Current Practice in Neurosciences Minimally Invasive Spine Surgery Part 2: Tumour, Trauma, Infection and Radiation Exposure Alok Ranjan, Rahul Lath, Umesh Srikantha 1 Senior Consultant Neurosurgeon, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, 1 Senior Consultant Neurosurgeon, Aster CMI Hospital, Bengaluru, Karnataka, India SEPTEMBER 2020 VOLUME 2, ISSUE 5

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
1
Ranjan, et al.: MISS for tumour, trauma and infection
Current Practice in Neurosciences
Minimally Invasive Spine Surgery Part 2: Tumour, Trauma, Infection
and Radiation Exposure
Alok Ranjan, Rahul Lath, Umesh Srikantha1
Senior Consultant Neurosurgeon, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, 1Senior Consultant Neurosurgeon, Aster CMI Hospital, Bengaluru, Karnataka, India
SEPTEMBER 2020
VOLUME 2, ISSUE 5
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
2
I. MISS for Spinal Tumours
Minimally invasive spine surgery (MISS) has been tried, used and established in various types of spinal tumours. Benign extradural tumours such as osteoid osteomas, dumbbell extradural neurofibromas, malignant extradural spinal tumours, intradural extramedullary spinal tumours and even intramedullary tumours have all been operated successfully through a minimally invasive approach. However, the minimally invasive approach has been found to be best suited for spinal metastases and small intradural extramedullary lesions without compromising the clinical outcome.
a. Spinal metastasis with or without spinal cord compressionMetastasis to the spine with or without spinal cord compression, causing neurologicalcompromise presents a special challenge. The goal of treatment quite often is to improve the quality of life. Also, at times, there is an urgency to start radiation and chemotherapy, and that needs quick healing of an operative wound. In a retrospective analysis, group of patients who had minimal open decompression with trans fascial pedicle screw fixationas opposed to traditional open operation, had less blood loss and postoperative painwithout compromising any other peri-operative measures and postoperative qualitymeasures.[1] In a consecutive single centre prospective study, 49 of 51 patients couldbe treated with percutaneous pedicle screw fixation with or without mini-open spinaldecompression. 55% of patients had significant improvement in KPS, and only twopatients required redo surgery for screw loosening, the biggest advantage being minimal blood loss.[2] In another comparative study, where MISS techniques were compared with an open traditional approach, there no significant difference in terms of complication andclinical outcomes, however, MISS group showed a clear and significant improvementin terms of the operation time, blood loss, early mobilization, postoperative pain andopioid use.[3]
b. MISS for intradural extramedullary tumoursThere have been attempts to minimize bone removal and optimize incision for removal of intradural tumours in the past, however, it did not do away with subperiosteal dissection of multifidus and retractor related muscle trauma.[4] There has been some variation in the MIS approach in the thoracolumbar spine where a midline trans-spinous approach has been used with good results. However, it definitely damaged the posterior tension band.[5] Aretrospective study of 45 consecutive cases treated either with open or minimally invasive approach (sequential dilator) showed that MIS approach had equivalent rates of gross totalremoval, perioperative complication rate and operative time as an open approach, buthad less blood loss and shorter hospitalization.[6] In the dorsal spine, intradural tumoursare eccentrically placed and are more amenable to a minimally invasive excision.[7] Mini-open (expandable tubular retractors) and minimally invasive (non-expandable tubularretractors) approaches have recently been used to resect extradural and intradural spinal tumours through reduced paraspinal tissue destruction.[8-10] There is Level III evidenceshowing that the use of minimally invasive surgery in the treatment of intradural spinaltumours translates into less blood loss, shorter operative time, shortened hospitalization, and a quicker return to daily activities.[6,11] Table 1 summarizes the results of the serieswhere the MIS approach has been used for spinal tumours.
Practical tipsIdeal tumourThe ideal tumour to be removed in MIS muscle splitting technique should be 2.5 cms or less in size, eccentrically and preferably dorsally placed especially in the dorsal spine. It is excellent for neurogenic intradural extramedullary tumours. Dural based tumours especially meningioma where the surgeon prefers to remove the dural attachment, requires an extra skill set. Removal of a very vascular tumour or very prominent vascular channels over rootlets requires extra skill, as bleeding in a confined space can be challenging. Potentially malignant lesions such as ependymoma which require en mass excision and where piecemeal decompression should be avoided so as to decrease the chances of drop metastasis, need expertise and careful planning.
Correct localizationAll MIS surgery relies on correct localization with intraoperative fluoroscopy. Again, fluoroscopic confirmation of the location is a must before dura is opened.
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
3
Ranjan, et al.: MISS for tumour, trauma and infection
Tabl
e 1:
Sum
mar
y of
ser
ies
with
the
MIS
app
roac
h to
spi
nal
tum
ours
Aut
hor
year
Num
ber
of
patie
nts
Loca
tion
of
path
olog
yS
yste
mM
ean
bloo
d lo
ssM
ean
OT
time
Mea
n ho
spita
l sta
yC
ompl
icat
ions
GTR
%Fo
llow
up
Out
com
e
Man
ion
et a
l.[9] 2
011
13ID
‑EM
Qua
dran
t an
d X
tube
155
2.5
h3.
1 da
ysW
rong
leve
l‑2, C
SF
leak
1P
sedo
men
ingo
cele
1C
onve
rsio
n 1
100%
1‑2
year
s10
0%
Nzo
kou
et a
l.[10] 2
013
13ID
‑EM
, ED
Spo
tligh
t21
918
9 m
in66
hou
rsN
il12
/13
2192
%W
ong
et a
l.[6] 2
015
27 o
pen
18 M
ISID
‑EM
Qua
dran
t55
8 m
l13
3 m
l24
1 M
ts25
6 M
ts6.
1 da
ys3.
9 da
ysC
SF
leak
3C
SF
leak
194
.4%
92.6
%13
.1 m
onth
16 m
onth
Haj
i et a
l.[12] 2
011
20 p
atie
nts
15‑ID
7‑E
D(2
0 P
atie
nts)
Qua
dran
t42
8 m
l21
0 m
in3
days
CS
F le
ak‑1
Foot
dro
p‑U
rinar
y re
tent
ion
68%
6 w
eeks
19/2
0 P
atie
nts
Pha
m e
t al.[1
3] 2
016
Met
a‑an
alys
is
9 st
udie
s To
tal
114
patie
nts
Poo
led
114
patie
nts
56‑2
38 m
l 18
4 to
25
6 m
in2.
