551 Received May 10, 2001; Accepted August 29, 2001 551 Neurol Med Chir (Tokyo) 41, 551¿555, 2001 Malignant Schwannoma of the Sciatic Nerve Originating in a Spinal Plexiform Neurofibroma Associated With Neurofibromatosis Type 1 —Case Report— Cahide TOPSAKAL, Ismail AKDEMIR, Murat TIFTIKCI, Ibrahim OZERCAN*, and Yunus AYDIN** Departments of Neurosurgery and *Pathology, Firat University, School of Medicine, Elazig, Turkey; **Department of Neurosurgery, Sisli Etfal Hospital, Istanbul, Turkey Abstract A 26-year-old man with neurofibromatosis type 1 (NF1) presented with a giant malignant schwannoma of the sciatic nerve. The differential diagnosis of malignant peripheral nerve sheath tumor (MPNST) was based on clinical, radiological, and histological evidence. The tumor apparently originated in a spi- nal plexiform neurofibroma. The lesion was resected totally without neural damage to the sciatic nerve. However, the tumor recurred within 2 months. The patient died of unknown factors probably associated with the spinal involvement. MPNST associated with NF1 has a poor prognosis due to recurrence or metastasis despite complete macroscopic removal. Key words: neurilemmoma, neurofibroma, malignant peripheral nerve sheath tumor, malignant schwannoma, von Recklinghausen's disease Introduction Neurofibromatosis (NF) is a disease of defective de- velopment of the neuroectodermal tissues and tends to involve multiple systems 22) with an incidence of 0.25–0.05%. 7,25) NF type 1 (NF1) is an autosomal dominant disease of the neurons and astrocytes 1,22,25) whereas type 2 (NF2) is a disease of the sheathing of the central nervous system. 1,22) Patients with NF1 tend to develop neurofibromas, whereas patients with NF2 harbor schwannomas. 1,18) NF1 is also asso- ciated with pheochromocytoma, 7,25) nephroblasto- ma, 3,32) ganglioneuroma, 5,32) brain tumors, 16,22) and non-neural crest malignancies such as rhab- domyosarcoma 3,32) and acute nonlymphoblastic leu- kemia. 3,19,32) Sarcoma developing within a peripheral nerve or a previous neurofibroma is rare and is known as malignant schwannoma, malignant peripheral nerve sheath tumor (MPNST), neurofibrosarcoma, or neu- rogenic sarcoma. 8,10,35) The true incidence is not known, 27) although reported as 4.6%, 10) but nerve sheath tumors account for 2–13% of soft tissue tumors. 12,26,35) MPNST is often associated with NF1 at an incidence of 4–53%. 5,7,10,12,31) Both types of NF account for 50–61% of cases of MPNST. 10,29) The criteria for the diagnosis of MPNST are as follows: Tumor originating from a nerve; association with a contiguous neurofibroma 8,34) ; possible association with NF1 10) ; and epineural invasion from within, 12) possibly containing heterologous mesenchymal and epithelial elements. 10) We report a case of giant MPNST associated with NF1 originating in a spinal plexiform neurofibroma. Case Report A 26-year-old man was admitted to our hospital on October 11, 1993 with a 25 × 30 cm mass on his posterior right thigh which had caused pain and slight weakness of the lower limb for 2 months and pruritus of the soles. Neurological examination found 4/5 motor strength in right plantar and dorsal flexion, but no other abnormalities. He had the stig- mata of NF1, but was otherwise intellectually and neurologically intact. His family contained no histo- ry of NF1. The patient had cafáe au lait spots and a few neurofibromas on the skin, but no Lisch nodules

Malignant Schwannoma of the Sciatic Nerve Originating in a Spinal Plexiform Neurofibroma Associated With Neurofibromatosis Type 1
Dec 26, 2022
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
DiovNT551
Neurol Med Chir (Tokyo) 41, 551¿555, 2001
Malignant Schwannoma of the Sciatic Nerve Originating in a Spinal Plexiform Neurofibroma Associated
With Neurofibromatosis Type 1
—Case Report—
Cahide TOPSAKAL, Ismail AKDEMIR, Murat TIFTIKCI, Ibrahim OZERCAN*, and Yunus AYDIN**
Departments of Neurosurgery and *Pathology, Firat University, School of Medicine, Elazig, Turkey; **Department of Neurosurgery, Sisli Etfal Hospital, Istanbul, Turkey
Abstract
A 26-year-old man with neurofibromatosis type 1 (NF1) presented with a giant malignant schwannoma of the sciatic nerve. The differential diagnosis of malignant peripheral nerve sheath tumor (MPNST) was based on clinical, radiological, and histological evidence. The tumor apparently originated in a spi- nal plexiform neurofibroma. The lesion was resected totally without neural damage to the sciatic nerve. However, the tumor recurred within 2 months. The patient died of unknown factors probably associated with the spinal involvement. MPNST associated with NF1 has a poor prognosis due to recurrence or metastasis despite complete macroscopic removal.
