Innervation and Neurotransmitter Localization in the Lung of the Nile bichir Polypterus bichir bichir GIACOMO ZACCONE,* ANGELA MAUCERI, MARIA MAISANO, ALESSIA GIANNETTO, VINCENZO PARRINO, AND SALVATORE FASULO University of Messina, Department of Animal Biology and Marine Ecology, Faculty of Science, Section of Comparative Neurobiology and Biomonitoring, Messina, Italy ABSTRACT Anatomical and functional studies of the autonomic innervation in the lung of dipnoan fishes and the bichirs are lacking. The present immu- nohistochemical studies demonstrated the presence of nerve fibers in the muscle layers of the lung of the bichir, Polypterus bichir bichir , and iden- tified the immunoreactive elements of this innervation. Tyrosine hydroxy- lase, acetylcholinesterase, and peptide immunoreactivity was detected in the intramural nerve fibers. Extensive innervation was present in the submucosa where adenylatecyclase/activating polypeptide 38, substance P, P 2 X 2 , and 5-hydroxytryptamine (5-HT)–immunoreactive nerve fibers mainly supplied blood vessels. A collection of monopolar neurons located in the submucosal and the muscular layers of the glottis expressed a vari- ety of various transmitters. These neurons may be homologous to gan- glion cells in the branchial and pharyngeal rami of the vagus in fishes. Nerves containing 5-HT and P 2 X 2 receptor immunoreactivity projected to the lung epithelium. Associated with neuroepithelial cells in mucociliated epithelium, were neuronal nitric oxide synthase–immunopositive axons. The physiological function of this innervation is not known. The present study shows that the pattern of autonomic innervation of the bichir lung may by similar in its elements to that in tetrapods. Anat Rec, 290:1166– 1177, 2007. Ó 2007 Wiley-Liss, Inc. Key words: autonomic nerves; NECs; nerve cell bodies; lung; muscle; epithelium; bichir The bichirs (Polypterus spp) belong to the family Poly- pteridae and are placed on an early evolutionary branch within the Actinopterygians. Although they have been assigned to higher level groups of bony fishes, the bichirs are among one of the primitive forms of the jawed verte- brates of Gnathostomes (Noack et al., 1996). The bichirs possess richly vascularized air sacs and develop as pouches of the pharynx and are more primitive than the lungs of dipnoan fishes. They are dual breathers and exchange O 2 and CO 2 in both aerial and aquatic environ- ments. During oxygen lack, the gills are not sufficient for gas exchange. Therefore, the bichirs must inhale air through air-sacs in addition to branchial respiration (Gra- ham, 1997). The dependence of teleost fishes on alterna- tive air-breathing structures is strongly correlated with their adaptative radiation and with the evolutionary ca- nalization of gas bladder structure (Graham, 1997). New structures derived from branchial chambers, which are found in more advanced teleost fishes, differ anatomically and embryologically from the archetypical polypterid lungs. Studies of air-breathing fishes have contributed to our comprehension of the many integrated changes involved in water/air transitions. However, compared with the lungs of dipnoan fishes (Holmgren et al., 1994), which do not receive any innervation from spinal autonomic nerves (Axelsson et al., 1989), the bichirs have developed *Correspondence to: Giacomo Zaccone, University of Messina, Department of Animal Biology and Marine Ecology, Faculty of Science, Section of Comparative Neurobiology and Biomonitor- ing, Via Salita Sperone 31, I-98166 Messina, Italy. Fax: 90-393409. E-mail: [email protected] Received 20 February 2007; Accepted 1 June 2007 DOI 10.1002/ar.20576 Published online in Wiley InterScience (www.interscience.wiley. com). Ó 2007 WILEY-LISS, INC. THE ANATOMICAL RECORD 290:1166–1177 (2007)