4 to
6.9
day
sC
SF
leak
or
psed
omen
ingo
cele
‑6 p
atie
nts
(4 re
quire
d re
oper
atio
n)
75‑1
00%
1.5
mon
ths
to 2
4 m
onth
s
Afa
thi e
t al.[1
4] 2
015
13 th
orac
ic4
lum
bar
1 ce
rvic
al
ID‑E
MQ
uadr
ant
<200
ml
95 m
in6
days
Mob
iliza
tion
15/1
8 in
1 d
ayni
lG
TR
100%
ID, i
ntra
dura
l; E
M, e
xtra
med
ulla
ry; I
M, i
ntra
med
ulla
ry; E
D, e
xtra
dura
l; O
T, o
pera
tion
time;
GTR
, gro
ss to
tal r
esec
tion
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
4
Skin incisionSkin incision should be placed in the paraspinal region and its laterality from midline depends upon the thickness of musculature. Skin stretch due to full retractor deployment has to be avoided to prevent poor wound healing and associated problems.
Retractor placementOnce initial localization is confirmed, initial dissection with one finger and stripping of muscle cleanly over the hemilaminae under microscopic visualization and, then deployment of dilators and expandable retractors not only minimizes muscle trauma in the depth but also prevents muscle creeping under the retractor blades. Retractor placement is the key and the intended tumour to be removed should be within the confines of retractor blade.
DrillingThe axis of visualization should be about 15 degrees lateral to medial as drilling under the spinous process overhang is very important. Care has to be taken to prevent damage to the facets.
Dural closureDura can be closed completely using 6-0 Vicryl. Attempts have to be made to do watertight dural repair, and use of fibrin glue, as well as dural substitutes, have been tried.
Figure 1 (Lumbar), Figure 2 (Thoracic) and Figure 3 (Cervical) show the MIS approach to spinal intradural extramedullary tumours.
II. MISS for Thoraco-Lumbar Fractures
MISS offers several advantages like reduced blood loss,[15-17] minimal injury to paraspinal muscles,[18] reduced post-operative pain and analgesic requirements,[17,19] lesser hospital stay,[17,20] early return to work,[19,21] lesser incidence of wound infections[17] and better extensor muscle function.[22] All these benefits acquire greater significance in an already injured patient, where further iatrogenic injury to the spine from a conventional/open surgery should be minimized and clinical recovery can be hastened.
With increasing experience, better equipment and technology (navigation, 3D imaging), most thoracolumbar injuries can be treated by minimally invasive techniques. However, from a practical point of view, not all thoracolumbar fractures are amenable to treatment by MISS. The following points can guide a surgeon as to the selection of approach and specific procedure, taking into consideration the AO fracture classification,[23] TLICS score,[24] and load sharing classification.[25]
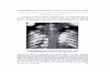
Figure 1: (a) Pre-operative T2 and T1 weighted sagittal MRI showing an intradural tumour at the L4 level; (b) Preoperative T2 weighted axial MRI; (c) Post-operative sagittal MRI showing a very localized operative area with
complete excision of the tumour; (d) Extent of hemilaminectomy in the T2 axial cuts
dc
ba
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
5
Ranjan, et al.: MISS for tumour, trauma and infection
AO Type A injuriesType A3 (Incomplete Burst) or rarely Type A1/A2 (Wedge/ Impaction/ Pincer) fractures which do not heal with adequate conservative treatment and/or have TLICS score >4 are best treated with posterior percutaneous pedicle screw fixation without fusion.[26,27]
[Figures 4 a-d and 5]. In rare A3 fractures with neurological deficits and load sharing classification score <7, a simple tube assisted decompression along with the removal of retro pulsed fragments can be done in addition to percutaneous fixation.[28] This type of fractures is perhaps the most common indications for a minimally invasive treatment strategy in common practice.[29] In patients where the kyphotic angulation is severe and doesn’t correct even after placing prone, it is better to consider open surgery to achieve good correction of kyphotic angulation and restoration of spinal balance.[30]
Type A4 (Complete burst) fractures with load sharing classification score <7 can be managed as above with posterior percutaneous pedicle screw fixation with additional tube assisted decompression, whenever indicated due to presence of neurological deficits.[31] Those A4 fractures where load sharing classification score is ≥7, will need anterior reconstruction in addition to posterior percutaneous pedicle screw stabilization. Anterior corpectomy and reconstruction can be achieved with either a posterolateral MIS approach (any level from T4-L5)[32,33] or a lateral retroperitoneal approach (fractures below L1)[34] [Figure 6]. In the thoracic region and thoracolumbar junction (T3-L2), a video-assisted thoracoscopic
Figure 2: (a) T1 weighted contrast enhanced sagittal MRI and axial; (b) showing the tumour at D12 level; (c) Left D12 hemilaminectomy and exposure of dura; (d) Tumour being delivered by a hook; (e) Tumor delivered outside; (f) Nerve
root attachment being cut
b
ec
f
a
d
Figure 3: (a) Fluoroscopic image of positioning of retractor system; T1 weighted sagittal (b) and axial (c) contrast MRI showing the tumour at C2 level. (d) Electrophysiological monitoring; (e) Quadrant retractor system introduced; (f)
Retractor system deployed; (g) Hemilaminectomy and drilling base of spine; (h) Tumor seen on opening dura; (i) Spinal cord seen after removal of tumor; (j) Dural closure; (k) Muscle layers opposing as the retractor is withdrawn
d
h
c
g
b
f
a
e
kji
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
6
technique[35,36] or a lateral transthoracic tube assisted technique[37] can be used. However, in extremely comminuted fractures with complete neurological injury, an open approach would be beneficial, as any dural tear can be more effectively managed, and also the benefits of a minimally invasive approach in these paraplegic patients may not be as significant as in other intact patients.
AO type B injuriesAll isolated type B injuries are also best treated by minimally invasive techniques. Since isolated Type B injuries are primarily a failure of posterior tension band, posterior
Figure 4: Examples of minimally invasive percutaneous pedicle screw stabilization with limited decompression for thoracolumbar fractures. (a and b) A case of non-healing, conservatively managed fracture for 5 weeks that underwent
pedicle screw stabilization with an index screw at fractured level. (c and d) shows follow up X-ray with partial loss of correction and acceptable segmental kyphosis. (e-h) A rare case of Type C fracture that underwent limited
decompression and segmental fusion, followed by reduction of translational injury by percutaneous pedicle screw stabilization
d
h
c
g
b
f
a
e
Figure 5: (a) MRI showing wedge fracture; (b) Longitude system being used; (c) Fluoroscopic image showing pedicle screws positioning; (d) Small skin incisions through which screws were placed
dc
ba
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
7
Ranjan, et al.: MISS for tumour, trauma and infection
percutaneous pedicle screw stabilization with or without fusion suffices in restoring spinal stability.[26,38] Even displaced fracture fragments in Type B1 fractures can be removed with tube assisted minimally invasive techniques. Type B injuries that occur in association with an additional Type A are best managed as dictated by the type of associated Type A fracture as described above.