Key words: neurilemmoma, neurofibroma, malignant peripheral nerve sheath tumor, malignant schwannoma, von Recklinghausen's disease
Introduction
Neurofibromatosis (NF) is a disease of defective de- velopment of the neuroectodermal tissues and tends to involve multiple systems22) with an incidence of 0.25–0.05%.7,25) NF type 1 (NF1) is an autosomal dominant disease of the neurons and astrocytes1,22,25)
whereas type 2 (NF2) is a disease of the sheathing of the central nervous system.1,22) Patients with NF1 tend to develop neurofibromas, whereas patients with NF2 harbor schwannomas.1,18) NF1 is also asso- ciated with pheochromocytoma,7,25) nephroblasto- ma,3,32) ganglioneuroma,5,32) brain tumors,16,22) and non-neural crest malignancies such as rhab- domyosarcoma3,32) and acute nonlymphoblastic leu- kemia.3,19,32)
Sarcoma developing within a peripheral nerve or a previous neurofibroma is rare and is known as malignant schwannoma, malignant peripheral nerve sheath tumor (MPNST), neurofibrosarcoma, or neu- rogenic sarcoma.8,10,35) The true incidence is not known,27) although reported as 4.6%,10) but nerve sheath tumors account for 2–13% of soft tissue
tumors.12,26,35) MPNST is often associated with NF1 at an incidence of 4–53%.5,7,10,12,31) Both types of NF account for 50–61% of cases of MPNST.10,29) The criteria for the diagnosis of MPNST are as follows: Tumor originating from a nerve; association with a contiguous neurofibroma8,34); possible association with NF110); and epineural invasion from within,12)
possibly containing heterologous mesenchymal and epithelial elements.10)
We report a case of giant MPNST associated with NF1 originating in a spinal plexiform neurofibroma.
Case Report
A 26-year-old man was admitted to our hospital on October 11, 1993 with a 25× 30 cm mass on his posterior right thigh which had caused pain and slight weakness of the lower limb for 2 months and pruritus of the soles. Neurological examination found 4/5 motor strength in right plantar and dorsal flexion, but no other abnormalities. He had the stig- mata of NF1, but was otherwise intellectually and neurologically intact. His family contained no histo- ry of NF1. The patient had caf áe au lait spots and a few neurofibromas on the skin, but no Lisch nodules
552
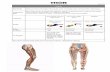
Fig. 1 Femoral angiogram of the lower limb show- ing moderate neovascularization in the field of the tumor (arrows) with the posterior dis- placement of femoral artery.
552
C. Topsakal et al.
or posterior subcapsular cataracts1,22) or other dis- eases associated with NF1.22,25,31) He had no history of previous radiotherapy or surgery at the site of lesion.
Angiography of the lower limb demonstrated moderate neovascular reaction and posterior dis- placement of the femoral artery20) (Fig. 1). Cranial computed tomography found no abnormalities. Preoperative magnetic resonance imaging of the thoracic and lumbar area revealed spinal plexiform neurofibromas causing enlargement of the interver- tebral foramina (Fig. 2).
He underwent surgery for the thigh mass on November 3, 1993. The tumor was found along the course of the nerve, infiltrating both proximally and distally into the sciatic nerve and the surrounding soft tissues. The mass was removed from the nerve trunk, and all functions were preserved (Fig. 3). Macroscopically, the cut surface was whitish, whorled, or homogeneous gray with areas of hemor- rhage and necrosis. Histological examination re- vealed infiltration into the surrounding muscle tis- sue, high cellularity with spindle cells containing nuclear mitotic figures and pleomorphism, and pseudopalisading and necrosis (Fig. 4A). Staining with periodic acid-Schiff and mucicarmine, and immunostaining for myoglobin, F8-related antigen, Gomori's reticulin, carcinoembryonic antigen, and keratin by the modified peroxidase-antiperoxidase method were all negative10) as well as staining for S-100 protein14,33) (Fig. 4B). The tumor was consid- ered grade 4 based on cellularity, nuclear pleomor- phism, mitotic rate, and necrosis and invasion.10,35)
Very few heterologous elements were noted. The di- agnosis was malignant schwannoma.
Postoperatively, he did well, but the tumor recurred at the site of operation after 2 months (Fig. 5). Hindquarter amputation and radiotherapy was planned, but he died of an unknown cause dur- ing the hospital stay.
