LIPIDS LUDITHA LUMAPAT-PE, MD, FPAAB CHAIR DEPARTMENT OF BIOCHEMISTRY

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
The fat speaks:
“With water, I say, “Touch me not”;
To the tongue, I am tasteful;
Within limits, I am dutiful;
In excess, I am dangerous!”
Lipids(Greek: lipos - fat)
1. Diverse group of compounds (structure)
2. Contains C, H, O
3. Organic substances, relatively insoluble in water (due to the predominance of hydrocarbon chains C-CH2-CH2-CH2-) in their structure
4. Soluble in organic solvents (alcohol, ether, chloroform & benzene
5. Actually or potentially related to fatty acids
6. Utilized by living cells
7. Not polymers, mostly small molecules
Four general functions of biologic lipids
1. A storage form of metabolic fuel (concentrated)
2. A transport form of metabolic fuel
3. A part of the outer coat between the body of the organism and the environment, providing protection in bacteria, plants, insects and vertebrates
4. Structural components of membranes
5. Source of fat soluble vitamins (A, D, E, K)
6. Protect the internal organs, serve as insulating materials and give shape and smooth appearance to the body.
7. As compounds of the inner mitochondrial membranes, lipids (phospholipids) participate in the electron transport chain
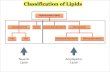
Commonly used classification of lipids and their general biologic functions:
Lipid Primary FunctionsFatty acids Energy sources, biosynthetic precursorsGlycerides Storage, transport, metabolic
intermediatesPhosphoglycerides Membrane componentsKetone bodies Energy sourcesSphingolipids Membrane componentsEicosanoids Modulators of physiologic activityCholesterol Membrane componentSteroid hormones Modulators of physiologic activity
I. Simple Lipids
• Esters of fatty acids with alcohols
2 types
1. Fats & oils - esters of fatty acids with glycerol
fat - solid at room temperature
oil - liquid
2. Waxes - esters of fatty acids (usually long chain) with alcohols other than glycerol
ex. Cetyl, meryl, merecyl alcohol
II. Complex (Compound) Lipids
• esters of fatty acids with alcohol containing additional groups such as phosphate, nitrogenous base, carbohydrates or protein, etc.
1. Phopholipids• lipids & phosphoric acid & nitrogeneous
base• (alcohol & FA)
1. a. glycerophospholipids• phospholipids with glycerol as alcohol• ex: cephalin
lecithin
1. B. sphingophospholipids• phospholipids with sphingosine as
alcohol• ex. Sphingomyeline
2. Glycolipids/Glycosphingolipids• FA + CHO +
III. Derived Lipids
• are derivatives obtained on the hydrolysis of group I and group 2 lipids which then retain the characteristics of the original lipids.
Examples:
1. Glycerol and other alcohols
2. FA
3. Mono and diacylglycerols
4. Lipid soluble vitamins - A, D, E, K
5. Steroid hormones
6. Hydrocarbons
7. Ketone bodies
8. Carotenoids
9. Squalene
10. Pentacosane
11. Terpenes
Fatty Acids
• simplest form of lipids • are carboxylic acids with hydrocarbon
chains• have a long hydrocarbon chain with a
terminal carboxylic acid group• have an even number of carbon atoms in
an unbranched chain
• properties of a FA depend on the chain length and the number of double bonds
• FA with shorter length - ↓ melting point • unsat FA have ↓ MP than sat FA • most common FA - Palmitic (16C)
stearic (18C)
• properties of a FA depend on the chain length and the number of double bonds
• FA with shorter chain length - ↓ melting point
• unsat FA have ↓ MP than sat FA • most common FA - Palmitic (16C)
Stearic (18C)
4 Major biological roles of FA
1. Membrane components (glycerophospholipids & sphingolipids
2. Several proteins are covalently modified by fatty acids
3. Act as energy stores (triacylglycerols) and fuel molecules
4. Fatty acid derivatives serve as hormones & intracellular second messengers
I. Saturated FA
• have the maximum number of hydrogens covalently bonded to the C chain of the R group
• no double bonds between the C atoms • all carbons are “Saturated” with
hydrogens
Gen. Formula - CH3 (CH2)n COOH
(n - even #)
II. Unsaturated FA
• has at least one double bond between carbons in the R group
• this decreases the number of hydrogen atoms that can bond to the carbon chain
• :. It is not saturated with hydrogens • it becomes more unsaturated as it has
