NORMAL IRON PHYSIOLOGY NIKHIL GUPTA MBBS - 2011

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
SOURCES OF IRON
Heme iron :-
• Liver
• Meat
• Poultry
• Fish
Non Heme iron:-• Leafy vegetables• Legumes• Beans• Cereals• Milk
DAILY REQUIREMENT
Children (ages 1-10): 7 to 10 mg per day.
Women (ages 19-50): 18 mg per day.
Pregnant Women: 27 mg per day.
Lactating Women: 9 to 10 mg per day.
Men (ages 19 and older): 8 mg per day
DISTRIBUTION
Total body iron = 3 to 5 grams
• 60 to 70 % - Hemoglobin.
• 15 to 30 % - stored in liver and RE system as ferritin and hemosiderin.
• 4 % - Myoglobin.
• 0.1 % - Blood plasma as transferrin.
ROLE OF IRON IN THE BODY
• Hematopoiesis.
• Found in Hemoglobin and myoglobin.
• Cytochrome P450 superfamily and catalase, which metabolize drugs and degrade hydrogen peroxide.
• Conversion of blood sugar to energy.
• Production of enzymes ,new cells, amino acids, hormones and neurotransmitters.
• Proper immune system functioning.
• Physical and mental growth.
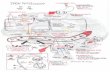
ABSORPTION
• 1-2 mg absorbed daily.
• From duodenum and upper jejunum.
• Heme iron is better absorbed than non heme iron.
• Ferric Iron(III) is reduced to ferrous iron(II) by
D cyt-b (duodenal cytochrome b).
• Taken up through the DMT1 (divalent metal transporter 1) protein.
• Heme iron is taken up through the HemeTransporter.
• Once in the enterocytes, iron is exported through the membrane protein ferroportin 1 into the plasma.
• Some of it can be stored as ferritin ,depending on the current iron requirement of the body.
• Iron(II) in the plasma is immediately oxidised to iron(III) by hephaestin or ceruloplasmin.
• The iron(III) binds to transferrin and is transported with the blood stream to the target cells for utilization.
FACTORS AFFECTING
ABSORPTION
Enhancers:-• Vitamin C• Cooking in iron
vessels• Gastric acid• Cysteine• Sugar• Amino acid• Lactate• Pyruvate
Inhibitors:-• Tannins• Phosphates• Oxalates• Pancreatic secretions• Antacids• Calcium• Tetracyclines
UTILIZATION
• Attachment of iron-transferrin complex to specific Transferrin receptors TfRs on RBCs and other cells.
• Complex engulfed by endocytosis.
• Iron dissociates from complex at acidic pH of endosomes.
• Released iron is utilized.
• Tf and TfR are returned to cell surface to carry fresh loads.
STORAGE
• In tissues-as ferritin & hemosiderin.
• In blood-as transferrin.
• Excess iron in the blood is deposited especially in liver hepatocytes & in the reticulo-endothelial cells of the bone marrow. This may lead to iron toxicity.
EXCRETION
• Daily excretion in adult male = 0.5-1 mg mainly as exfoliated GI mucosal cells , RBCs and in bile.
• Very little in urine and sweat.
• In women, additional menstrual loss of blood may bring iron loss average upto 1.5 mg per day.
FP
Ferroportin
Hc
• Mediated by hepcidin -produced by the liver in response to increased iron availability or stores.
• Hepcidin downregulatesferroportin in enterocytes-blocks iron absorption from the intestine.
REGULATION OF IRON
LEVELS
DEFICIENCY OF IRON
CAUSES-chronic bleeding. 1.excessive menstrual bleeding.2.GIT bleeding (ulcers, hemorrhoids, Ulcerative
Colitis etc.).inadequate intake.substances (in diet or drugs) interfering with
iron absorption.malabsorption syndromes.Inflammation.
SYMPTOMS
anemia
fatigue
dizziness
pallor
hair loss
irritability
weakness
brittle or grooved nails
glossitisGLOSSITIS
BRITTLE GROOVED NAILS
WHEN DOES IRON BECOME A
PROBLEM?
• Normally 3 – 5 g of iron in the body.
• Tissue damage when total body iron is 7 – 15 g.
• 3 commonly encountered forms of chronic overload:
1- Primary haemochromatosis
2- Transfusion-associated haemochromatosis
3- Dietary causes
EFFECTS OF IRON
OVERLOAD
• Cardiac failure
• Liver cirrhosis/fibrosis/cancer
• Diabetes mellitus
• Infertility
• Growth failure
1. Primary Haemochromatosis (chronic iron toxicity)• Excessive absorption of iron from the gut• Iron accumulates in the liver, heart and pancreas &
damages these organs by free radical production• gives the skin a bronze color
Therapy:Phlebotomy (removal of 0.5 l of blood): a decrease of iron in the circulation leads to iron mobilisation from stores
2. Secondary haemochromatosis
• Due to multiple frequent blood transfusions
• in thalassemia major, sickle cell anaemia
Therapy: iron chelators
3. Dietery causes (Acute iron poisoning)
• among people who are exclusively cooking in iron pots
• due to ingestion of iron tablets (15-20) - fatal poisoning in young children.
• Vomiting, diarrhoea, cyanosis, hemetemesis, convulsions, acidosis, shock, death
Therapy: iron chelator-desferoxamine
Related Documents