Discussion
Malignant transformation of neurofibroma to malignant schwannoma occurs in 2–29% of patients with NF1,2,5,9,11) but origin from a large nerve trunk is less common in patients with NF1.8,34) Some trans- formations occur after radiotherapy10,11,31,32,36,37) or previous surgery for a benign neurofibroma. The pleiotropic effect of the NF allele on chromosome 174,22) is responsible for increasing the risk for both neural crest and nonneural crest malignancies.1,3,5,7,
16,18,22,25,32) Development of malignant schwannoma from neurofibroma is associated with inactivation of the both NF1 (tumor suppressor gene) alleles, and by
partial inactivation of the other tumor suppressor gene p53 located elsewhere on the centromere of chromosome 17.4,17) Neurofibromas increase in size under the control of the sex steroids in both sexes, directly or through mediation by nerve growth fac- tor (NGF),23) whose receptor is located on the distal arm of chromosome 17.28) The onset of malignant schwannoma may be attributable to the abnormal and continuous stimulation of nerve cells sensitive to NGF.21,26)
MPNST lesions tend to occur more centrally in patients with NF1,11,13,31) and peripherally9,10,12,13) or evenly distributed in patients without NF1.31) There is a male8,36) or female10,31) preponderance or even distribution8,12) in NF1. Patients with MPNST and NF1 are younger than patients without NF1 (28.7–36 years vs. 39.7–48 years)10,31) or no age/sex difference is noted,8,12) and the mean age is 34 years.10,13,18) The most common origin is the sciatic nerve followed by the brachial plexus,6,8,10,13) spinal nerve roots, and the vagus, femoral, median, sacral plexus, popliteal, obturator, posterior tibial, and ulnar nerves,8,10,38)
and the vulva, posterior mediastinum, or retroperi- toneal locations.5,9,10,12,13) Association with intes- tinal,5,24) spinal,5,16,22,24) and segmental forms of NF
553
Fig. 2 A: Sagittal T1-weighted magnetic resonance (MR) image displaying the intraspinal extension of the neurofibroma. B: Sagittal T2-weighted MR image demonstrating the enlarged inter- vertebral foramina at almost every level. C: Coronal T1-weighted MR image revealing the plexiform nature of the neurofibromatosis clearly.
Fig. 3 Photograph of the 25× 30 cm tumor mass removed from the sciatic nerve. The irregu- lar shaped tumor resembles the plexiform type of neurofibroma.
553
Malignant Schwannoma
(NF5)25,30) have also been reported. Development of malignant schwannoma from a
Schwann cell is extremely uncommon.11) A malig- nant spindle cell lesion containing sarcomatous cells in a whorled pattern in a nerve or a neurofibro- ma is called malignant schwannoma,13) and is characterized by high cellularity, with spindle- shaped or polygonal cells with ill-defined borders,
nuclear pleomorphism, perivascular cell prolifera- tion, high mitotic activity, and infiltration of adja- cent tissue.12,26,29) High-grade sarcomas usually oc- cur in NF1 (82% grade 3 or 4), and display cellular pleomorphism, brisk mitotic activity, prominent nucleoli, microvascular proliferation, necrosis, and palisading,10,31) as in our case. Fascicles of spindle cells may resemble fibrosarcoma or a storiform pat- tern of cell orientation without giant cells may resemble malignant fibrous histiocytoma.8,10,34)
MPNST is composed of heterologous mesenchymal and epithelial elements.11,12) A wide range of metaplasia is attributed to the neurilemmal origin.27,34) Multipotential ectomesenchyme is responsible for the divergent differentiation.
Sarcomas arising from the nerves are less malig- nant and metastasize less frequently than other forms of sarcoma. However, MPNST generally causes local recurrence and metastases even after macroscopic total removal.8,29) Recurrence occurs at least once within 2–73 months in 45–78% of cases associated with NF1.10,13) Metastasis occurred in 39–65%10,13) of patients with NF1 and 16%10) without NF1. Hematogenous dissemination to the lungs, soft tissue, bone, liver, intra-abdominal cavity, adrenal glands, diaphragm, mediastinum, brain, ovaries, kidneys, and retroperitoneum9,10,12,26,29,31) and in- traneural dissemination9,15) have been reported.
554
Fig. 4 A: Photomicrograph of the malignant schwannoma showing significant cellulari- ty with mitosis and pleomorphism as well as invasion into the muscle tissue and some necrosis. HE stain, ×200. B: Pho- tomicrograph showing absence of S-100 pro- tein immunostaining with a small artifact area. ×100.
Fig. 5 Photograph of the recurrent tumor at the operation site. Note the cutaneous neu- rofibromas on the opposite lower limb and the many caf áe au lait spots on the buttocks.