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Innervation and NeurotransmitterLocalization in the Lung of the Nile
bichir Polypterus bichir bichirGIACOMO ZACCONE,* ANGELA MAUCERI, MARIA MAISANO,
ALESSIA GIANNETTO, VINCENZO PARRINO, AND SALVATORE FASULOUniversity of Messina, Department of Animal Biology and Marine Ecology, Faculty of
Science, Section of Comparative Neurobiology and Biomonitoring, Messina, Italy
ABSTRACTAnatomical and functional studies of the autonomic innervation in
the lung of dipnoan fishes and the bichirs are lacking. The present immu-nohistochemical studies demonstrated the presence of nerve fibers in themuscle layers of the lung of the bichir, Polypterus bichir bichir, and iden-tified the immunoreactive elements of this innervation. Tyrosine hydroxy-lase, acetylcholinesterase, and peptide immunoreactivity was detected inthe intramural nerve fibers. Extensive innervation was present in thesubmucosa where adenylatecyclase/activating polypeptide 38, substanceP, P2X2, and 5-hydroxytryptamine (5-HT)–immunoreactive nerve fibersmainly supplied blood vessels. A collection of monopolar neurons locatedin the submucosal and the muscular layers of the glottis expressed a vari-ety of various transmitters. These neurons may be homologous to gan-glion cells in the branchial and pharyngeal rami of the vagus in fishes.Nerves containing 5-HT and P2X2 receptor immunoreactivity projected tothe lung epithelium. Associated with neuroepithelial cells in mucociliatedepithelium, were neuronal nitric oxide synthase–immunopositive axons.The physiological function of this innervation is not known. The presentstudy shows that the pattern of autonomic innervation of the bichir lungmay by similar in its elements to that in tetrapods. Anat Rec, 290:1166–1177, 2007. � 2007 Wiley-Liss, Inc.
Key words: autonomic nerves; NECs; nerve cell bodies; lung;muscle; epithelium; bichir
The bichirs (Polypterus spp) belong to the family Poly-pteridae and are placed on an early evolutionary branchwithin the Actinopterygians. Although they have beenassigned to higher level groups of bony fishes, the bichirsare among one of the primitive forms of the jawed verte-brates of Gnathostomes (Noack et al., 1996). The bichirspossess richly vascularized air sacs and develop aspouches of the pharynx and are more primitive than thelungs of dipnoan fishes. They are dual breathers andexchange O2 and CO2 in both aerial and aquatic environ-ments. During oxygen lack, the gills are not sufficient forgas exchange. Therefore, the bichirs must inhale airthrough air-sacs in addition to branchial respiration (Gra-ham, 1997). The dependence of teleost fishes on alterna-tive air-breathing structures is strongly correlated withtheir adaptative radiation and with the evolutionary ca-nalization of gas bladder structure (Graham, 1997). Newstructures derived from branchial chambers, which are
found in more advanced teleost fishes, differ anatomicallyand embryologically from the archetypical polypteridlungs. Studies of air-breathing fishes have contributed toour comprehension of the many integrated changesinvolved in water/air transitions. However, compared withthe lungs of dipnoan fishes (Holmgren et al., 1994), whichdo not receive any innervation from spinal autonomicnerves (Axelsson et al., 1989), the bichirs have developed
*Correspondence to: Giacomo Zaccone, University of Messina,Department of Animal Biology and Marine Ecology, Faculty ofScience, Section of Comparative Neurobiology and Biomonitor-ing, Via Salita Sperone 31, I-98166 Messina, Italy. Fax:90-393409. E-mail: [email protected]
Received 20 February 2007; Accepted 1 June 2007
DOI 10.1002/ar.20576Published online in Wiley InterScience (www.interscience.wiley.com).
� 2007 WILEY-LISS, INC.
