operating conditions given in Table 6.2. From the
product distribution obtained from the industry, three sets of data are selected for
78

estimating the rate coefficients. The first set of data is at the low temperatiire, the second
set is at moderate temperature, and the third set of data is at high temperature.
Table 6.2 Typical Operating Conditions for Product Distribution
Quantity Numerical Value
Space velocity (1/hr) 0.75-1.25
Hydrogen flow (SCFB) 4000-5000
Temperature (°F) 550-650
Pressure (psig) 500-1500
These three sets of data are selected primarily to capture the temperature
dependence of the rate coefficients while keeping the other operating conditions the
same. A sequential quadratic programming (SQP) software (Gill et al., 1986) is used for
parameter estimation. The model has 19 rate coefficients, which are listed in Table 6.3.
There are about 226 components and lumps in the model. The 19 rate coefficients are the
decision variables. Two nonlinear consfraints, amount of hydrogen consumed and total
moles of products, are used to bracket the numerical values for the rate coefficients
imtially. It was very difficuft to get a feasible set of conditions for 19 rate constants. The
reactor model produced infeasible product flow rates during many mns due to unreahstic
moves calculated by the nonlinear programming software. Cycling around a particular set
of decisions variables have also been observed during several mns. In fact, the software
took three to four days of computing time to reahze that it was not able to improve the
given guess values for the rate coefficients. We stopped this exercise and switched to the
mode of isothermal reactor simulation without the optimization program. Several
simulation mns were carried out to find the sensitivity of the each of the decision
variables. In this exercise, it has been identified that there are only five decision
variables, which are sensitive to the product distiibution. The five decision variables are
79

given m Table 6.4. These five rate coefficients are for the main reactions such as
isomerization and cracking in hydrocracking.
Table 6.3 Single Event Rate Coefficients
Reaction Type
Metiiyl shift (MS)
Protonated cyclo-propane (PCP)
Acyclic/exocyclic beta Scission (Cr)
Endocyclic beta scission (Endo)
Cyclization (Cy)
Dealkylation (Dealk)
Alkylation (Alk)
Disproportionation (Disp)
Single Event Rate Coefficient
kMs(s;s), kMs(s;t), kMs(t;s), kMs(t;t)
kpcp(s;s), kpcp(s;t), kpcp(t;s), kpcp(t;t)
kcr(s;s), kcr(s;t), kcr(t;s), kcr(t;t)
kgn
key
koealkfs), kDealk(t)
kAlk(s), kAlk(t)
I^Disp
Table 6.4 Single Event Rate Coefficients Sensitive to Product Distribution
Reaction Type Single Event Rate Coefficient
Protonated cyclo-propane (PCP)
Acyclic/exocyclic beta scission (Cr)
Endocyclic beta scission
kpcp(s;s)
kcr(s;t), kcr(t;s), kcr(t;t)
ksn
A reasonable set of initial guesses obtained through the isothermal simulation
mode has been used in the parameter estimation using the optimization software to get
the estimates for the five rate coefficients. A similar exercise has been carried out for the
other two sets of product distribution. The normalized molar flow rates from the
industrial data and the model predictions for three cases: low, moderate, and high
80

temperatures are shown in Figures 6.2, 6.3, and 6.4. The rate coefficients estimated at
three different temperatures are used to generate the Arrhenius' law graph of ilnik) vs.
I/T). The slope and intercept calculated from the Arrhenius' law graphs give values for
the activation energy and frequency factor. As per the confidentiality agreement with the
industry, the numerical value of the rate coefficients, activation energies, and the
frequency factors are not reported in the dissertation. The normalized Arrhenius' law
graphs for the single event rate coefficients kpcp(s;s), kcr(s;t), kcr(t;s), kcr(t;t), and kgn are
shown in Figures 6.5-6.9, respectively. The Arrhenius' law graphs show the data points
along with their linear approximations. The R^ (square of the sample correlation
coefficient) information regarding the linear fit to the data in the Arrhenius' law plot for
all the five rate coefficients are given in Table 6.5. The R^ information can be interpreted
as the interrelation between Inik) predicted by the linear fit and ln(k) obtained from
parameter estimation. In other words, R value is an indication regarding how good
(higher the R^ better the fit) the rate coefficients obey the Arrhenius' law.
81

0 50 100
Components
150 200
^ Industrial Data ^ Model Predicted
Figure 6.2 Industrial Data versus Model Predictions at a Low Temperature
100 150
Components
A Industrial Data ^ Model Predicted
Figure 6.3 Industrial Data versus Model Predictions at a Moderate Temperature
82

0 50 100 150
Components
^ Industrial Data > Model Predicted
200
Figure 6.4 Industiial Data versus Model predictions at a High Temperature
T3
. N
75 E o -0.5
Normalized 1/T(K)
Figure 6.5 Normalized Arrhenius Law Plot for Single Event Rate Coefficient kpcp(s;s)
83

0.9 0.92 0.94 0.96
Normalized 1/T (K)
0.98
Figure 6.6 Normahzed Arrhenius Law Plot for Single Event Rate Coefficient kcr(s;t)
1.2
1
f 0.8
.§ 0.6 CO
E b 0.4 ^
0.2
0 0.9 0.92 0.94 0.96 0.98
Nomnalized 1/T(K)
1.02
Figure 6.7 Normahzed Arrhenius Law Plot for Single Event Rate Coefficient kcr(t;s)
84

1.2
1 -I
r 0.8 •D 0)
^ 0.6 -CO
b 0.4 -I
0.2
0
0.9 0.92 0.94 0.96 0.98
Nomnalized 1/T (K)
1.02
Figure 6.8 Normalized Arrhenius Law Plot for Single Event Coefficient kcr(t;t)
0.9 0.92 0.94 0.96 0.98
Nomnalized 1/T (K)
1.02
Figure 6.9 Normahzed Arrhenius Law Plot for Single Event Coefficient kgn
85