554
C. Topsakal et al.
MPNST has never metastasized to the regional lymph node.9,12)
The presence of NF1, multiple or plexiform type neurofibromas, tumor size over 5 cm, and extent of resection and recurrence are poor prognostic fac- tors.5,10,12,13,31) The 5-year survival is 10–47% and the 10-year survival is 30% and 39% in cases with NF1 and 47–75% in cases without NF1,2,10,12,26,31,35) re- spectively. Location and histological type (divergent differentiation/grade) are not major prognostic fac- tors5,8,10,13,26,31) although patients with distal extremi- ty lesions do better than those with head and neck lesions.10,31) Paravertebral malignant schwannomas had a worse outcome than in any other location.12)
Prior local radiotherapy or surgery does not affect the prognosis.8,10) Patients with NF1 are younger, have malignant schwannomas centrally located, and a shorter 5-year survival than patients without NF1.31)
Radical tumor excision without damage to the nerve is possible because the tumor arises from only one fiber, displacing the rest to one side,30) but usu- ally entails local recurrence. Muscle group excision and if necessary amputation5,9,10,12,31) combined with high-dose radiation therapy10,31) should be contem- plated. However, radiotherapy is palliative and does not effect the survival.2,10) Chemotherapy is incon- clusive for the treatment of malignant schwanno- ma.2,31) Treatment for spinal lesions is palliative, and the patients usually die of complications developing after involvement of the spinal cord.5)
References
1) Aoki S, Barkovich AJ, Nishimura K, Kjos BO, Machida T, Cogen P, Edwards M, Norman D: Neu- rofibromatosis types 1 and 2: cranial MR findings. Radiology 172: 527–534, 1989
2) Ariel IM: Tumors of the peripheral nervous system. CA Cancer J Clin 33: 282–299, 1983
3) Bader JL, Miller RW: Neurofibromatosis and child- hood leukemia. J Pediatr 92: 925–929, 1978
4) Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D, Bishop DT, Carey J, Baty B, Kivlin J, Willard H, Waye JS, Greig G, Leinward L, Nakamura Y, O'Connell P, Leppert M, Lalouel JM, White R, Skolnick M: Gene for von Recklinghausen neu- rofibromatosis is in the pericentromeric region of chromosome 17. Science 236: 1100–1102, 1987
5) Brasfield RD, Das Gupta TK: Von Recklinghausen's disease: a clinicopathological study. Ann Surg 175: 86–104, 1972
6) Brown RW, Tornos C, Evans HL: Angiosarcoma aris- ing from malignant schwannoma in a patient with neurofibromatosis. Cancer 70: 1141–1144, 1992
555555
Malignant Schwannoma
7) Crowe FW, Schull WJ, Neel JV: A Clinical, Pathologi- cal and Genetic Study of Multiple Neurofibromatosis. Springfield, Ill, Charles C Thomas Publisher, 1956, pp 1–176
8) D'Agostino AN, Soule EH, Miller RH: Sarcomas of the peripheral nerves and somatic soft tissues associ- ated with multiple neurofibromatosis (von Reckling- hausen's disease). Cancer 16: 1015–1027, 1963
9) Das Gupta TK, Brasfield RD: Solitary malignant schwannoma. Ann Surg 171: 419–428, 1970
10) Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM: Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 57: 2006–2021, 1986
11) Enzinger FM, Weiss SW: Soft Tissue Tumors. St Louis, The CV Mosby, 1983, pp 625–656
12) Ghosh BC, Ghosh L, Huvos AG, Fortner JG: Malig- nant schwannoma. A clinicopathologic study. Can- cer 31: 184–190, 1973
13) Guccion JG, Enzinger FM: Malignant schwannoma associated with von Recklinghausen's neurofibroma- tosis. Virchow Arch A Pathol Anat Histol 383: 43–57, 1979
14) Hayashi K, Takahashi K, Sonobe H, Ohtsuki Y, Taguchi K: The distribution of alpha and beta sub- units of S-100 protein in malignant schwannomas arising from neurofibromatosis of von Reckling- hausen's disease. Virchows Arch A Pathol Anat Histopathol 411: 515–521, 1987
15) Heffner RR: Hydromyelia in von Recklinghausen's disease with neurofibrosarcoma. Conn Med 33: 311–313, 1969
16) Herrmann J: Sarcomatous transformation in multiple neurofibromatosis (von Recklinghausen's disease). Ann Surg 131: 206–217, 1950
17) Lothe RA, Saeter G, Danielsen HE, Stenwig AE, Hoyheim B, O'Connell P, Borresen AL: Genetic alter- ations in a malignant schwannoma from a patient with neurofibromatosis (NF1). Pathol Res Pract 189: 465–474, 1993
18) Martuza RL, Eldridge R: Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med 318: 684–688, 1988
19) McEvoy MW, Mann JR: Neurofibromatosis with leu- kaemia. Br Med J 3: 641, 1971
20) Menendez LR, DiCesare PE, Soto C: Neurofibroma in a patient with von Recklinghausen's disease seen as a malignant schwannoma. A case report. Clin Or- thop 254: 298–302, 1990
21) Mentzel T, Katenkamp D: Intraneural angiosarcoma and angiosarcoma arising in benign and malignant peripheral nerve sheath tumours: clinicopathological and immunohistochemical analysis of four cases. Histopathology 35: 114–120, 1999
22) Mulvihill JJ, Parry DM, Sherman JL, Pikus A, Kaiser- Kupfer MI, Eldridge R: NIH conference. Neu- rofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neu- rofibromatosis). An update. Ann Intern Med 113: 39–52, 1990