AO type C injuriesThese are the most severe injuries with either a 3-column displacement or dislocation and are also commonly associated with a neurological deficit. Though some type C injuries can be reduced and stabilized with minimally invasive techniques [Figure 4 e-h], these complex fractures often need correction in all three dimensions and hence are best managed with conventional/open techniques.
Minimally invasive treatment options can be applied to most thoracolumbar fractures. In fact, several studies mentioned above have reported favourable short-term results and promoting early recovery without compromising fracture healing rates when treated with minimally invasive techniques [Table 2].
III. MISS for Spinal Infections
Reducing injury and preserving paraspinal muscles and midline tension band is also of paramount importance in spinal infections, where these structures are already subject to inflammation, injury, and destruction by the disease process itself. The role of MIS in spinal infections has grown significantly, whether it be for diagnostic biopsy, decompressing neural structures or for maintaining/restoring spinal stability.
Biopsy and sample collectionPercutaneous full endoscopic technique is one of the ideal methods to collect a sample for microbiological studies and histopathological examination. Transforaminal endoscopy [Figure 7] is the least invasive among minimally invasive techniques and can be done under local analgesia and offers the advantage of collecting an adequate quantity of infected/necrotic tissue under direct vision, thus increasing the positivity rate of culture/other diagnostic tests as compared to other sampling techniques, for example, the CT guided transpedicular biopsy.[47-49] Percutaneous CT guided transpedicular/trans-costovertebral biopsy is preferable in infections involving the thoracic spine.[50] Similar advantages also exist for a tube assisted sampling done via a posterior interlaminar/translaminar route, but the procedure may have to be done under general anesthesia
Figure 6: Examples of MIS corpectomy for anterior reconstruction. (a-d) A type A4 injury with load sharing classification score >7 in a young girl, who underwent minimally invasive posterolateral corpectomy and pedicel screw stabilization; (e-h) A failed posterior pedicle fixation device for an A4 fracture, that underwent minimally invasive, retroperitoneal L2
corpectomy and anterior reconstruction followed by extension of posterior construct
d
h
c
g
b
f
a
e
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
8
Table 2: Studies com
paring
open versus percutaneou
s pedicle screw fixatio
n for thoracolum
bar trauma
Stu
dyTy
pe o
f st
udy
No.
of c
ases
(o
pen/
Per
c)Ty
pe o
f #F/
u (m
onth
s)D
urat
ion
of
surg
ery
(min
s)
(ope
n/P
erc)
Blo
od lo
ss (m
l) O
pen/
Per
cH
ospi
tal s
tay
(day
s) O
pen/
Per
c
Scr
ew
mal
posi
tion
(No
of e
vent
s) O
pen/
Per
c
Infe
ctio
ns (n
o of
ev
ents
) Ope
n/P
erc
Gra
ss (2
006)
[39]
P, O
S24
/33
TL#
Mai
nly
T12‑
L2N
R10
0/85
870/
400/
11/
1
Wild
(200
7)[4
0]R
, OS
11/1
0TL
#M
ainl
y A
O ty
pe A
360
80/8
738
0/19
00/
00/
0
Hua
ng (2
008)
[41]
R, O
S30
/30
TL#
2480
/80
292/
7512
/8W
ang
(201
0)[4
2]P
, OS
21/1
7TL
# Ty
pe A
11.6
161/
9730
4/83
14/1
12/
00/
0Ti
an (2
011)
[43]
P, R
CT
50/4
7TL
#12
122/
125
204/
911/
0Ji
ang
(201
1)[3
0]P
, RC
T30
/31
TL#
5989
/79
145/
7911
/10
1/2
1/0
Son
g (2
012)
[44]
R, O
S32
/20
TL#
1290
/82
330/
6821
/12
5/2
0/0
Bro
nsar
d (2
013)
[45]
R, O
S30
/30
TL#;
Mai
nly
T11‑
L2M
ainl
y M
ager
l A3
2514
8/83
318/
501/
03/
0
Gro
ssba
ch (2
013)
[26]
P, O
S27
/11
TL#
AO
B1,
2 m
ost c
omm
on9
(per
c)18
(ope
n)25
7/19
549
8/93
11/8
0/0
1/0
Lee
(201
3)[2
7]R
, OS
27/3
2TL
#D
enis
type
A o
r B30
(per
c)39
(ope
n)15
4/83
684/
262
1/1
2/0
Van
ek (2
014)
[46]
P, O
S19
/18
TL#;
Mos
tly T
12, L
1M
ainl
y A
324
60/5
333
1/56
0/0
1/0
Wan
g (2
017)
[29]
P, O
S39
/61
TL#;
Mai
nly
L1A
O T
ype
A20
140/
9831
1/87
18/1
3½
1/0
P, p
rosp
ectiv
e; R
, ret
rosp
ectiv
e; O
S, o
bser
vatio
nal s
tudy
; RC
T, ra
ndom
ised
con
trol t
rial;
Per
c, p
ercu
tane
ous;
#, f
ract
ure;
f/u,
Fol
low
up
dura
tion;
min
s, m
inut
es; m
l – m
illili
tres;
TL#
, tho
raco
lum
bar f
ract
ure
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
9
Ranjan, et al.: MISS for tumour, trauma and infection
and tissue invasiveness is more as compared to full endoscopic technique, and hence is reserved for cases where simultaneous decompression/ debridement is necessary.