more C- to -C double bonds
Ex. Linoleic Acid Oleic Acid
Linolenic Acid • also called Essential FA
Nomenclature
FA - named according to the number of carbon atoms in the chain and the number and position of any double bonds
Common FA
1. Palmitate (C16:0)
2. Stearate (C18:0)
3. Oleate (C18:1)
4. Linoleate (C18:2)
5. Linolenate (C18:3)
6. Arachidonate (C20:4)• the double bonds in a FA are usually in
the CIS configuration
Names and Formulas of Some Common Fatty Acids
Fatty Acid Formulae No. ofdoublebonds
No. ofCarbonatoms
Palmitate CH3(CH2)14COO- None 16Stearate CH3(CH2)16COO- None 18Oleate CH3(CH2)7 CH = CH(CH2)7COO- 1 18Linoleate CH3(CH2)4 (CH = CH CH2)2(CH)6COO- 2 18Linolenate CH3 CH2 (CH = CH CH2)3 (CH2)6 COO- 3 18Arachidonate CH3 (CH2)4 (CH = CH CH2)4 (CH2)2 COO- 4 20
1. Systematic names for FA are made by adding ‘OIC acid’ on to the name of the parent hydrocarbon
• based on the hydrocarbon from which it is derived
• sat FA end with a suffix - ANOIC (e.g., Octanoic Acid)
• unsat FA end with a suffix - ENOIC (e.g., Octadecanoic Acid)
2. However, as FA are ionized at physiologic pH, they are usually written as RCOO-, and have names ending in ‘ate’ rather than ‘oic acid’
Ex: a C18 Sat FA → Octadecanoate
a C18 mono-unsaturated FA → octadecenoate
a C18 FA with 2 double bonds → octadecadienoate
3. There is also a shorthand rotation to show the number of carbon atoms and the position of any double bonds in the structure
A FA with 18 carbons & no double bond → 18:0
a FA with 18 carbons & 2 double bonds → 18:2
4. The carbon atoms in FA are numbered from the carboxylic acid residue, and so the position of double bonds can be described using the number of the first carbon involved in the bond
Ex. ∆9 shows a double bond between carbons 9 & 10 of the FA chain
5. The general rule is that the total number of carbon atoms are written first, followed by the number of double bonds and finally the (first carbon) position of double bonds, starting from the carboxyl ends
Ex. Palmitic Acid - 16:0
Oleic Acid - 18:1; 9
Arachidonic - 20:4; 5, 8, 11, 14 or ∆9 - indicates that the double bond is between 9 and 10 of the FA
6. The configuration of the double bonds in most unsaturated FA is CIS; so called because the 2 hydrogens on the carbon atoms of either side of the double bond are on the same side of the molecules.
(Latin, cis = on this side of)
Thus, the full systematic name of linoleate is cis, cis - ∆9, ∆12 - octadecadienoate
7. During the degradation of FA some trans-isomers are formed where the hydrogens on the carbon atoms either side of the double bond are on opposite sides of the molecule
(Latin, trans = across)
8. The presence of cis rather than trans double bonds in naturally occuring unsat fatty acids ensure that lipids containing FA have a lower melting points and are therefore fluid at physiological temperatures
9. Length of Hydrocarbon chain of FA 3 groups
1. Short chain - less than 6 carbons
2. Medium chain - with 8 to 14 carbons
3. Long chain - with 16 to 24 carbons
Classification as to source:
1. Nonessential FA• all nonessential FA can be synthesized
from products of glucose oxidation• they do not have to be obligatory
included in the diet
2. Essential FA • Linoleic (18:2:∆9,12) & Lenolenic
(18:3:∆9, 12, 15) families must be obtained from the diet
• cannot be synthesized by the body, therefore should be supplied in the diet
• there are no human enzyme systems that can introduce a double bond beyond the ninth carbon atom (9-10 position) of a FA chain, and all double bonds that are introduced are separated by three-carbon intervals
This rule, combined with the fact that fatty acid elongation only occurs by two-carbon additions, makes it impossible to synthesize de novo certain polyunsaturated FA
Physical Properties
1. Are detergent-like due to their amphipathic nature; they have nonpolar (CH2) and polar (-COOH) ends and in biphasic systems, they orient with the polar end associated with water and the nonpolar end associated with the hydrophobic phase.
2. The melting point of FA is related to chain length and the degree of unsaturation. The longer the chain length, the higher the melting point, and the greater the number of double bonds, the lower the melting points.