23) Perez-Polo JR, Hall K, Livingston K, Westlund K: Steroid induction of nerve growth factor synthesis in cell culture. Life Sci 21: 1535–1544, 1977
24) Pulst SM, Riccardi VM, Fain P, Korenberg JR: Familial spinal neurofibromatosis: clinical and DNA linkage analysis. Neurology 41: 1923–1927, 1991
25) Riccardi VM: Von Recklinghausen neurofibromato- sis. N Engl J Med 305: 1617–1627, 1981
26) Risio M, Coverlizza S, Digirolamo P, Leli R, Saccia A: Malignant schwannoma: a frequent evolution of von Recklinghausen's disease. Panminerva Med 29: 283–288, 1987
27) Russel DS, Rubinstein LJ: Pathology of Tumours of the Nervous System, ed 4. Baltimore, Williams & Wilkins, 1977, pp 26–28, 372–401
28) Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Faryniarz AG, Chao MV, Huson S, Korf BR, Parry DM, Pericak-Vance MA: Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell 49: 589–594, 1987
29) Seppala MT, Haltia MJ: Spinal malignant nerve- sheath tumor or cellular schwannoma? A striking difference in prognosis. J Neurosurg 79: 528–532, 1993
30) Sieb JP, Schultheiss R: Segmental neurofibromatosis of the sciatic nerve. Case report. Neurosurgery 31: 1122–1125, 1992
31) Sordillo PP, Helson L, Hajdu SI, Magill GB, Kosloff C, Golbey RB, Beattie EJ: Malignant schwannoma — clinical characteristics, survival, and response to therapy. Cancer 47: 2503–2509, 1981
32) Stay EJ, Vawter G: The relationship between nephroblastoma and neurofibromatosis (von Reck- linghausen's disease). Cancer 39: 2550–2555, 1977
33) Stefansson K, Wollmann R, Jerkovic M: S-100 protein in soft-tissue tumors derived from Schwann cells and melanocytes. Am J Pathol 106: 261–268, 1982
34) Stout AP: The malignant tumors of the peripheral nerves. Am J Cancer 25: 1–36, 1935
35) Trojanowski JQ, Kleinman GM, Proppe KH: Malig- nant tumors of nerve sheath origin. Cancer 46: 1202–1212, 1980
36) Vieta JO, Pack GT: Malignant neurilemmomas of peripheral nerves. Am J Surg 82: 416–431, 1951
37) Woodruff JM, Chernik NL, Smith MC, Millett WB, Foote FW: Peripheral nerve tumors with rhab- domyosarcomatous differentiation (malignant ``Tri- ton'' tumors). Cancer 32: 426–439, 1973
38) Zivot ML, Pitzer S, Pantig-Felix L, Nathan LE: Malig- nant schwannoma of the medial plantar branch of the posterior tibial nerve. J Foot Surg 29: 130–134, 1990
Neurol Med Chir (Tokyo) 41, 551¿555, 2001
Malignant Schwannoma of the Sciatic Nerve Originating in a Spinal Plexiform Neurofibroma Associated
With Neurofibromatosis Type 1
—Case Report—
Cahide TOPSAKAL, Ismail AKDEMIR, Murat TIFTIKCI, Ibrahim OZERCAN*, and Yunus AYDIN**
Departments of Neurosurgery and *Pathology, Firat University, School of Medicine, Elazig, Turkey; **Department of Neurosurgery, Sisli Etfal Hospital, Istanbul, Turkey
Abstract
A 26-year-old man with neurofibromatosis type 1 (NF1) presented with a giant malignant schwannoma of the sciatic nerve. The differential diagnosis of malignant peripheral nerve sheath tumor (MPNST) was based on clinical, radiological, and histological evidence. The tumor apparently originated in a spi- nal plexiform neurofibroma. The lesion was resected totally without neural damage to the sciatic nerve. However, the tumor recurred within 2 months. The patient died of unknown factors probably associated with the spinal involvement. MPNST associated with NF1 has a poor prognosis due to recurrence or metastasis despite complete macroscopic removal.
Key words: neurilemmoma, neurofibroma, malignant peripheral nerve sheath tumor, malignant schwannoma, von Recklinghausen's disease
Introduction
Neurofibromatosis (NF) is a disease of defective de- velopment of the neuroectodermal tissues and tends to involve multiple systems22) with an incidence of 0.25–0.05%.7,25) NF type 1 (NF1) is an autosomal dominant disease of the neurons and astrocytes1,22,25)
whereas type 2 (NF2) is a disease of the sheathing of the central nervous system.1,22) Patients with NF1 tend to develop neurofibromas, whereas patients with NF2 harbor schwannomas.1,18) NF1 is also asso- ciated with pheochromocytoma,7,25) nephroblasto- ma,3,32) ganglioneuroma,5,32) brain tumors,16,22) and non-neural crest malignancies such as rhab- domyosarcoma3,32) and acute nonlymphoblastic leu- kemia.3,19,32)
Sarcoma developing within a peripheral nerve or a previous neurofibroma is rare and is known as malignant schwannoma, malignant peripheral nerve sheath tumor (MPNST), neurofibrosarcoma, or neu- rogenic sarcoma.8,10,35) The true incidence is not known,27) although reported as 4.6%,10) but nerve sheath tumors account for 2–13% of soft tissue
tumors.12,26,35) MPNST is often associated with NF1 at an incidence of 4–53%.5,7,10,12,31) Both types of NF account for 50–61% of cases of MPNST.10,29) The criteria for the diagnosis of MPNST are as follows: Tumor originating from a nerve; association with a contiguous neurofibroma8,34); possible association with NF110); and epineural invasion from within,12)
possibly containing heterologous mesenchymal and epithelial elements.10)
We report a case of giant MPNST associated with NF1 originating in a spinal plexiform neurofibroma.