Table 6.5 R^ for Arrhenius' Law Plots
Single Event Rate Coefficient R ' (Sample Correlation Coefficient)
kpcp(s;s) 0.9301
kcr(s;t) 0.9306
kcr(t:s) 0.9461
kcr(t;t) 0.8634
kEn 0.981
6.2.1 Discussion
The rate coefficients obtained from parameter estimation are single event rate
coefficients. Recall that the single event rate coefficients are not the same as the
elementary rate coefficients. A single event rate coefficient when multiplied by the
"number of single events (n^)" yields the corresponding elementary rate coefficient for
that particular elementary reaction.
The single event model has 19 rate coefficients: 4 for methyl shift, 4 for
protonated cyclopropane (PCP), 4 for beta scission, 1 for endocyclic cracking, 2 for
alkylation, 2 for dealkylation, 1 for cyclization, and 1 for disproportionation. The
parameter estimation resuhs show that there are 5 rate coefficients, which are sensitive to
the industiial product distribution. The five are: 1 rate coefficient for PCP isomerization,
3 rate coefficients for beta scission, and 1 rate coefficient for endocyclic cracking. The
resuhs from parameter estimation seem to be reasonable in that the five rate coefficients
cover the major reactions in hydrocracking, namely isomerization and cracking of acychc
molecules and cyclic molecules. The rate coefficients also cover some degree of cracking
in aromatic side chains. The aromatics follow the path of saturation to become naphthenic
molecules and undergo both exocyclic and endocyclic cracking, which will fiuiher
participate in isomerization and beta scission reactions.
86

The rate coefficients for the alkylation and dealkylation reactions for the aromatic
components are insensitive because most of the aromatics are saturated to become
naphthenic components. For the same reason, the single event rate coefficient for the
endocyclic beta scission, which is responsible for ring opening in naphthenic
components, has become a sensitive rate coefficient. The rate coefficients for cyclization
and disproportionation reactions associated with the naphtheno-aromatic components are
insensitive because naphtheno-aromatic components are found in very small amounts
compared to normal-paraffins, iso-paraffins, and naphthenes in the industrial product
distribution.
At this point of time, it is unclear why the 4 rate coefficients for the methyl shift
reactions ikMs(s;s), kMs(s;t), kMs(t;s), and kMs(t;t)) and 3 rate coefficients for PCP
isomerization reactions ikpcp(s;t), kpcp(t;s), and kpcp(t;t)) are insensitive to the industrial
product distribution. The insensitivity of the rate coefficients may mean that the
corresponding reaction paths do not contribute to the net rate of formation at which the
products are formed. One possible reason may be that the synthetic product distribution
generated by the stmcture oriented lumping (SOL) approach may not have considered the
same components and/or lumps that are generated in single event approach. It is possible
that the rate parameters are biased towards the lumps that are considered in the SOL
model. Also it may be possible that the reactions associated with the insensitive rate
coefficients are not significant with respect to the SOL product distiibution.
It can be seen from Figures 6.2-6.4 that the model predictions do not fit perfectly
with the industrial data. The important reason behind the mismatch is due to not having
the correct number of single events, «e for the cracking reactions such as acyclic,
exocyclic and endocyclic beta scissions. The «e factors are calculated algorithmically for
the other isoermization elementary steps. The calculation of a number of single events for
the various cracking reactions is one ofthe important areas for the fixture research.
The single event rate coefficients estimated in the present work are used to
develop a hydrocracker model. The details of the reactor model and reactor simulation
results are discussed in the next chapter.
87

CHAPTER 7
HYDROCRACKER SIMULATIONS
The hydrocracking of vacuum gas oil (VGO) fractions is performed in three-
phase (gas-solid-liquid) fixed bed trickle flow reactors except for the H-oil process (Yan,
1980). The three phases correspond to a fixed bed of porous catalyst particles, a vapor
phase that mainly consists of hydrogen and vaporized light hydrocarbons, and a liquid
phase tiiat consists of mainly vacuum gas oil components, sulfur- and nitrogen-containing
components and dissolved gases (e.g., hydrogen, hydrogen sulfide, ammonia, and light
hydrocarbons). In commercial applications, trickle and pulse flow features are the mostly
likely flow regimes.
The use of trickle bed reactors in industrial processing was reviewed, by
Satterfield (1975). In trickle flow, the gas phase is continuous and the liquid phase is
dispersed. The liquid flows over the catalyst particles as a laminar film or as rivulets. The
pulsed flow regime is attained at higher liquid and gas throughputs. It is commonly
accepted that in commercial hydroprocessing reactors all the particles are completely
wetted when the gas and liquid are adequately distributed (Shah, 1979). Froment et al.
(1994) proposed a one-dimensional heterogeneous model with both liquid and gas phase
in plug flow for a hydrodesulfurization reactor. The reactor model considers material and
energy balance equations for the gas, liquid, and solid phases. In this work, because of
the unavailability ofthe two-phase data (vapor and liquid), a pseudo-homogeneous model
is formulated to simulate the hydrocracking reactor.
The following assumptions have been made in the development of the reactor
model:
1. A plug flow pattern exists in the trickle flow reactor.
2. Heat losses are negligible and the commercial reactors operate under adiabatic
conditions.
3. Diffusional resistances are absent.
4. Steady state operation is considered.
5. Pressure drop in the reactor is negligible.
88

6. Catalyst deactivation is neglected.
With the above-mentioned assumptions, the following homogeneous material and
energy balance equations are given below for the hydrocracking reactor.
1 dF. ' - = PBY^[JArj i = l,2,...,Nc (7.1) Q dz j^^
mCp dT ^
^ ^ = ^.S0(-A«,) (7.2)
where Q is the cross sectional area of the reactor, F, is the molar flow rate of the
component or lump /, z is the axial coordinate in the reactor, p^ is the bulk density ofthe
catalyst, S[j,i] is the stoichiometric coefficient ofthe component or lump i for reaction y,
r, is the net rate of formation ofthe component or lump i in reaction^', and Nc is the total
number of components and lumps. The net rate of formation of a component or lump i is
calculated using the lumping coefficients described in the previous chapter. The
physisorption of the component or lump / is accounted for by Langmuir adsorption. In
Equation (7.2), m is the mass flow rate ofthe hydrocarbon and hydrogen feed, Cp is the
specific heat capacity, T is the reactor temperature, and AHj is the heat of reaction for
reaction j . The hetero-molecules such as sulfiir-, oxygen-, and nitrogen-containing
components are not considered in the feed, rather the reactor feed is completely made of
carbon and hydrogen only.
The rate parameters, namely the activation energies and frequency factors are
estimated from synthetic industrial data as explained in Chapter 6. The estimated rate
parameters are used in the model. The simulation results present a quahtative assessment
about the hydrocracking rather than a quantftative one. The feed and product streams are
described by 226 paraffinic, naphthenic, and aromatic components and lumps. The
material and energy balance equations are integrated along the length of the catalyst bed
by using Gear's method (Gear, 1971) or the package LSODE (Byme and Hindmarsh,
1987) for solving stiff ordinary differential equations.
89