Decompression and/or debridementMinimally invasive techniques can also achieve adequate decompression and debridement in select cases of spinal infections. Epidural granulation tissue causing neural compression can effectively be decompressed through a tubular retractor, even when extending to multiple levels.[51,52] Ventral epidural compression can also be accessed and decompressed through a transforaminal endoscope.[48] In both these techniques, adequate irrigation can also be performed to wash away necrotic material, decrease bacterial load, improve clinical symptoms and assist in effectively controlling the infection.[49,53] [Figure 8a] Reports of using bilateral transforaminal ports to maximize decompression and debridement by means of irrigation through one port and suction through the other have also been described.[54]
Maintaining/restoring spinal stabilitySpinal infections with a destructive course can often result in spinal instability. Stand-alone percutaneous pedicle screw stabilization can be done with ease in cases with intractable axial pain due to spinal instability in the absence of neural compression or focal deficits.[51,55,56] If needed, a percutaneous biopsy or a minimally invasive interbody fusion can also be performed at the same sitting.[57] [Figure 8b-e] With increasing experience, one can add a decompression procedure or a full-fledged posterolateral minimally invasive corpectomy and anterior reconstruction for cases with gross anterior column destruction and neural compression.[58.59] [Figure 8f-i] In L2-L5 levels, anterior reconstruction can be done from a lateral retroperitoneal trans-psoas/anterior to psoas approaches as well.[60]
Video-assisted thoracoscopy[61] or a minimally invasive transthoracic approach[62] can be used as an effective alternative to drain the paraspinal abscess, decompress the neural elements and achieve anterior column reconstruction in destructive lesions of the thoracic spine.
IV. Radiation Exposure in Minimally Invasive Spine Surgery
Minimally invasive spine procedures require multiple fluoroscopic exposures and result in a higher radiation dose when compared to the open procedures.[63,64] This could have potentially harmful effects for the patient, the surgeon and operating room personnel. This would include both deterministic (once time dose – like skin erythema) and stochastic
Figure 7: Sagittal (a) and axial (b) contrast T1 weighted images showing post-operative discitis/vertebral osteomyelitis with maximal involvement of the L3-L4 level. (c) lateral and (d) antero-posterior fluoroscopic image showing
endoscopic port in the L3-4 disc via a transforaminal route for biopsy and cultures
db
ca
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
10
effects (cumulative dose effect – like malignancies). Mariscalco et al,[65] in a prospective controlled trial, concluded that surgeons in minimally invasive microdiscectomy as opposed to open discectomy were exposed to more radiation to thyroid/eye (P=0.01), chest (P=0.013), and hand (P=0.006) (level 2 evidence). However, one would need 1623 MIS micro discectomies per year to exceed the exposure limit for whole-body radiation. Ahn et al.[66] in another prospective study, concluded that a surgeon can perform 291 PLED annually without a protective shield to exceed the annually allowed dose of radiation (Level 2 evidence). In another prospective cohort study by Wang et al.,[67] MI TLIF had X-ray exposure time of 84 seconds versus 37 seconds in open TLIF group (P < 0.05) (level 2 evidence). Fransen,[68] in a prospective observational study found that fluoroscopic time for open pedicle screw placement was 8 seconds per screw versus 27 seconds per screw for percutaneous pedicle screw placement which was 3.8 times higher.
Grelat et al.[69] compared the group with fluoroscopy (average duration 3.718 minutes, average exposure 12µSv on the thorax, 1168 µSv on the main hand, and 179 µSv on the lens as compared to O-Arm where it was measured zero. Kim et al[70] in their meta-analysis (8 cohort studies) of radiation-related risks in MI TLIF compared with traditional open surgery, concluded that MI TLIF group was exposed to 2.4 fold more radiation as compared to the open TLIF group.
Figure 8: Illustrative examples showing some of the applications of MISS in treating spinal infections. (a) Thorough irrigation through an infant feeding tube placed in the disc space being performed after percutaneous transforaminal
endoscopic biopsy and decompression. (b-e) A refractory case of Staph aureus infection at L5-S1 disc space in a patient of chronic liver disease treated with L5-S1 MIS-TLIF and L4-Ilium percutaneous stabilization. (b) Pre-op T1W sagittal MRI image; (c) Minimally invasive Iliac screws being placed; (d) Intra-op C-arm image showing guidewires
and tubular retractor in place; (e) Post-op X-ray at 1 year follow up. (f-i) A case of tubercular collapse of L1 with neural compression being treated with minimally invasive corpectomy, anterior cage placement and D11-L3 percutaneous
pedicle screws. (f) Pre-op T2W sagittal MRI image; (g) Intra-op image showing stabilizing screws and rod on one side with the expandable retractor in place for corpectomy and cage placement; (h and i) Final screw and cage construct at
the end of procedure
d
ih
c
g
b
f
a
e
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
11
Ranjan, et al.: MISS for tumour, trauma and infection
Radiation limit and its consequencesGuidelines from the 1990 International Commission on Radiological Protection (ICRP) allow occupational radiation exposure to accumulate to maximum annual radiation exposure of 150 mSv to the crystal lens, 500 mSv to the skin or hands, 20 mSv as the effective dose to the whole body, and peak exposure limit of 2 rem for a five year average.[71] Mastrangelo et al.[72] reported a fivefold increase in their lifetime cancer risk in the orthopaedic surgeon, as compared to non-orthopaedic surgeons in a hospital setting. Cranial radiation exposure remains a major risk factor for the development of cataract and brain tumours such as meningioma and glioma.[73]
Methods to reduce radiationMethods to reduce radiation exposure to the surgeon and OT personnel include avoiding continuous fluoroscopy, lead apron covering the entire torso including the genitals and thyroid collar. The surgeon’s hand must be kept away from the operative field (ideally more than 3 feet) during fluoroscopy and stay away from the radiation-emitting side, preferably on the cranial side of the patient.[74] Some authors have suggested a protocol with low dose pulse images or digital spot images to decrease the fluoroscopy times and radiation doses.[75,76] Mulconrey[77] in a prospective study (level 4) concluded that the surgeon who intermittently stood away from radiation source was exposed to 1225 mRem and assistant who stood 3 feet away was exposed to 369mRem dose, cranial end of the table received 92 mRem and caudal end 150 mRem.