Case Report
A 26-year-old man was admitted to our hospital on October 11, 1993 with a 25× 30 cm mass on his posterior right thigh which had caused pain and slight weakness of the lower limb for 2 months and pruritus of the soles. Neurological examination found 4/5 motor strength in right plantar and dorsal flexion, but no other abnormalities. He had the stig- mata of NF1, but was otherwise intellectually and neurologically intact. His family contained no histo- ry of NF1. The patient had caf áe au lait spots and a few neurofibromas on the skin, but no Lisch nodules
552
Fig. 1 Femoral angiogram of the lower limb show- ing moderate neovascularization in the field of the tumor (arrows) with the posterior dis- placement of femoral artery.
552
C. Topsakal et al.
or posterior subcapsular cataracts1,22) or other dis- eases associated with NF1.22,25,31) He had no history of previous radiotherapy or surgery at the site of lesion.
Angiography of the lower limb demonstrated moderate neovascular reaction and posterior dis- placement of the femoral artery20) (Fig. 1). Cranial computed tomography found no abnormalities. Preoperative magnetic resonance imaging of the thoracic and lumbar area revealed spinal plexiform neurofibromas causing enlargement of the interver- tebral foramina (Fig. 2).
He underwent surgery for the thigh mass on November 3, 1993. The tumor was found along the course of the nerve, infiltrating both proximally and distally into the sciatic nerve and the surrounding soft tissues. The mass was removed from the nerve trunk, and all functions were preserved (Fig. 3). Macroscopically, the cut surface was whitish, whorled, or homogeneous gray with areas of hemor- rhage and necrosis. Histological examination re- vealed infiltration into the surrounding muscle tis- sue, high cellularity with spindle cells containing nuclear mitotic figures and pleomorphism, and pseudopalisading and necrosis (Fig. 4A). Staining with periodic acid-Schiff and mucicarmine, and immunostaining for myoglobin, F8-related antigen, Gomori's reticulin, carcinoembryonic antigen, and keratin by the modified peroxidase-antiperoxidase method were all negative10) as well as staining for S-100 protein14,33) (Fig. 4B). The tumor was consid- ered grade 4 based on cellularity, nuclear pleomor- phism, mitotic rate, and necrosis and invasion.10,35)
Very few heterologous elements were noted. The di- agnosis was malignant schwannoma.
Postoperatively, he did well, but the tumor recurred at the site of operation after 2 months (Fig. 5). Hindquarter amputation and radiotherapy was planned, but he died of an unknown cause dur- ing the hospital stay.
Discussion
Malignant transformation of neurofibroma to malignant schwannoma occurs in 2–29% of patients with NF1,2,5,9,11) but origin from a large nerve trunk is less common in patients with NF1.8,34) Some trans- formations occur after radiotherapy10,11,31,32,36,37) or previous surgery for a benign neurofibroma. The pleiotropic effect of the NF allele on chromosome 174,22) is responsible for increasing the risk for both neural crest and nonneural crest malignancies.1,3,5,7,
16,18,22,25,32) Development of malignant schwannoma from neurofibroma is associated with inactivation of the both NF1 (tumor suppressor gene) alleles, and by
partial inactivation of the other tumor suppressor gene p53 located elsewhere on the centromere of chromosome 17.4,17) Neurofibromas increase in size under the control of the sex steroids in both sexes, directly or through mediation by nerve growth fac- tor (NGF),23) whose receptor is located on the distal arm of chromosome 17.28) The onset of malignant schwannoma may be attributable to the abnormal and continuous stimulation of nerve cells sensitive to NGF.21,26)
MPNST lesions tend to occur more centrally in patients with NF1,11,13,31) and peripherally9,10,12,13) or evenly distributed in patients without NF1.31) There is a male8,36) or female10,31) preponderance or even distribution8,12) in NF1. Patients with MPNST and NF1 are younger than patients without NF1 (28.7–36 years vs. 39.7–48 years)10,31) or no age/sex difference is noted,8,12) and the mean age is 34 years.10,13,18) The most common origin is the sciatic nerve followed by the brachial plexus,6,8,10,13) spinal nerve roots, and the vagus, femoral, median, sacral plexus, popliteal, obturator, posterior tibial, and ulnar nerves,8,10,38)
and the vulva, posterior mediastinum, or retroperi- toneal locations.5,9,10,12,13) Association with intes- tinal,5,24) spinal,5,16,22,24) and segmental forms of NF
553
Fig. 2 A: Sagittal T1-weighted magnetic resonance (MR) image displaying the intraspinal extension of the neurofibroma. B: Sagittal T2-weighted MR image demonstrating the enlarged inter- vertebral foramina at almost every level. C: Coronal T1-weighted MR image revealing the plexiform nature of the neurofibromatosis clearly.
Fig. 3 Photograph of the 25× 30 cm tumor mass removed from the sciatic nerve. The irregu- lar shaped tumor resembles the plexiform type of neurofibroma.