7.1 Profiles along the Length ofthe Catalvst Bed
The catalyst bed in the reactor is divided into four parts of equal length. The bed
length is often decided by the maximum temperature increase allowed by the design
limitation, hi between the catalyst beds, cold hydrogen is injected to absorb the
exotiiermic heat of reaction. Details about the reactor feed have been covered in chapter
6. Figure 7.1 displays the temperature profile in each catalyst bed for a typical inlet
temperature of 608 K. All four catalyst beds have the same inlet temperature. The
increase in tiie temperature is normally in the range of 25-30 K (Laxminarsimhan, 1989;
Mohanty, 1984). A temperature increase of 30 K is considered the maximum limit in any
of the catalyst beds. The temperature increase in the beds decreases after the second bed
due to a decrease in the reaction rate. The bed lengths and the inlet temperatiires to the
individual beds may be optimized to achieve desired product profiles.
The temperature increase in the individual beds with respect to different inlet
temperatures is given in Table 7.1.
Table 7.1 Temperature Increase in Catalyst Beds with Different Inlet Temperatures
Inlet Temperature (K) Temperature Increase in Beds (K)
583.0 5.978, 6.094, 6.388, 6.629
593.0 10.436, 11.059, 11.463, 11.545
603.0 19.314, 20.456, 19.941, 17.208
608.0 27.389, 28.457, 23.457, 15.754
609.0 29.567, 30.362, 23.452, 14.860
The increase in the temperature almost doubles for a change in the inlet temperature of
about 10 K. Beyond an inlet temperature of 609 K the second bed temperature increases
more than 30 K.
90

635 -
^ 630 -
3 625 -
2 g_ 620 -i 615 -1—
610 -
605 -
/
y
J
1
1
/
1 / V
/ ^ 1 ' 1
_ - ^ — , 1 0.2 0.4 0.6
Normalized Bed Length
0.8
Figure 7.1 Temperature Profile along the Catalyst Bed
Figure 7.2 shows the hydrogen consumption along the length of the catalyst beds
for different inlet temperatures. It is very difficult to estimate hydrogen consumed in the
hydrocracking reactions using a lumped model approach. With a detailed molecular
model such as the present one, it is not difficult to calculate the consumption of
hydrogen. Since the chemical stmcture of the components and lumps are known, a
hydrogen balance on the feed and products will yield the hydrogen consumption. An
increase in the operating temperature causes an increase in the rate of the cracking
reactions, which serve to increase the hydrogen consumption. The cracking reactions, in
which hydrogen is consumed, reduce the boiling range of the feed stock. The molecular
weight of the reaction mixture steadily reduces along the length of the reactor, which is
the main purpose of hydrocracking.
The various components and lumps considered in the model can be broadly
grouped under common oil fractions such as liquefied petroleum gases (LPG), gasoline,
middle distillates (MDS), and residue. The grouping based on the carbon number is given
in Table 7.2.
91

Table 7.2 Components and Lumps in Terms of Oil Fractions
Carbon Number Range Oil Fraction
C3-C4
C5-C10
C11-C24
C24+
Liquefied petroleum gases (LPG)
Gasoline
Middle distillates (MDS)
Residue
O •t-j
Q. E ^ ^ - v
CO «-
o ^
0) ^-^ O) O L_ • D
>» X
500
400
300
200
100
U 0 0.2 0.4 0.6 0.8
Normalized Be6 Length
•593 K .603 K .608K
Figure 7.2 Hydrogen Consumption
92

The fractions listed in Table 7.2 consist of paraffinic, napthenic, aromatic
components, and lumps. For example, the gasoline fraction contains all possible
molecules belonging to the carbon numbers between C5 and Cio. Figure 7.3 shows how
these oil fractions evolve along the catalyst bed. The LPG fraction mainly consists of
light gases, propane, n-butane, and iso-butane. The molar flow rates of propane and
butane are initially zero because the feedstock does not contain any of these components.
Once the hydrocracking reactions start, the heavier molecules crack to produce lighter
fractions. The propane, butane and pentane fractions cannot undergo cracking reactions
by themselves by the mles of the carbenium ion chemistry. For a beta scission to occur,
the reactant carbenium ion must have a tertiary beta carbon with respect to the carbon
atom bearing the positive-charge. The propane, butane, and pentane molecules cannot
have both a positive charge and a tertiary carbon atom simultaneously. For this reason,
the molar flow rates of these components continuously grow until they reach an
equilibrium value with respect to the particular reactor and operating conditions. If the
hydrocracking reactions are carried out for a sufficiently long time, all the paraffins and
isoparaffins in the reaction mixture will be converted into propane, butane, and pentane
fractions. This is the reason for the continuous increase in the molar flow rate ofthe LPG
fraction. Although the number of moles of the hght gases increases along the reactor, the
light gases do not make up a significant percentage of the total mass of the cracked
products, in actual practice, due to their low molecular weights. The molar flow rate of
the gasoline fraction also continuously increases as the cracking proceeds and reaches a
steady value towards the end of the fourth bed. If there is a further increase in the inlet
temperature, the gasoline components might undergo cracking to become propane,
butane, and pentane fractions, which will lead to the loss of valuable gasoline
components to LPG fraction. The amount of lighter components determines the octane
number and Reid vapor pressure (RVP) specifications for the gasoline fraction.
93

250
(/) $ o u.
ar
o "^
150
100
50
U
0.2 0.4 0.6 0.8
Normalized Bed Length
LPG •GASOLINE •MDS •RESIDUE
Figure 7.3 Evolution of Various Oil Fractions
The fraditional lumped models based on boiling point description will be unable
to provide information on the chemical constituents present in a particular lump. This
information is very important for design, operation, and optimization of hydrocracking
reactors. The present approach relies on to the molecular level detail and provides precise
information about the chemical species present in the reaction mixture. Figure 7.4
illusfrates how the components, propane, butane, and iso-butane present in the LPG
fraction evolves along the length ofthe catalyst bed. Figures 7.5 and 7.6 show how molar
flows ofthe paraffinic components such as n-pentane, n-hexane, n-heptane etc. present in
the gasoline fraction vary in the catalyst beds, hi Figure 7.5, the molar flow rate of n-
pentane continues to increase through the reactor because this component does not
participate in the cracking reactions, whereas the molar flow rates of components with
higher carbon numbers initially increase and then decrease after going through a
maximum point.
94

, 80
0.2 0.4 0.6 0.8
Normalized Bed Length
• Propane > •n-Butane Iso-butane
Figure 7.4 Components in LPG fraction
0.2 0.4 0.6 0.8
Normalized Bed Length
• n-Pentane •n-Hexane' •n-Heptane
Figure 7.5 Paraffinic Components in Gasoline Fraction I
95