Navigation Navigation with intraoperative optical tracking systems like Stealth (Medtronics) and Vector Vision (BrainLab) are being used increasingly in minimally invasive spine surgery to decrease the fluoroscopy time and radiation during surgery and improve the accuracy of pedicle screw placement. However, their accuracy is enhanced by adding an intraoperative imaging platform. There are three major imaging platforms with their advantages and disadvantages, with which majority of navigation procedures are performed (a) the O-Arm system which gives 2D and 3D imaging with an O shaped imaging tool, which can wrap around the operating table completely to give 3600 visualization of anatomy and works with Stealth station (Medtronics) (b) The Airo system (BrainLab) which has a mobile large-bore intraoperative CT system which works with BrainLab navigation system and (c) Ziem visionTM system.[78] Tian et al.[79] in their meta-analysis in 2009, found that CT based navigation system had an accuracy of 90% versus fluoroscopic guided accuracy of 85% for pedicle screw system. In a retrospective observational study on 152 consecutive patients, there was significantly reduced pedicle screw misplacement in O-Arm navigation versus 3D-C-Arm navigation versus 2D-fluoro guided placement. O-Arm navigation significantly reduced pedicle screw misplacement (1.23%) compared to 3D-C-Arm navigation (7.29%, P = 0.0082) and 2D-fluoro guided placement (5.16%, P = 0379). 3D-C-Arm navigation was associated with lower procedural radiation exposure of the patient (0.4 mSv) than O-Arm navigation (3.24 mSv) or 2D-fluoro guidance (1.5 mSv) [Table 3]. Operative time was comparable between the three modalities.[80]
Table 3a: Estimated radiation exposure in 3 different modalities*2D Fluoro 3D CT Fluoro O‑Arm
Intra‑procedural 1.5 mSv 0.4 mSv 3.2 mSvWith post procedural imaging 10.5 mSv 9.4 mSv 8.1 mSvmSv, milli Sievert. *Modified from Tajsic et al.[80]
Table 3b: Estimated cancer risk2 D Fluoro 3 D CT O‑Arm
Intra‑procedural 1:12,000 1:40,000 1:6000With post‑operative imaging 1:1800 1:2000 1:2200
Table 3c: Estimated cancer riskEstimated dose (mSv) Estimated cancer risk
CT lumbar spine 9 1:2000CT staging 48 1:380CT trauma series 54 !:340mSv, milli Sievert
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
12
Robotics Robotics in spine surgery has been a newer phenomenon and the three most widely used systems are Mazor X (Medtronics and Mazor Robotics, Memphis, USA), Excelsius GPS (Globus Medical Inc, PA, USA), and ROSA (Medtech Surgical Inc., NY, USA). Mazor robotics was introduced in 2004 and has had several upgrades and it is the more widely used system currently.[81] Hyun et al[82] in their randomized controlled trial found that robot-guided pedicle screw placement significantly reduced (a) radiation exposure, (b) length of hospital stay, (c) significantly reduced encroachment of proximal screw from facet (logically reducing the chance of adjacent segment disease in the long term). Patel et al.[83] in their cadaveric study found 58 % rate of facetal capsule violation by Jamshidi needle in fluoroscopic assisted percutaneous pedicle screw insertion. Kantelhardt et al,[84] in a retrospective study, observed that the average x-ray exposure per screw was 77 seconds for conventional fluoroscopic assisted procedures and 34 seconds in robot-guided procedures. Roser et al,[85] in a prospective randomized controlled trial, noted a similar decrease in radiation by almost half in the robot-assisted and navigation groups compared with the fluoroscopy assisted freehand group. Hyun et al[82] found a lower mean fluoroscopy time per screw (FT) of 3.5 seconds in the robot-assisted group compared to 13.3 seconds in the fluoroscopy group. C-arm output in millisieverts (mSv) was 0.13 versus 0.27 in the robotic and fluoroscopy groups respectively. In their review article Pennington et al.[86] have looked at the surgeon and patient exposure to radiation during image guided thoracolumbar fusion procedures. They included studies with conventional fluoroscopy without navigation, fluoroscopy with pre-op CT based navigation, intra-operative CT based navigation and robotic-assisted instrumentation. They concluded that all image-guided procedures were associated with radiation exposure to the surgeons which was well below the recommended safety levels. However, the intraoperative CT based navigation systems had the least radiation exposure to the surgeons. The patients, however, received the lowest radiation in the fluoroscopy with the navigation group and highest radiation in the intraoperative CT group [Table 4]. Robotic-assisted spine surgery using the Renaissance hexapod device (Mazor) or the ROSA robot have demonstrated increased accuracy in placement of the pedicle screws and decreasing the fluoroscopy times.[87,88]
References
1. Saadeh YS, Elswick CM, Fateh JA, Smith BW, Joseph JR, Spratt DE, et al. Analysis of outcomes between traditional open versus mini-open approach in surgical treatment of spinal metastasis. World Neurosurg. 2019 Oct;130:e467-e474.
2. Hamad A, Vachtsevanos L, Cattell A, Ockendon M, Balin B. Minimally invasive spinal surgery for the management of symptomatic spinal metastasis. Br J Neurosurg. 2017;31:526-530.
3. Miscusi M, Polli FM, Forcato S, Ricciardi L, Frati A, Cimmati M, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: Surgical technique and early clinical results. J Neurosurg Spine 2015;22:518-25.
4. Turel M, D’souza WP, Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: An analysis of 164 patients. Neurosurg Focus 2015;39:E9.
5. Raygor KP, Than KD, Chou D, Mummaneni PV. Comparison of minimally invasive transspinous and open approaches for thoracolumbar intradural-extramedullary spinal tumors. Neurosurg Focus 2015;39:E12.
6. Wong AP, Lall RR, Dahdaleh NS, Lawton CD, Smith ZA, Wong RH, et al. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus 2015;39:E11.
Table 4: Radiation exposure duration and doses with different techniquesTechnology Fluoroscopy Time
Per screwPatient Dose
Per screwSurgeon Dose
Per screwConventional Fluoroscopy 11.1±9.0 sec 0.26±0.38 mSv 6.0±7.9×10−3 mSvFluroscopy with navigation (pre‑op CT)
7.20±3.93 sec 0.027±0.010 mSv 1.8±2.5×10−3 mSv
Intraop CT with navigation 19.96±17.09 sec 1.20±0.91 mSv 0±0 mSvRobotic assisted 20.07±17.22 sec 0.04±0.30 mSv 2.0±4.0×10−3 mSvmSv – milli Sievert
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
13
7. Tumialan LM. Theodore N, Narayanan M, Marciano FF, Nakaji P. Anatomic basis for minimally invasive resection of intradural extramedullary lesions in dorsal spine. World Neurosurg 2018;109:e770-777.
8. Fessler RG: Minimally invasive resection of intradural-extramedullary spinal neoplasms. Neurosurgery 2006;58(1 Suppl):ONS52-ONS58.
9. MannionRJ,NowitzkeAM,EfendyJ,WoodMJ:Safetyandefficacyofintraduralextramedullaryspinal tumor removal using a minimally invasive approach. Neurosurgery 2011;68 (1 Suppl Operative):208-216.