553
Malignant Schwannoma
(NF5)25,30) have also been reported. Development of malignant schwannoma from a
Schwann cell is extremely uncommon.11) A malig- nant spindle cell lesion containing sarcomatous cells in a whorled pattern in a nerve or a neurofibro- ma is called malignant schwannoma,13) and is characterized by high cellularity, with spindle- shaped or polygonal cells with ill-defined borders,
nuclear pleomorphism, perivascular cell prolifera- tion, high mitotic activity, and infiltration of adja- cent tissue.12,26,29) High-grade sarcomas usually oc- cur in NF1 (82% grade 3 or 4), and display cellular pleomorphism, brisk mitotic activity, prominent nucleoli, microvascular proliferation, necrosis, and palisading,10,31) as in our case. Fascicles of spindle cells may resemble fibrosarcoma or a storiform pat- tern of cell orientation without giant cells may resemble malignant fibrous histiocytoma.8,10,34)
MPNST is composed of heterologous mesenchymal and epithelial elements.11,12) A wide range of metaplasia is attributed to the neurilemmal origin.27,34) Multipotential ectomesenchyme is responsible for the divergent differentiation.
Sarcomas arising from the nerves are less malig- nant and metastasize less frequently than other forms of sarcoma. However, MPNST generally causes local recurrence and metastases even after macroscopic total removal.8,29) Recurrence occurs at least once within 2–73 months in 45–78% of cases associated with NF1.10,13) Metastasis occurred in 39–65%10,13) of patients with NF1 and 16%10) without NF1. Hematogenous dissemination to the lungs, soft tissue, bone, liver, intra-abdominal cavity, adrenal glands, diaphragm, mediastinum, brain, ovaries, kidneys, and retroperitoneum9,10,12,26,29,31) and in- traneural dissemination9,15) have been reported.
554
Fig. 4 A: Photomicrograph of the malignant schwannoma showing significant cellulari- ty with mitosis and pleomorphism as well as invasion into the muscle tissue and some necrosis. HE stain, ×200. B: Pho- tomicrograph showing absence of S-100 pro- tein immunostaining with a small artifact area. ×100.
Fig. 5 Photograph of the recurrent tumor at the operation site. Note the cutaneous neu- rofibromas on the opposite lower limb and the many caf áe au lait spots on the buttocks.
554
C. Topsakal et al.
MPNST has never metastasized to the regional lymph node.9,12)
The presence of NF1, multiple or plexiform type neurofibromas, tumor size over 5 cm, and extent of resection and recurrence are poor prognostic fac- tors.5,10,12,13,31) The 5-year survival is 10–47% and the 10-year survival is 30% and 39% in cases with NF1 and 47–75% in cases without NF1,2,10,12,26,31,35) re- spectively. Location and histological type (divergent differentiation/grade) are not major prognostic fac- tors5,8,10,13,26,31) although patients with distal extremi- ty lesions do better than those with head and neck lesions.10,31) Paravertebral malignant schwannomas had a worse outcome than in any other location.12)
Prior local radiotherapy or surgery does not affect the prognosis.8,10) Patients with NF1 are younger, have malignant schwannomas centrally located, and a shorter 5-year survival than patients without NF1.31)
Radical tumor excision without damage to the nerve is possible because the tumor arises from only one fiber, displacing the rest to one side,30) but usu- ally entails local recurrence. Muscle group excision and if necessary amputation5,9,10,12,31) combined with high-dose radiation therapy10,31) should be contem- plated. However, radiotherapy is palliative and does not effect the survival.2,10) Chemotherapy is incon- clusive for the treatment of malignant schwanno- ma.2,31) Treatment for spinal lesions is palliative, and the patients usually die of complications developing after involvement of the spinal cord.5)
References
1) Aoki S, Barkovich AJ, Nishimura K, Kjos BO, Machida T, Cogen P, Edwards M, Norman D: Neu- rofibromatosis types 1 and 2: cranial MR findings. Radiology 172: 527–534, 1989
2) Ariel IM: Tumors of the peripheral nervous system. CA Cancer J Clin 33: 282–299, 1983
3) Bader JL, Miller RW: Neurofibromatosis and child- hood leukemia. J Pediatr 92: 925–929, 1978
4) Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D, Bishop DT, Carey J, Baty B, Kivlin J, Willard H, Waye JS, Greig G, Leinward L, Nakamura Y, O'Connell P, Leppert M, Lalouel JM, White R, Skolnick M: Gene for von Recklinghausen neu- rofibromatosis is in the pericentromeric region of chromosome 17. Science 236: 1100–1102, 1987
5) Brasfield RD, Das Gupta TK: Von Recklinghausen's disease: a clinicopathological study. Ann Surg 175: 86–104, 1972
6) Brown RW, Tornos C, Evans HL: Angiosarcoma aris- ing from malignant schwannoma in a patient with neurofibromatosis. Cancer 70: 1141–1144, 1992
555555
Malignant Schwannoma