0.2 0.4 0.6 0.8
Normalized Bed Length
• n-Octane • n-Nonane > •n-Decane
Figure 7.6 Paraffinic Components in Gasoline Fraction E
Figures 7.7 and 7.8 show the molar flow profiles for the iso-paraffinic lumps
present in the middle distillates (MDS) fraction. The C12, C15, C18, and C24 fractions
are lumps, which contain all possible iso-paraffins corresponding to the definition of that
particular lump. The C12 and CI5 fractions are not present in the feed stock. The molar
flow of C24 fraction initially increases due to its formation from the other heavier
components and subsequently undergoes cracking to produce the lighter components.
Similar profiles are presented in Figure 7.8 for di-naphthenic lumps, C25, C29, C31, and
C33, which are present in the residue. The purpose of showing these profiles is to
illustrate that the present approach can give a high-resolution product spectmm that in
most cases may exceed the analytical capabilities of most refineries.
96

0.2 0.4 0.6 0.8
Normalized Bed Length
C12 -C15 • C18' • C24
Figure 7.7 Iso-paraffinic Lumps in MDS Fraction
-S 0.5 o
0.2 0.4 0.6 0.8
Normalized Bed Length
.C25 .C29' .C31 C33
Figure 7.8 Di-naphthenic Lumps in Residue
97

An important feature of the hydrocracking process is its ability to isomerize the
feed components before they undergo cracking reactions. Isomerization is very important
because tiie isomerization products are highly branched components, which are valuable
(high octane number) in any product fraction. For example, the normal octane, a linear
molecule with no side chains has a motor-octane number (MON) of-17.0, whereas the
highly branched isomer of n-octane, 2,2,4-trimethyl pentane (iso-octane) has a motor
octane number of 100.0 (Nelson, 1969). From n-octane hydroisomerization and
hydrocracking in Chapter 5, the linear molecules do not crack without undergoing
isomerization reactions. The various product fractions from the hydrocracking process
exhibit high ratios of iso-paraffins to normal paraffins (Froment, 2002).
Figure 7.9 shows the molar ratio of iso-paraffins to normal paraffins for gasoline
fraction at different temperatures. The iso/normal ratio varies from 2.5 to 4 in the
gasoline fraction. A high molar ratio of iso-paraafins to normal paraffins is a typical
characteristic of the hydrocracking process. As the operating temperature increases, the
iso/normal ratio also increases due to an increase in the isomerization reaction rate. The
presence of the branched components helps to raise the octane number in a product
fraction such as gasoline.
98

0.2 0.4 0.6 0.8
Normalized Bed Length
• T = 6 0 8 K • T = 5 9 3 K •T = 583 K
Figure 7.9 Molar Ratio of Iso-paraffins to Normal Paraffins in Gasoline Fraction
7.2 Optimization
The following section discusses the optimization study carried out using the
reactor model. The current profit optimization study is not intended to cover the entire
range of possibilities but to highlight a few important features ofthe single event model.
The reactor inlet temperature is very sensitive to product distribution. As the inlet
temperature is increased, the reaction rates are accelerated leading to increased
production of products and rapid depletion of the heavy components in the feed. The
product distribution is grouped under four major categories, which are liquefied
pefroleum gases (LPG), gasoline, middle distillates (MDS), and residue. From the
simulation results of the previous section, it was shown that as the reactor inlet
temperature is increased, the LPG fraction increases, and the gasoline fraction approaches
a steady value; middle distillates initially increase and then slowly decay; and the amount
of residue continuously decreases. As the inlet temperature is increased beyond a certain
value, the high-valued components in the gasoline fraction start to undergo cracking
reactions to become low-valued components in the LPG fraction. The relative differences
in the formation and consumption of the feed and product molecules imply an optimum
99

inlet temperature for which the net profit from the products is maximized. However, the
optimization will not be meaningful if we do not consider the constraints. The inlet
temperature cannot be increased if the temperature increase in the catalyst bed violates
the design limitation. The temperature increase makes the gasoline fraction lighter and
the amount of lighter components allowed in this fraction is dictated by the Reid vapor
pressure (RVP) (Gary and Handwerk, 1984). The optimization problem is formulated to
maximize the net profit while satisfying the constraints on bed temperature increase and
RVP specification on gasoline. Reactor inlet temperature is the decision variable. The
prices for the various oil fractions are taken from Li (2000). The profit objective fimction
and consfraints are as follows:
objf=Ypy-llPuyj i J
Gasoline RVP<RVP\ (7.3)
Bed AT < ATI
where Pp is the price for product /, F, is the molar flow of product i, P^ is the price for
utility 7, Ui is the molar flow of utilityy , and RVPl and ATI are the upper limits on
gasoline RVP and temperature increase in a catalyst bed.
Figure 7.10 shows how the profit function changes with respect to the inlet
temperature. The profit reaches a maximum value for the inlet temperature of 608.5 K
and then decreases for further increases in the temperature. The profit reduces because
the components in the gasoline range undergo cracking reactions to produce the light
products whose economic value are less than that of the gasoline fraction. The
temperature increase in the bed violates the constraint beyond 609 K. At about 613 K, the
increase in the bed temperature reaches a value of 41.79 K.
100

•a 0)
S o E o o
o
570 580 590 600 610
Inlet Temerature (K)
620
Figure 7.10 Profit Optimization
The product distiibution for inlet temperattires 598 K, 608 K, 613 K, and 618 K
are shown in Table 7.3. The relative increase in the gasoline fraction decreases with
respect to the increase in the inlet temperature beyond 608 K. The components in the
gasoline fraction undergoes cracking with ftirther increase in the inlet temperature (see
Table 7.3), which causes the profit function to decrease.
Table 7.3 Product Distribution for Different Inlet Temperatures
Temperature
(K)
598.0
608.0
613.0
618.0
LPG
(kmol/hr)
68.164
154.119
201.661
239.657
Gasoline
(kmol/hr)
155.708
222.139
230.489
226.921
MDS
(kmol/hr)
58.728
23.302
11.008
4.9780
Residue
(kmol/hr)
3.517
0.214
0.0104
0.00007
101