10. Nzokou A, Weil AG, Shedid D. Minimally invasive removal of thoracic and lumbar spinal tumors using a nonexpandable tubular retractor. J Neurosurg Spine 2013;19:708-715.
11. Gandhi RH, German JW. Minimally invasive approach for the treatment of intradural spinal pathology. Neurosurg Focus 2013;35:E5.
12. Haji FA, Cenic A, Crevier L, Murty N, Reddy K. Minimally invasive approach for the resection of spinal neoplasm. Spine (Phil Pa 1976) 2011;36:E1018-E1026.
13. Pham MH, Chang KE, Liu JC, Hsieh PC. Minimally invasive surgery for intradural extramedullary spinal tumors. A comprehensive review with illustrative clinical cases. World Spinal Column J 2016;2:84-96.
14. Afathi M, Peltier E, Adetchessi T, Grallion T, Dufour H, Fuentes S. Minimally invasive transmuscular approach for the treatment of benign intradural extramedullary spinal cord tumours: Technical notes and results. Neurochirurgie 2015;61:333-338.
15. StarkweatherAR,Witek-JanusekL,NockelsRP,PetersonJ,MathewsHL.Themultiplebenefitsof minimally invasive spinal surgery: Results comparing transforaminal lumbar interbody fusion and posterior lumbar fusion. J Neurosci Nurs. 2008;40:32-39.
16. Wong AP, Smith ZA, Stadler JA, Hu XY, Yan JZ, Li XF, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Neurosurgery Clinics of NA. 2014;25(2):279-304.
17. Tian NF, Wu YS, Zhang XL, Xu HZ, Chi YL, Mao FM. Minimally invasive versus open transforaminal lumbar interbody fusion: A meta-analysis based on the current evidence. Eur Spine J. 2013;22:1741-1749.
18. StevensKJ, SpencinerDB,GriffithsKL, KimKD,Zwienenberg -LeeM,AlaminT,et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19:77-86.
19. Parker SL, Lerner J, McGirt MJ. Effect of minimally invasive technique on return to work and narcotic use following transforaminal lumbar inter-body fusion. Professional Case Management. 2012;17:229-235.
20. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: Evaluating initial experience. International Orthopaedics (SICOT). 2009;33:1683-1688.
21. Granger E, Prada S, Bereczki Z, Weiss M, Wade C, Davis R. Return-to-duty rates following minimally invasive spine surgery performed on active duty military patients in an ambulatory surgery center. Mil Med. 2018;24:769.
22. Bresnahan LE, Smith JS, Ogden AT, Quinn S, Cybulski GR, Simonian N, et al. Assessment of paraspinal muscle cross-sectional area after lumbar decompression. Clinical Spine Surgery 2017;30(3):E162-E168.
23. ReinholdM,AudigéL,SchnakeKJ,BellabarbaC,DaiLY,OnerFC.AOspineinjuryclassificationsystem: A revision proposal for the thoracic and lumbar spine. European Spine journal 2013;22(10):2184-2201.
24. Vaccaro AR, Lehman RA, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, et al. A new classificationofthoracolumbarinjuries:Theimportanceofinjurymorphology,theintegrityofthe posterior ligamentous complex, and neurologic status. Spine. 2005;30(20):2325-2333.
25. McCormackT,KaraikovicE,GainesRW.Theloadsharingclassificationofspinefractures.Spine.1994;19:1741-1744.
26. Grossbach AJ, Dahdaleh NS, Abel TJ, Woods GD, Dlouhy BJ, Hitchon PW. Flexion-distraction injuries of the thoracolumbar spine:Open fusionversus percutaneouspedicle screwfixation.Neurosurgical Focus. 2013;35(2):E2.
27. Lee JK, Jang J-W, Kim T-W, Kim T-S, Kim S-H, Moon S-J. Percutaneous short-segment pedicle screw placement without fusion in the treatment of thoracolumbar burst fractures: Is it effective? Comparativestudywithopenshort-segmentpediclescrewfixationwithposterolateralfusion.ActaNeurochir (Wien). 2013;155:2305-12-discussion 2312.
28. Walker CT, Xu DS, Godzik J, Turner JD, Uribe JS, Smith WD. Minimally invasive surgery for thoracolumbar spinal trauma. Ann Transl Med. 2018;6:102-102.
29. Wang H, Zhou Y, Li C, Liu J, Xiang L. Comparison of open versus percutaneous pedicle screw fixationusingtheSextantsysteminthetreatmentoftraumaticthoracolumbarfractures.ClinicalSpine Surgery. 2017;30:E239-E246.
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
14
30. Jiang XZ, Tian W, Liu B, Li Q, Zhang GL, Hu L, et al. Comparison of a paraspinal approach with a percutaneous approach in the treatment of thoracolumbar burst fractures with posterior ligamentous complex injury: A prospective randomized controlled trial. J Int Med Res. 2012;40:1343-1356.
31. MaciejczakA,BarnasP,DudziakP,Jagiełło-BajerB,LitworaB,SumaraM.Posteriorkeyholecorpectomy with percutaneous pedicle screw stabilization in the surgical management of lumbar burst fractures. Operative Neurosurgery 2007;60:232-242.
32. MaciejczakA,BarnasP,DudziakP,Jagiełło-BajerB,LitworaB,SumaraM.Minimallyinvasivetranspedicular dorsal stabilization of the thoracolumbar and lumbar spine using the minimal access non-traumatic insertion system (MANTIS): Preliminary clinical results in 52 Patients. J Neurol Surg A Cent Eur Neurosurg 2012;73(06):369-376.
33. Sasani M, Ozer AF. Single-stage posterior corpectomy and expandable cage placement for treatment of thoracic or lumbar burst fractures. Spine. 2009;34:E33-E40.
34. Smith WD, Ghazarian N, Christian G. Acute and hyper-acute thoracolumbar corpectomy for traumatic burst fractures using a mini-open lateral approach. Spine. 2018;43:E118-E124.
35. Khoo LT, Beisse R, Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: A series of 371 consecutive cases. Neurosurgery. 2002;51(5 Suppl):S104-S117.
36. Kim SJ, Sohn M-J, Ryoo J-Y, Kim Y-S, Whang CJ. Clinical analysis of video-assisted thoracoscopic spinal surgery in the thoracic or thoracolumbar spinal pathologies. J Korean Neurosurg Soc. 2007;42:293-299.