Functions of EFA
1. required for the membrane structure and function
2. Transport of cholesterol
3. Formation of lipoproteins
4. Prevention of fatty liver
5. Also needed for the synthesis of eicosanoids
EFA deficiency → results in toad skin or phrynoderma
phrynoderma - characterized by a) the presence of horny eruptions on the posterior and lateral parts of limbs, on the back and buttocks; b) loss of hair; c) poor wound healing
Isomerism in UFA
UFA - exhibit geometric isomerism depending on the orientation of the groups around the double bond axis
cis transfiguration - if the atoms or acyl groups are present on the same side of the double bond
trans configuration - if the groups occur on the opposite side
Oleic Acid(cis form)
Elaidic Acid(trans form)
H
C
(CH2)7 COOH
C
H (CH2)7 CH3
H
C
(CH2)7 COOH
C
H3 C (CH2)7
H
• cis isomers are less stable
• most of the naturally occurring UFAcids exist as cis isomers
Hydroxy FA
• some of the FA are hydroxylated
∀ β-hydroxybutyric acid, one of the ketone bodies produced in metabolism is a simple example of hydroxy fatty acids
Cyclic FA
• with cyclic structures are rare• example is chaulmoogric acid found in
chaulmoogra oil (used in leprosy treatment) contains cyclopentenyl ring
Eicosanoids
• compounds related to eicosa-polyenoic acid and include prostaglandins, prostacyclins, leukotrienes, thromboxanes
Properties of Triacylglycerols
1. Hydrolysis• triacylglycerols undergo stepwise
enzymatic hydrolysis to finally liberate free fatty acids and glycerol
• the process of hydrolysis, catalyzed by lipases is important for digestion of fat in the GIT and fat mobilization from adipose tissues
2. Saponification • the hydrolysis of triacylglycerols by alkali
to produce glycerol and soaps is known as saponification
Triacylglycerols + 3 NaOH → Glycerol + 3 R-COONa (soaps)
3. Rancidity• term used to represent the deterioration of
fats and oils resulting in an unpleasant taste
• fats containing UFA are more susceptible • occurs when fats and oils are exposed to
air, moisture, light, bacteria, etc.• hydrolytic rancidity occurs due to partial
hydrolysis of triacylglycerols by bacterial enzymes
• oxidative rancidity → due to oxidation of uFA → resulting to the formation of unpleasant products as dicarboxylic acids, aldehydes, ketones, etc.
• oxygen is required for oxidative rancidity which occurs through the formation of intermediates → peroxides
• rancid fats and oils are unsuitable for human consumption
Antioxidants
• substances which can prevent the occurrence of oxidative rancidity
Examples:
Tocopherols (vit. E) added in small
Hydroquinone amounts to
Gallic acid commercial
α-naphthol preparations of
fats & oils
Antioxidants in food preservation
Propyl gallate
Butylated Hydroxyanisole (BHA)
Butylated Hydroxytoluene (BHT)
4. Lipid Peroxidation in vivo• in the living cells, lipids undergo oxidation
to produce peroxides and free radicals that can damage the tissueAntioxidants in the cells
Vitamin E
Urate
Superoxide Dismutase
Triacylglycerols (Triglycerides) Neutral Fats
• triesters of glycerol and 3 fatty acids • FA are converted to triglycerides for
transport between tissues and for storage of metabolic fuel
• adipocytes - the fat deposits in fat cells are the main stores of metabolic fuel in humans
• a very large proportion of ingested fats are stored as triglycerides in the fat droplets (serve long term needs for metabolic fuel ) of the adipocytes
Advantages over other forms of fuel
1. Are light (less dense than water). They provide a concentrated form of fuel because their complete combustion to CO2 and water releases 9 kcal/g as opposed to 4 kcal/gm for carbohydrate.
2. Because they are water insoluble, triglycerides present no osmotic problems to the cell even when stored in large amounts.