A detailed molecular model can play an important role in such constrained
optimization applications. For example, consider maximizing the production of the
gasoline fraction. The lumped models will be able to predict that the gasoline fraction
will increase with an increase in the inlet temperature but they will not be able to predict
critical specifications like RVP and octane number accurately. Because of this, even if
the lumped model predicts that the gasoline fraction is maximized, the product
specifications may violate a critical consfraint such as the RVP. Since the chemical
stmctures of the species that are present in the products are known in the single event
model, it is possible to estimate the performance properties in a very rigorous way. The
ability to calculate the performance properties (e.g., RVP, octane number) of a product
fraction is very valuable for process modeling and optimization.
102

CHAPTER 8
SUMMARY AND CONTRIBUTIONS
8.1 Summary
The present research work began with the following broad objectives in mind:
1. To develop a detailed, fimdamental, and molecular level model for a hydrocracker;
2. To estimate the rate parameters from the data obtained from an industrial
organization;
3. To use the model to understand the complex nature ofthe hydrocracking process.
The single event kinetics method was used to develop the kinetic model because
the lumped models appear to have significant limitations. Unit optimization and refinery-
wide optimization need high-fidelity models. The single event approach employs a
microscopic analysis ofthe chemistry ofthe hydrocracking process and a methodology to
fransform the information from the complex chemistry into an actual model building
exercise. In effect, the fimdamental concepts from fransition state theory and quantum
chemistry are used directly in this approach. It is surprising to realize that the concept of
locating a fransition stmcture using quantum chemical principles can actually be used in
building a kinetic model for an industrial process. The single event approach can be used
to elevate the fraditional modeling approach for complex reactor systems such as
hydrocracking and catalytic cracking from a primitive level of boiling point lumps to a
sophisticated level of individual molecules.
To illusfrate the complexity of the single event approach as compared to the
fraditional lumping approaches, consider the model building exercise in the lumped
approach. The feedstock and the products are divided into a finite number of lumps. The
pathways between the lumps are selected based on the observed product distiibution
while using known principles of cracking chemistry. Once the reaction pathways are
chosen, rate parameters are estimated from the information from the feestocks and
products.
103

The single event approach is more complex and the increased complexity
associated with the single event kinetics does come with a price. The reaction pathways
are too complicated to generate analytically. A computer algorithm is needed to generate
the reaction networks.
Developing the software for generating the reaction network is one contribution
from this work. The fimdamentals of the carbenium ion chemistry are embedded in the
network generation algorithms. The reaction pathways are neither obtained from
published information nor from experimentation. Rather, they are constmcted based on
the general principles of carbenium ion chemisfry that are relevant to cracking systems
using a computer algorithm.
An important part of the network generation software is the algorithms for
standardized labeling. The standardized labehng algorithms permit a unique Boolean
relation matrix for a particular carbenium ion or an olefin. The approach is general and
can be used for standardized labeling of acyclic and cyclic hydrocarbon species. This
software with little or no modifications can be used for (1) developing reaction pathways
for other complex acid-catalyzed processes such as catalytic cracking, alkyaltion, and
reforming; and (2) developing reaction networks for model component studies. The
model component studies involve hydrocracking the pure components and analyzing their
product distributions. The model component studies are not only for exploring and
understanding the chemical pathways but also for estimating the single event parameters.
The rate coefficient for an elementary reaction is obtained by multiplying the
single event rate coefficient with a factor called "number of single events." The
calculation of the number of single events requires knowledge of the geometry of the
fransition stmcture or activated complex formed in an elementary reaction. The geometry
of the activated complex can be determined using a quantum chemical package. The
program GAMESS (General Atomic and Molecular Electronic Stmcture System)
(Schmidt, 1993) was used to identify the activated complex for a hydride shift reaction.
The number of single events calculated using the stmctiure of the activated complex is
consistent with the published information.
104

Since the chemisfry in the kinetic model is represented in terms of the
fundamental elementary reactions, the single event model is invariant in the rate
parameters with respect to feedstock composition and operating conditions. The rate
coefficients can be used with any type of feedstocks as long as there are no major
changes in the catalyst properties for which the rate parameters are identified.
One of the primary objectives was to estimate the single event rate coefficients
from the data obtained from the industry. An industrial synthetic product distribution was
generated using stinctiue oriented lumping (SOL) approach (Quann and Jaffe, 1992). A
partially hydrogenated vacuum gas oil (VGO) was the feedstock (Froment, 1999). The
single event model had 19 rate coefficients: 4 for methyl shift isomerization, 4 for PCP
isomerization, 4 for beta scission, 1 for endocyclic scission, 2 for alkylation, 2 for
dealkylation, 1 for cyclization, and 1 for disproportionation. Parameter estimation resuhs
show that there are 5 rate coefficients, which are sensitive to the industrial product
distiibution. The five are: 1 rate coefficient for PCP isomerization, 3 rate coefficients for
beta scission, and 1 rate coefficient for endocyclic cracking. The results are reasonable
because these rate coefficients cover the major reactions, namely isomerization, cracking
of acyclic molecules and naphthenic molecules. The rate coefficients also cover some
degree of cracking in the aromatic side chains. The aromatics follow the path of
saturation to become naphthenic molecules and undergo both exocyclic and endocyclic
cracking, which will ftirther participate in isomerization and beta scission reactions.
The rate coefficients for the alkylation and dealkylation reactions for the aromatic
components are insensitive because most of the aromatics are saturated resulting in the
formation of naphthenic components. The rate coefficients for the cyclization and
disproportionation reactions associated with the naphtheno-aromatic components are
insensitive because naphtheno-aromatic components are found in very small amounts
compared to n-paraffins, iso-parffins, and naphthenes in the industrial product
distribution.
It is found that the 4 rate coefficients for the methyl shift reactions ikMs(s;s),
kusisit), kMs(t;s), and kMs(t;t)) and the 3 rate coefficients for PCP isomerization reactions
105