37. Smith WD, Dakwar E, Le TV, Christian G, Serrano S, Uribe JS. Minimally invasive surgery for traumatic spinal pathologies: A mini-open, lateral approach in the thoracic and lumbar spine. Spine. 2010;35(26 Suppl):S338-S346.
38. Dhall SS, Wadhwa R, Wang MY, Tien-Smith A, Mummaneni PV. Traumatic thoracolumbar spinal injury: An algorithm for minimally invasive surgical management. Neurosurgical Focus. 2014;37:E9.
39. Grass R, Biewener A, Dickopf A, Rammelt S, Heineck J, Zwipp H. [Percutaneous dorsal versus open instrumentation for fractures of the thoracolumbar border. A comparative, prospective study]. Unfallchirurg. 2006;109:297-305.
40. Wild MH, Glees M, Plieschnegger C, Wenda K. Five-year follow-up examination after purely minimally invasive posterior stabilization of thoracolumbar fractures: A comparison of minimally invasive percutaneously and conventionally open treated patients. Arch Orthop Trauma Surg. 2007;127:335-343.
41. Huang QS, Chi YL, Wang XY, Mao FM, Lin Y, Ni WF, et al. [Comparative percutaneous with openpediclescrewfixationinthetreatmentofthoracolumbarburstfractureswithoutneurologicaldeficit].ChineseJournalofSurgery2008;46:112-114.
42. WangHW,LiC-Q,ZhouY,ZhangZF,WangJ,ChuTW.Percutaneouspediclescrewfixationthrough the pedicle of fractured vertebra in the treatment of type A thoracolumbar fractures using Sextant system: An analysis of 38 cases. Chinese Journal Traumatology. 2010;13:137-145.
43. Tian W, Han X, He D, Liu B, Li Q, Li ZY, et al. The comparison of computer assisted minimally invasive spine surgery and traditional open treatment for thoracolumbar fractures. Chinese Journal of Surgery 2011;49:1061-1066.
44. Song H-P, Lu J-W, Liu H, Zhang C. Case-control studies between two methods of minimally invasive surgery and traditional open operation for thoracolumbar fractures. Chinese Journal or Orthopaedics and Traumatology. 2012;25:313-316.
45. Bronsard N, Boli T, Challali M, Dompsure R de, Amoretti N, Padovani B, et al. Comparison betweenpercutaneousandtraditionalfixationoflumbarspinefracture:Intraoperativeradiationexposure levels and outcomes. Orthop Traumatol Surg Res. 2013;99:162-168.
46. Vanek P, Bradac O, Konopkova R, de Lacy P, Lacman J, Benes V. Treatment of thoracolumbar trauma by short-segment percutaneous transpedicular screw instrumentation: Prospective comparative study with a minimum 2-year follow-up. Journal of Neurosurgery: Spine. 2014;20:150-156.
47. Yang S-C, Fu T-S, Chen L-H, Chen W-J, Tu Y-K. Identifying pathogens of spondylodiscitis: Percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res. 2008;466:3086-3092.
48. Wang X, Zhou S, Bian Z, Li M, Jiang W, Hou C, et al. Unilateral percutaneous endoscopic debridement and drainage for lumbar infectious spondylitis. Journal of Orthopaedic Surgery and Research. 2018;13(1):306-308.
49. Yang SC, Chen WJ, Chen HS, Kao YH, Yu SW, Tu YK. Extended indications of percutaneous endoscopic lavage and drainage for the treatment of lumbar infectious spondylitis. Eur Spine J. 2014;23:846-853.
50. Nourbakhsh A, Grady JJ, Garges KJ. Percutaneous spine biopsy: A meta-analysis. J Bone Joint Surg Am. 2008;90:1722-1725.
51. Kandwal P, Garg B, Upendra B, Chowdhury B, Jayaswal A. Outcome of minimally invasive surgery in the management of tuberculous spondylitis. Indian J Orthop. 2012;46:159-164.
52. Turel MK, Kerolus M, Deutsch H. The role of minimally invasive spine surgery in the management
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
15
of pyogenic spinal discitis. J Craniovertebr Junction Spine. 2017;8:39-43. 53. Murata Y, Kanaya K, Wada H, Wada K, Shiba M, Hatta S, et al. Clinical outcome of percutaneous
drainage for spondylodiscitis. J Neurological Surg Part A: Central European Neurosurgery.2014;75:7-11.
54. Hsu LC, Tseng TM, Yang SC, Chen HS, Yen CY, Tu YK. Bilateral portal percutaneous endoscopic debridement and lavage for lumbar pyogenic spondylitis. Orthopedics. 2015;38(10):e856-e863.
55. Jain A, Jain R, Kiyawat V. Evaluation of outcome of posterior decompression and instrumentedfusion in lumbar and lumbosacral tuberculosis. Clin Orthop Surg. 2016;8:268-273.
56. BlondelB,FuentesS,MetellusP,AdetchessiT,DufourH.Percutaneousinternalfixationinthemanagement of lumbar spondylitis: Report of two cases. Orthop Traumatol Surg Res. 2009;95:220-223.
57. Duan K, Qin Y, Ye J, Zhang W, Hu X, Zhou J, et al. Percutaneous endoscopic debridement withpercutaneouspediclescrewfixationforlumbarpyogenicspondylodiscitis:Apreliminarystudy.International Orthopaedics (SICOT). 2020;44:495-502.
58. Kim DH, O’Toole JE, Ogden AT, Eichholz KM, Song J, Christie SD, et al. Minimally invasiveposterolateral thoracic corpectomy. Neurosurgery. 2009;64(4):746-753.
59. Smith ZA, Wong AP, Ahmadieh El TY, Aoun SG, Haque R, Bendok BB, et al. Minimally invasive thoracic corpectomy: 3-dimensional operative video of a posterolateral approach to decompressionand anterior column reconstruction. Neurosurgery. 2013;73(2 Suppl Operative):ons141-ons141.
60. Srikantha U, Lokanath YK, Hari A, Nirmala S, Varma RG. Minimally invasive lateral transpsoas approach for lumbar corpectomy and stabilization. Surg Neurol Int. 2019;10:153.
61. Lü G, Wang B, Li J, Liu W, Cheng I. Anterior debridement and reconstruction via thoracoscopy-assisted mini-open approach for the treatment of thoracic spinal tuberculosis: Minimum 5-yearfollow-up. Eur Spine J. 2012;21:463-469.
62. Lall RR, Smith ZA, Wong AP, Miller D, Fessler RG. Minimally invasive thoracic corpectomy:Surgical strategies for malignancy, trauma, and complex spinal pathologies. Minimally InvasiveSurgery. 2012;2012(7):1-10.