7) Crowe FW, Schull WJ, Neel JV: A Clinical, Pathologi- cal and Genetic Study of Multiple Neurofibromatosis. Springfield, Ill, Charles C Thomas Publisher, 1956, pp 1–176
8) D'Agostino AN, Soule EH, Miller RH: Sarcomas of the peripheral nerves and somatic soft tissues associ- ated with multiple neurofibromatosis (von Reckling- hausen's disease). Cancer 16: 1015–1027, 1963
9) Das Gupta TK, Brasfield RD: Solitary malignant schwannoma. Ann Surg 171: 419–428, 1970
10) Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM: Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 57: 2006–2021, 1986
11) Enzinger FM, Weiss SW: Soft Tissue Tumors. St Louis, The CV Mosby, 1983, pp 625–656
12) Ghosh BC, Ghosh L, Huvos AG, Fortner JG: Malig- nant schwannoma. A clinicopathologic study. Can- cer 31: 184–190, 1973
13) Guccion JG, Enzinger FM: Malignant schwannoma associated with von Recklinghausen's neurofibroma- tosis. Virchow Arch A Pathol Anat Histol 383: 43–57, 1979
14) Hayashi K, Takahashi K, Sonobe H, Ohtsuki Y, Taguchi K: The distribution of alpha and beta sub- units of S-100 protein in malignant schwannomas arising from neurofibromatosis of von Reckling- hausen's disease. Virchows Arch A Pathol Anat Histopathol 411: 515–521, 1987
15) Heffner RR: Hydromyelia in von Recklinghausen's disease with neurofibrosarcoma. Conn Med 33: 311–313, 1969
16) Herrmann J: Sarcomatous transformation in multiple neurofibromatosis (von Recklinghausen's disease). Ann Surg 131: 206–217, 1950
17) Lothe RA, Saeter G, Danielsen HE, Stenwig AE, Hoyheim B, O'Connell P, Borresen AL: Genetic alter- ations in a malignant schwannoma from a patient with neurofibromatosis (NF1). Pathol Res Pract 189: 465–474, 1993
18) Martuza RL, Eldridge R: Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med 318: 684–688, 1988
19) McEvoy MW, Mann JR: Neurofibromatosis with leu- kaemia. Br Med J 3: 641, 1971
20) Menendez LR, DiCesare PE, Soto C: Neurofibroma in a patient with von Recklinghausen's disease seen as a malignant schwannoma. A case report. Clin Or- thop 254: 298–302, 1990
21) Mentzel T, Katenkamp D: Intraneural angiosarcoma and angiosarcoma arising in benign and malignant peripheral nerve sheath tumours: clinicopathological and immunohistochemical analysis of four cases. Histopathology 35: 114–120, 1999
22) Mulvihill JJ, Parry DM, Sherman JL, Pikus A, Kaiser- Kupfer MI, Eldridge R: NIH conference. Neu- rofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neu- rofibromatosis). An update. Ann Intern Med 113: 39–52, 1990
23) Perez-Polo JR, Hall K, Livingston K, Westlund K: Steroid induction of nerve growth factor synthesis in cell culture. Life Sci 21: 1535–1544, 1977
24) Pulst SM, Riccardi VM, Fain P, Korenberg JR: Familial spinal neurofibromatosis: clinical and DNA linkage analysis. Neurology 41: 1923–1927, 1991
25) Riccardi VM: Von Recklinghausen neurofibromato- sis. N Engl J Med 305: 1617–1627, 1981
26) Risio M, Coverlizza S, Digirolamo P, Leli R, Saccia A: Malignant schwannoma: a frequent evolution of von Recklinghausen's disease. Panminerva Med 29: 283–288, 1987
27) Russel DS, Rubinstein LJ: Pathology of Tumours of the Nervous System, ed 4. Baltimore, Williams & Wilkins, 1977, pp 26–28, 372–401
28) Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Faryniarz AG, Chao MV, Huson S, Korf BR, Parry DM, Pericak-Vance MA: Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell 49: 589–594, 1987
29) Seppala MT, Haltia MJ: Spinal malignant nerve- sheath tumor or cellular schwannoma? A striking difference in prognosis. J Neurosurg 79: 528–532, 1993
30) Sieb JP, Schultheiss R: Segmental neurofibromatosis of the sciatic nerve. Case report. Neurosurgery 31: 1122–1125, 1992
31) Sordillo PP, Helson L, Hajdu SI, Magill GB, Kosloff C, Golbey RB, Beattie EJ: Malignant schwannoma — clinical characteristics, survival, and response to therapy. Cancer 47: 2503–2509, 1981
32) Stay EJ, Vawter G: The relationship between nephroblastoma and neurofibromatosis (von Reck- linghausen's disease). Cancer 39: 2550–2555, 1977
33) Stefansson K, Wollmann R, Jerkovic M: S-100 protein in soft-tissue tumors derived from Schwann cells and melanocytes. Am J Pathol 106: 261–268, 1982
34) Stout AP: The malignant tumors of the peripheral nerves. Am J Cancer 25: 1–36, 1935
35) Trojanowski JQ, Kleinman GM, Proppe KH: Malig- nant tumors of nerve sheath origin. Cancer 46: 1202–1212, 1980
36) Vieta JO, Pack GT: Malignant neurilemmomas of peripheral nerves. Am J Surg 82: 416–431, 1951
37) Woodruff JM, Chernik NL, Smith MC, Millett WB, Foote FW: Peripheral nerve tumors with rhab- domyosarcomatous differentiation (malignant ``Tri- ton'' tumors). Cancer 32: 426–439, 1973
38) Zivot ML, Pitzer S, Pantig-Felix L, Nathan LE: Malig- nant schwannoma of the medial plantar branch of the posterior tibial nerve. J Foot Surg 29: 130–134, 1990
Related Documents