ikpcp(s;t), kpcp(t;s), and kpcp(t;t)) are insensitive to the industrial product distribution.
The possible reasons for the insensitivity ofthe rate coefficients are as follows:
1. The reaction paths corresponding to the insensitive rate coefficients do not contribute
to the net rate of formation ofthe products.
2. The synthetic product disfribution generated by the stmcture oriented lumping (SOL)
approach may not have considered the same components and/or lumps that are
generated in the single event approach.
3. The rate parameters are biased towards the lumps that are considered in the SOL
model.
4. The reactions associated with the insensitive rate coefficients are not significant with
respect to the SOL product distiibution.
The single event rate coefficients estimated at different temperatures are used to
develop the Arrhenius' Law graphs. The activation energies and frequency factors are
calculated from these graphs. The single event model is inserted into a homogeneous
adiabatic reactor model and the resulting continuity equations are integrated along the
length of the catalyst beds to obtain the temperature profile, composition profiles, and
hydrogen consumption profile through the reactor. The simulation results from the
homogeneous reactor model are consistent with the industrial practice and pubhshed
results in this area.
The estimation of hydrogen consumption is very complicated in lumped models.
The lumped models will yield the information about the amount of carbon and hydrogen
present in a particular lump. Since the chemical stmcture of the pure components and
lumps is known in the single event model, a simple hydrogen balance on the feed and the
products yields the overall hydrogen consumption.
A profit optimization study was carried to maximize the net profit from the
various product fractions without violating the constraint on the Reid vapor pressure
(RVP) and a consfraint on the increase in the bed temperature. The study showed that
beyond a certain inlet temperature (335°C), the components from the high-valued
gasoline fraction start to undergo cracking reactions to become low-valued light gases
106

such as propane and butane. The molecular nature ofthe single event model allows one to
calculate the critical specifications such as RVP and octane number of a product fraction.
8.2 Contributions
The confributions of this research are as follows:
1. Standardized labeling procedures for acyclic and cyclic hydrocarbon species. The
labeling algorithms are a part ofthe software developed in this work.
2. A methodology to address alkene carbenium ions generated in endocyclic beta
scission of naphthenes.
3. Elementary reaction networks for the hydrocracking of VGO feed components.
4. Modification of lumping coefficient calculations to have separate parts for
consumption and generation of various pure components and lumps.
5. Modification of the algorithm proposed by Bahanas and Froment (1985) to reduce the
memory requirements to one half of its original value.
8.3 Recommendations
The following recommendations are proposed for fiiture study.
1. The lumping coefficients calculations require a factor called "number of single
events." The reliable estimation of this factor may be done using a quantum chemical
package. In the present work, we were not able to get this factor for all the cracking
reactions. In fact, considering the complexity ofthe feedstock such as vacuum gas oil
(VGO) it is almost impossoble to calculate number of single events for each
individual reaction. There needs to be a systematic approach to address this problem.
2. The present work considers a homogeneous reactor model for the hydrocracking
process. There are difficulties associated with developing a heterogeneous model for
the hydrocracker. The fluid phase equilibria is described by using Henry's law
coefficients. No clear methodology is available in the open literature for estimating
107

Henry's law coefficients for various components present in the reaction mixture for a
complex feed such as VGO. This is tme for the adsorption coefficients also, ft will be
an interesting exercise to compare simulation results from the homogeneous and
heterogeneous models.
3. The present research considers the second stage hydrocracking process where
isomerization and cracking reactions dominate. However, the reactions involving
hetero-molecules (sulfiir, nitrogen, and oxygen compounds) such as desulfurization
and denifrogenation occur in the first stage of hydrocracking. ft is recommended to
include reactions for the hetero-molecules to develop the complete model for a
hydrocracker.
4. It is recommended to include the hydrogenolysis reaction (cleavage by hydrogen) in
the kinetic model. The hydrogenolysis reaction is responsible for producing methane
and ethane. The carbenium ion chemistry mles do not allow the formation of methane
and ethane molecules in hydrocracking. Inadequate metal loading on the catalyst or
catalyst poisoning promotes hydrogenolysis reactions.
5. The present approach does not consider the catalyst deactivation process. The catalyst
aging occurs in hydrocracking very slowly due to the very high partial pressure of
hydrogen present in the reactor. The deactivation model can also be built along the
same lines followed in the present work. It is important to realize that the catalyst
deactivation by coke formation is also due to chemical reactions similar to alkylation,
isomerization etc.
6. The methodology to extend single event approach to alkylation, fluidized catalytic
cracking (FCC), and reforming are summarized briefly below:
6.1. Alkylation is a process of combining iso-butane with hght olefins such as
propylene, butylene, and pentylene to form high-octane components, which are
excellent blending stocks for gasoline pool. The alkylation reactions occurring in
aqueous phase in presence of sulfuric acid or hydrofluoric acid are based on
carbenium ion chemistry. Protonation, deprotonation, hydride shift, methyl shift,
PCP, hydride transfer, cracking, diproportionation and polymerization are the
108

major reactions in alkyahion process. This process is an interesting candidate for
applying single event kinetics.
6.2. Fluidized catalytic cracking (FCC) is carried out in a riser reactor with VGO
feed. The reactions occur in the gas phase. Physical adsorption reaches
equilibrium conditions unlike hydrocracking. The catalyst does not contain metal
components (though impurities deposit metal components on the catalyst);
hydrogenation dehydrogenation equilibrium need not be considered. The cracked
product will have olefins, which are to be considered in the product distribution.
In addition to the elementary steps considered for hydrocracking, hydride
absfraction, hydride fransfer, and protolytic scission are to be included to model
FCC reactions. An interesting point to note: the carbenium ions are generated in
FCC by hydride abstraction and also by the protonation of olefins present in the
feed.
6.3. Reforming reactions are carried out in fixed bed reactors with naphtha as the
feed. The catalyst is bi-functional. The gas phase reactions follow the same
carbenium ion chemistry ftindamentals. Dehydrogenation, isoermization,
dehydrocychzation, and hydrocracking are the important reactions. The single
event concepts can be applied to develop the reformer model. The naphtha feed,
which is much lighter than the feed to a hydrocracker, will make modeling and
parameter estimation exercise easier than for a hydrocracker.
On a final note, we strongly believe that the present research will act as a catalyst
in stimulating the commitment of oil companies to improve their analytical capabilities
and also in developing a mutual tmst between the industiy and academia to share
information and research results.
109