63. Phan K, Rao PJ , Andrew C. Kam AC, Mobbs RJ. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: Systematic review andmeta-analysis. Eur Spine J 2015;24:1017-1030.
64. Khan NR, Clark AJ, Lee SL,Venable GT,Rossi NB, Foley KT. Surgical outcomes for minimallyinvasive vs open transforaminal lumbar interbody fusion: An updated systematic review and meta-analysis. Neurosurgery 2015;77:847-874.
65. Mariscalco MW, Yamashita T, Steinmetz MP, Krishnaney AA, Lieberman IH, Mroz TE.Radiation exposure to the surgeon during open Lumbar microdiscectomy and minimally invasive microdiscectomy: A prospective controlled trial. Spine (Phila Pa 1976)2011;36:255-260.
66. Ahn Y, Kim CH, Lee JH, Lee SH, Kim JS. Radiation exposure to the surgeon during percutaneous endoscopic lumbar discectomy: A prospective study. Spine (Phila Pa 1976) 2013;38:617-25.
67. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesisgrades 1 and 2. Eur Spine J 2010;19:1780-1784.
68. Fransen P. Fluroscopic exposure in modern spine surgery. Acta Orthopaedica Belgica 2011;77:386-9.69. Grelat M, Zairi F, Quidet M, Marinho P, Allaoui M, Assaker R. Assessment of the surgeon radiation
exposureduringminimallyinvasiveTLIF:ComparisonbetweenfluoroscopyandO-Armsystem.(Neurochirurgie 2015;61:255-259.
70. Kim CH, Lee CH, Kim KP. How high are radiation- related risks in MI TLIF compared withtraditional open surgery?: A meta-analysis and dose estimates of ionizing radiation. Clin SpineSurg 2016;29:52-59.
71. International Commission on Radiological Protection (1991) 1990 Recommendations of theInternational Commission on Radiological Protection. ICRP publication 60. Ann ICRP 21(1-3):1-201.
72. Mastrangelo G, Fedeli U, Fadda E, Giovanazzin A, Scoizzato L, Saia B. Increased cancer riskamong surgeons in an orthopaedic hospital. Occup Med (Lond) 2005;55:498-500.
73. Mohammad LM, Messegee J, Chohan MO, Taylor CL. Fluoroscopic cranial radiation exposurein spine surgery: A prospective single centre evaluation in operating room personnel. Int J SpineSurg 2019;22:28-32.
74. El Tecle NE, El Ahmadieh TY, Patel BM, Lall RR, Bendok BR, Smith ZA. Minimizing radiation exposure in minimally invasive spine surgery: Lessons learned from neuroendovascular surgery.Neurosurg Clin N Am 2014;25:247-260.
75. Funao H, Ishii K, Momoshima S, Iwanami A, Hosogane N, Watanabe K, et al. Surgeons exposure to radiation in single- and multi-level minimally invasive transforaminal lumbar interbody fusion; A prospective study. PLoS One 2014;9(4):e95233.
123456789
1011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556
Ranjan, et al.: MISS for tumour, trauma and infection
16
76. Clark JC, Jasmer G, Marciano FF, Tumialán LM. Minimally invasive transforaminal lumbar interbodyfusionsandfluoroscopy:Alow-doseprotocoltominimizeionizingradiation.NeurosurgFocus. 2013;35:E8.
77. Mulconrey DS. Fluroscopic radiation exposure in spinal surgery: In vivo evaluation for operating room personnel. Clinical Spine Surgery 2016;29:e331-335.
78. Virk S, Qureshi S. Navigation in minimally invasive spine surgery. J Spine Surg 2019;5(suppl 1):S25-S30.
79. Tian NF Xu HZ. Image guided pedicle screw insertion accuracy: A meta-analysis. Int Orthop 2009;33:895-903.
80. Tajsic T, Patel K, Farmer R, Mannion RJ, Trivedi RA. Spinal navigation for minimally invasive thoracicandlumbosacralspinefixation:Implicationsforradiationexposure,Operativetime,andaccuracy of pedicle screw placement. Eur Spine J 2018;27:1918-1924.
81. Staub BN. The use of robotics in minimally invasive spine surgery. J Spine Surg 2019;5(Suppl 1):S31-S40.
82. HyunSJ,KimKJ,JahngTA,KimHJ.Minimallyinvasiveroboticversusopenfluoroscopic-guidedspinal instrumented fusions: A randomized controlled trial. Spine (Phila Pa 1976) 2017;42:353-358.
83. Patel RD, Graziano GP, Vanderhave KL, Patel AA, Gerling MC. Facet violation with placement of percutaneous pedicle screws. Spine (Phila Pa 1976)2011;36:E1749-1752.
84. Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V: Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J 2011;20:860-868.
85. Roser F, Tatagiba M, Maier G. Spinal robotics: Current applications and future perspectives. Neurosurgery 2013;72(Suppl1):12-18.
86. Pennington Z, Cottrill E, Westbroek EM, Goodwin ML, Lubelski D, Ahmed AK, Sciubba DM. Evaluation of surgeon and patient radiation exposure by imaging technology in patients undergoing thoracolumbar fusion: Systematic review of the literature. Spine Journal 2019;19(8):1397-1411.
87. Schröder ML, Staartjes VE. Revisions for screw malposition and clinical outcomes after lumbar robot-guided lumbar fusion for spondylolisthesis. Neurosurg Focus 2017;42:E12.
88. Chenin L, Peltier J, Lefranc M. Minimally invasive transforaminal lumbar interbody fusion with theROSA(TM)Spinerobotandintraoperativeflat-panelCTguidance.ActaNeurochir(Wien)2016;158:1125-8.
Core Editorial Committee
Dr. Vedantam Rajshekhar, CMC Vellore- Chairperson
Dr. P. Sarat Chandra, AIIMS, New Delhi- Editor,Neurology India
Dr. D.Muzumdar, KEM Hosptial, Mumbai - Member
Dr. Ashish Suri, AIIMS, New Delhi - Member
Dr. Dwarakanath Srinivas, NIMHANS, Bengaluru - Member
Dr. Pravin Salunke, PGIMER, Chandigarh - Member
Ex-officio members
President- Dr. Lokendra Singh
President Elect - Dr. V.P. Singh
Secretary - Dr. N.Muthukumar
Treasurer - Dr. Daljit Singh
Related Documents