PHOSPHOLIPIDS
• major lipid constituents of cellular membranes, high concentrations in the lipids of glandular organs, blood plasma, egg yolk and in the sees of legumes
• comprise 40% → erythrocyte membrane
95% → inner mitochondrial
membrane
20% cardiolipin (phosphoglyceride)
• contain phosphorous, have a backbone of glycerol (e.g., phosphoglycerides) or sphingosine (e.g., sphingomyelin)
• are triesters of glycerol 3-phosphate in which two esters have been formed between the 2 hydroxyl groups & FA side chain (R1 & R2), and a third ester has been formed between the phosphate group and a hydroxyl containing compound X
Phosphoglycerides are named and classified according to the nature of the alcohol esterifying the glycerol phosphate
Major classes of phosphoglycerides/phospholipids
1. Phosphatidate
2. Phosphatidylethanolamine
3. Phosphatidylcholine
4. Phosphatidylserine
5. Phosphatidylglycerol
6. Diphosphatidylglycerol
7. Phosphatidylinositol
Glycoglycerolipids
• similar to the phosphoglycerides in that they have hydrophobic and polar parts, the latter being provided by a carbohydrate moiety rather than by an esterified phosphate
Sphingolipids
• are built from long-chain, hydroxylated bases rather than from glycerol
• 2 such bases are found in animals
a. sphingosine (more common)
b. dihydrosphingosine (sphingonine)• when the amino group of sphingosine or
sphingosine is acylated with a FA - the product is ceramide
2 classes of sphingolipid - (the 1o hydroxyl group is substituted in one of 2 ways to give 2 classes of sphingolipid
a. phosphosphingolipids
b. glycosphingolipids
• in phosphosphingolipids - the 1o hydroxyl group is esterified with choline phosphate
• the lipid is known as sphingomyelin• in glycosphingolipids - the 1o hydroxyl
group is substituted with a carbohydrate• glycosphingolipids that contain the sugar
sialic acid in the carbohydate portion are called gangliosides
Biological Functions
Phospholipids• compose the membrane of cells• plasma membrane is partly phospholipid• polar ends of the phospholipid molecules
are attracted toward water• the remaining parts of these molecules
are nonpolar and are oriented away from water
• much of the plasma membrane is a phospholipid bilayer
• there are 2 parallel layers of phospholipids
• the polar ends (heads) - hydrophilic they are along the outside and inside of the membrane surfaces where water is present
• the nonpolar ends (tails) are hydrophobic they are directed toward each other at the interior of the membrane, away from the water
1. Phosphatidic Acid• simplest phospholipid• intermediate in the synthesis of
triacylglycerols and phospholipids
2. Lecithin (Phosphatidylcholine)• most abundant in the cell membranes • chemically (Greek: egg yolk) a
phosphatidic acid with choline as the base
• Dipalmitoyl Lecithin - an important phosphatidylcholine found in lungs, a surface active agent and prevents the adherence of inner surface of the lungs due to surface tension; thereby preventing collapse
• in infants especially premature infants, Respiratory Distress Syndrome is a disorder characterized by the absence of Dipalmitoyl Lecithin
3. Cephalins (Phosphatidylethanolamine)• Ethanolamine is the nitrogeneous base• participate in blood clotting
4. Phosphatidylinositol• myo-inositol is attached• important component of cell membranes• act as second messengers for hormonal
action
5. Phosphatidylserine• serine as the base
6. Plasmolagen• brain and muscle contain a good
concentration• plasmolagen is the resultant compound
when a fatty acid is attached by an ether linkage at C1 of glycerol in the glycerophospholipids
• Phosphatidaleethanolamine - most important which is similar in structure to phosphatidylethanolamine but for the ether linkage in place of ester
• choline, inositol and serine - may substitute ethanolamine to give other plasmolagens
7. Cardiolipin• first isolated from the heart muscle• the only phosphoglyceride that possesses
antigenic properties• as to structure, consists of 2 molecules of
phosphatidic acid held by an additional glycerol through phosphate groups
• an important component of inner mitochondrial membrane
II. Sphingomyelins
• sphingosine - the amino alcohol present and is attached by an amide linkage to a fatty acid to produce ceramide
• the alcohol group of sphingosine is bound to phosphorylcholine in sphingomyelin structure
• sphingomyelins are important constituents of myelin and are found in good quantity in brain and nervous tissues
Phospholipases
• group of enzymes that hydrolyse phospholipids
• 4 distinct phospholipases A1, A2, C and D
Glycolipids
• glycosphingolipids are important constituents of cell membrane and nervous tissues (brain)
• cerebrosides - simplest form of glycolipids
• contain a ceramide and 1 more sugars
marielvillanueva
Highlight
• gangliosides - predominantly found in ganglions are the most complex form of glycosphingolipids
• are the derivatives of cerebrosides and contain one or more molecules of N-acetylneuraminic acid (NANA), the most important sialic acid
marielvillanueva
Highlight
Lipoproteins
• molecular complexes of lipids with proteins
• transport vehicles for lipids in the circulation
• 5 types
chylomicrons
VLDL - very low density lipoproteins
LDL - low density lipoproteins
HDL - high density lipoproteins
Free fatty acid - albumin complexes
Example 5.1
The following are some biologically important fatty acids
1. Saturated
Palmitic Acid Stearic Acid
CH3 (CH2)14 (COOH) CH3 (CH2)16 COOH
16:0 18:0
2. In unsaturated fatty acids, the double bond nearly always has the cis conformation
Palmitoleic Acid
CH3 (CH2)5 CH = CH (CH2)7 COOH
16:1∆9
3. In polyunsaturated FA, the double bonds are rarely conjugated
Linoleic Acid
CH3 (CH2)4 CH = CH CH2 CH = CH (CH2)7 COOH
18:2∆9,12
A number notation used widely for indicating the structure of a FA is shown under the names of the FA.
To the left of the colon is shown the number of C atoms in the acid: to the right, the number of double bonds.
Related Documents