BIBLIOGRAPHY
Amundson, N.R., Acrivos, A., "Steady State Fractionation of Multicomponent Complex Mixtures in an Ideal Cascade," Chem. Eng Sci, 1955, 4, 29.
Aris, R., Gavalas, G., "Theory of Reactions in Continuous Mixtures," Phil. Trans. Roy. Soc, 1966, A260, 351.
Astarita, G., Ocone, R., "Lumping Non-linear Kinetics," AIChE J., 1988, 34, 1299.
Baltanas, M. A., Froment, G. F., "Computer Generation of Reaction Networks and Calculation of Product Distributions in the Hydroisomerization and Hydrocracking of Paraffins on Pt-containing Bifunctional Catalysts," Comp. & Chem. Eng, 1985,9, 1,71-81.
Baltanas, M. A., Vansina, H., Froment, G. F., "Hydroisomerization and Hydrocracking. 5. Kinetic Analysis of Rate Data for n- Octane," Ind. Eng. Chem., Prod. Res. Dev. 1983,22,531-539.
Baltanas,M. A ., Van Raemdonck, K. K., Froment, G. F., Mohedas, S. R., "Fundamental Kinetic Modeling of Hydroisomerization and Hydrocracking on Noble-Metal Loaded Faujasities. 1. Rate Parameters for Hydroisomerization," Ind. Eng. Chem. Res. 1989, 28, 899-910.
Benson, S.W., Cmikshank, F.R., Golden, D.M., Haugen, G.R., O' Neal, H.E., Rodgers, A.S., Shaw, R., Walsh, R., "Addivity Rules for the Estimation of Thermochemical Properties," Chem. Rev., 1969, 69, 279-323.
Briano, J.G., Glandt, E.C., "Statistical Thermodynamics of Disperse Fluids," J. Chem. Phys., 1988, 80, 3336.
Brouwer, D.M., Hoogeveen, H., "The Importance of Orbital Orientation as a Rate-Controlling Factor in Intramolecular Reactions of Carbonium Ions," Rec. Trav. Chim., 1970,89,211.
Campbell, D.R., Wojciechowski, B.W., "Catalytic Cracking of Cumene on Aging Catalysts I. The Mechanism ofthe Reaction," /. Catal., 1971, 20, 217-222.
Chou, M.Y., Ho., T.C., "Lumping Coupled Non-linear Reactions in Continuous Mixtures," AIChE J., 1989, 35, 4, 533-538.
Chou, M.Y., Ho., T.C., "Continuum Theory for Lumping Nonlinear Reactions Mixtiires," ^/C/i^J.,1988, 34, 1519.
110

Cicarelli, P., Astarita, G., Gallifiioco, A., "Continuous Kinetic Lumping of Catalytic Cracking Processes," .4/C;?£ J., 1992, 38, 7, 1038-1044.
Clymans, P. J., Froment, G.F., "Computer-Generation of Reaction Paths and Rate Equations in the Thermal Cracking of Normal and Branched Paraffins," Comp. & Chem. Eng., 1984, 8, 2, 137-142.
Corma, A., Miguel, P. J., Orchilles, A.V., Koermer, G.S., "Cracking of Long-Chain Alkyl Aromatics on USY Zeolite Catalysts," J. Catal, 1992, 135,45-59.
Dewachtere, N.V., Santella, F., Froment, G.F., "Application of a Single Event Kinteic Model in the Simulation of an hidusfrial Riser Reactor for the Catalytic Cracking of Vacuum Gas Oil," Chem. Eng. Sci., 1999, 54, 3653-3660.
Egan, C.J., Langlois, G.E., White, R.J., "Selective Hydrocracking of C9-to C12-Alkylcyclohexanes on Acid Catalysis. Evidence for the Paring Reaction," /. Am. Chem. Soc, 1961, 84, 1204-1212.
Feng, W., Vynckier, E., Froment, G. F., "Single-Event Kinetics of Catalytic Cracking,"/nJ. Eng Chem. Res., 1993, 32, 2997-3005.
Froment, G. F., "Kinetic Modeling of Acid-Catalyzed Oil Refining Processes," Catalysis Today, 1999, 52, 153-163.
Froment, G.F., Depauw, G.A., Vanrysselberghe, V., "Kinetic Modeling and Reactor Simulation in Hydrodesulfurization of Oil Fractions," Ind. Eng. Chem. Res. 1994, 33, 2975-2988.
Gary, J.H., Handwerk, G.E., Petroleum Refining - Technology and Economics, Marcel Dekker, hic, New York, 1984.
Gates, B.C., Katzer, J.R., Schuft, G.C., Chemistry of Catalytic Process, McGraw Hill, New York, 1979.
Gear, C.W., Numerical Initial Value Problems in Ordinary Differential Equations, Prentice Hall, Englewood Chffs, NJ, 1971.
Gill, P.E., Murray, W., Saunders, M.A., Wright, M.H., User's Guide for NPSOL ™ (Version 4.0) A Fortran Package for Non-linear Programming, Systems Optimization Laboratory, Stanford, Cahfomia, 1986.
Govindhakannan, J., "Continuum Lumping Model for Hydrocracker Simulation", hitemal Report, Texas Tech University, 1999.
I l l

Greensfelder, B.S., Voge, H.H., Good, G.M., "Catalytic and Thermal Cracking of Pure Hydrocarbons-Mechanisms of Reaction," Ind Eng Chem., 1949, 41, 11, 2573-2 j o 4 .
Hillewaert L. P., Dierickx, J. L., Froment, G. F., "Computer Generation of Reaction Schemes and Rate Equations for Thermal Cracking," AIChE J., 1988, 34, 1, 17-
Jaffe, S.B., Personal Communication, Lubbock, TX, NJ, 2002.
Jacob, S.M., Gross, B., Voltz, S.E., Weekman, V.W., "A Lumping and Reaction Scheme for Catalytic Cracking," /C/z£ J., 1976, 22, 701-713.
Kane, L.A., Ed., "Hydrocracker Advanced Control", Hydrocarbon Processing, August 2002,111-113.
Klein, M.T., Neurock, M., Nigam. A., Libanati, C, "Monte Carlo Modeling of Complex Reaction Systems: An Asphaltene Example," Proc. Mobil Workshop on Chemical Reactions in Complex Mixtures, Van Nostrand Reinhold, New York, 1991, 126-142.
Krambeck, F.J., "Computer and Modem Analysis in Reactor Design," Ind. Chem. Eng. Symp. Ser., 1984, A260, 351.
Krishna, R., Saxena, A.K., "Use of an Axial-Dispersion Model for Kinetic Description of Hydrocracking," Chem. Eng. Sci., 1989, 44, 3, 703-712.
Laxminarasimhan, C.S., Verma, R.P.,Ramachandran, P.A., "Continuous Lumping Model for Simulation of Hydrocracking," AIChE J., 1996 42, 9, 2645-2653.
Laxminarasimhan, C.S., Sau, M., Verma, R.P., "Modeling Heat Effects of VGO Hydrocracking," International Symp. on the Adv. In Catal, and Processes for Heavy Oil Conversion, ACS, San Francisco, CA, 1997, 416-419.
Leach, A.R., Molecular Modeling - Principles and Applications, Longman, Harlow, England, 1996.
Levsen, K., Fundamental Aspects of Organic Mass Spectrometry, Weinheim, New York, Verlag Chemie, 1978.
Li, X., Refinery-wide Optimization, Ph.D. Dissertation, Texas Tech University, Lubbock, TX, 2000.
112

Liguras, D.K., Allen, D.T., "Stmctural Models for Catalytic Cracking," Ind Eng Chem. Res., 1989, 28, 665-673.
Luss, D., Hutchinson, P., "Lumping of Mixtures with Many Parallel «th Order Reactions," Chem. Eng J., 1971, 2, 172.
Mohanty, S., Kunzru, D., Saraf, D.N., "Hydrocracking: A Review," Fuel, 1990, 69, 1467-1473.
Martens, G.G., Marin, G.B., Martens, J.A., Jacobs, P A., Baron, G.V.A., "Fundamental Kinetic Model for Hydrocracking of Cg to Cn AUcanes on Pt/US-Y Zeolites," J. Catal., 2000, 195, 253-267.
Martens, J. A., Jacobs, P.A., Theoretical Aspects of Heterogeneous Catalysis, Van Nosfrand Reinhold, New York, 1990, 52-109.
Martens, G. G., Thybaut, J. W., Marin, B.G., "Single-Event Rate Parameters for the Hydrocracking of Cycloalkanes on Pt/US-Y Zeolites," Ind. Eng. Chem. Res. 2001,40,1832-1844.
McCoy, B.J., Balasubramanium, "Continuous Mixture Kinetics of Coke Formation from Ohfinic Oligomers," AIChE J., 1995, 41, 2.
McKetta, J., Petroleum Processing Handbook, Marcel Dekker, Inc., New York, 1992.
Meyers, R.A., Handbook of Petroleum Processes, McGraw-Hill, New York, 1996
Minderhoud, J.K., Van Veen, J.A.R., Hagen, A.P., "Hydrocracing in the Year 2000: A
Strong Interation between Technology Development and Market Requirements," Hydrotreatment and Hydrocracking of Oil Fractions, Elsevier Science B.V., 1999, 3-20.
Mohanty, S., Saraf, D.N., and Kunzm, D. "Modeling of a Hydrocracking Reactor," Fuel Processing Technology, Elsevier Science Pubhshers B. V., 1991, 29, 1-17.
Nelson W.L., Petroleum Refinery Engineering, 4* Ed., McGraw-Hill, New York, 1969.
Olah, G.A., "Carbocations and Electrophilic Reactions", Angew. Chem. Internal Edit, 1973, 12, 3, 173-254.
Olah, G.A., Friedel-Crafts and Related Reactions, Wiley, New York, 1964.
Quader, S.A., Singh, S., Wiser, W.H., Hill, G.R., "Hydrocracking of Petroleum Oils," J. Inst. Pterol, 1970, 56, 550.
113

Quann, R. J., Jaffe, S.B., "Stmcture-Oriented Lumping: Describing the Chemistry of Complex Hydrocarbon Mixtures," Ind Eng Chem. Res., 1992, 31, 2483-2497.
Shah, Y.T., Gas-liquid-solid Reactor Design, McGraw-Hill, New York, 1979.
Schmidt, M.W., Balridge, K. K., Boatz, J. A.;Elbert, S. T., Gordon, M. S., Jensen, J. H., Koseki, S., Matsunaga, N., Nguyen, K. A., Su, S., Windus, T. L., Dupuis, M., Montgomery, Jr., J. A. "General Atomic and Molecular Electronic Stmctiire System," J. Comput. Chem., 1993, 14, 11, 1347-1363.
Schweitzer, J. M., Galtier, P., Schweich, D., "A Single Events Kinetic Model for the Hydrocracking of Paraffins in a Three-Phase Reactor," Chem. Eng. Sci., 1999, 54, 2441-2452.
Svoboda, G.D., Vynckier, E., Debrabandere, B., Froment, G.F., "Single-Event Rate Parameters for Paraffin Hydrocracking on a Pt/US-Y Zeolite," Ind Eng Chem. Res. 1995, 34, 3793-3800.
Stangeland, B. E., "A Kinetic Model for the Prediction of Hydrocracker Yields," Ind. Eng. Chem., Process Des. Dev., 191 A, 13. 1, 71-76.
Stangeland, B.E., Kittrell, J.R., "Jet Fuel Selectivity in Hydrocracking," Ind. Eng. Chem., Process Des. Dev., 1972, 11, 16.
Steijns, M., Froment, G.F., "Hydroisomerization and Hydrocracking. 2. Product Distributions from n-Decane and n-Dodecane," Ind. Eng. Chem., Prod. Res. Dev., 1981, 20, 654-660.
Tom, T.B., Mosby, J.F., Gutberlet, L.C., "Hydrocracking for Distillates," Symp. Adv. in Distillate and Residual Oil Technol, ACS Meeting, New York, 1972.
Vansina, H., Baltanas, M. A., Froment, G. F. "Hydroisomerization and Hydrocracking. 4. Product Distribution from n-Octane and 2,2,4-Trimetiiylpentane," Ind. Eng. Chem., Prod Res. Dev. 1983, 22, 526-531.
Vynckier, E., Froment, G.F., "Modeling ofthe Kinetics of Complex Process Based upon Elementary Steps. Kinectics and Thermodynamic Lumping of Muhicomponent Mixtures," Kinetic and Thermodynamic Lumping of Multicomponent Mixtures, Elsevier Science Pubhshers B.V., Amsterdam, 1991, 131-161.
Weekman, V.W., Nace, D. M., "Kinetics of Catalytic Cracking Selectivity in Fixed, Moving and Fluid Bed Reactors," /C/JJ^ J., 1970, 16, 397.
114

Weitkamp, J., Jacobs. P. A., Award Symposium on Fundamentals of Catalysis and Thermal Reactions, 181" National Meeting of the American Chemical Society, Aflanta, GA, March 29-April 3, 1981.
Yan, T. Y. "Dynamics of a Trickle-Bed Hydrocracker with a Quenching System," Can. J. Chem. Eng, 1980, 58, 259-266.
Zabicky, J., The Chemistry ofAlkenes, Volume 2, Interscience Publishers, John Wiley & Sons, New York, 1970.
Zeeman, R.J., Amundson, N.R., "Continuous Polymerization Sequels," Chem. Eng. Sci., 1965,20,331.
Zhorov, Y.M., Panchenkov, G.M., Tatarintseva, G.M., Kuzmin, S.T., Zenkovskii, S.M., "Chemical Scheme and Stmcture of Mathematical Description of Hydrocracking,"/nf. Chem. Eng, 1971, 11(2), 256.
115
Related Documents