THE ANATOMICAL RECORD 290:1166–1177 (2007)
a more complex autonomic pathway due to the presenceof an adrenergic component of intramural innervation(Zaccone et al., 2006c). Occurrence of the ganglion cells isanother aspect of the autonomic nervous system of thebichir (Zaccone et al., 2006a) that is similar to neurons ofthe ganglionic plexuses present in the mammalian respi-ratory tract. Autonomic nerves control vascular resistanceby the release of neuroactive substances such as cathecol-amines and neuropeptides, which act on the smooth mus-cle of blood vessels and play a key role in modulatingblood flow.Gills, accessory respiratory organs, and lungs are
organized in series with the systemic vasculature and,therefore, the entire cardiac output must pass throughthe respiratory circulation (Olson, 1998). The innerva-tion of the airways in mammals resembles that of theupper gastrointestinal tract in that the vagus nerve isthe principal source of the parasympathetic input to thelung and the sympathetic nerves arise from the sympa-thetic trunk. Accumulated morphological, physiological,and pharmacological data indicate that a parasympa-thetic cholinergic innervation results in the contractionof airway smooth muscle and a sympathetic adrenergicoutput to muscle is sparse or absent (Black, 1997).There is growing evidence that nitric oxide (NO) func-
tions as a primary nonadrenergic noncholinergic (NANC)neurotransmitter in the relaxation of lung muscle and in-hibitory responses are also mediated by vasoactive intes-tinal polypeptide (VIP). The lung visceral muscle of Pro-topterus is contracted by acetylcholine (Ach) and vagalnerve stimulation (Abrahamsson et al., 1979), but thereis no evidence of NANC transmission, which is a com-mon feature of lung visceral muscle in Tetrapods (Camp-bell and McLean, 1994). In the respiratory tract of uro-deles, NO is a bioactive substance involved in inhibitoryneurotransmission in the pulmonary nervous system,where it may be colocalized with VIP (Adriaensen et al.,1994).Histochemical studies have localized NO to a discrete
subset of extra- and intramural neurons in primarilyparasympathetic as well as enteric ganglia in mamma-lian vertebrates. Parasympathetic ganglia are foundalong intrapulmonary airways. Neurons of these gangliacontain Ach, as well as various peptides, including VIP,galanin, and substance P (Undem and Myers, 1997). Inthe respiratory tract of several mammalian species, ni-tric oxide synthase (NOS) has been demonstrated in asubpopulation of ganglion cells located in the posteriorwall of the trachea and the extrapulmonary bronchi(Diaz de Rada et al., 1993). Also in these regions various
neuropeptides associated with sensory nerves includingsubstance P have been observed in nerve terminals ofautonomic ganglia (Kummer et al., 1992).By using double-label immunofluorescence methods,
we have investigated the occurrence of peptide and non-peptide trasmitters associated with the autonomicnerves, intrinsic neurons, and ganglionic structuresincluding the presence of neuroepithelial cells, in thelung of the Nile bichir Polypterus bichir bichir.
MATERIALS AND METHODSAnimals and Tissue Preparation
Ten specimens of Polypterus bichir bichir (35–40 cmtotal length, six males and four females) were obtainedfrom a local supplier, maintained in aquaria at 278C,and fed amphibian larvae and goldfish. Maintenanceand killing of the fish used in this study followed theguidelines of animal care and experimentation of Mes-sina University. Adult specimens were anesthesized with0.01% ethyl 3-aminobenzoate methanesulfonate (MS222, Sigma). The ventral glottis with right and left lungwere perfused with 4% paraformaldehyde in 0.1 phos-phate buffer (pH 7.4). Tissue was dissected, degassedand immediately immersed in the same fixative for 2–4hr. Thereafter, all specimens were dehydrated in anascending series of ethanol and routinely embedded inparaplast.
Immunoperoxidase Labeling
Techniques for immunolabeling and immunoperoxi-dase were previously reported for catfish gill and am-phibian epidermal tissues (Zaccone et al., 2003, 2006b).Deparaffinized and rehydrated sections were treatedwith 0.1 M phosphate-buffered saline (PBS, pH 7.4), con-taining 1% bovine serum albumin at room temperature(RT) for 30 min to block endogenous peroxidase and con-secutively incubated with the primary antisera (Table 1)overnight at 48C in a moist chamber. The antigen-anti-body complexes were visualized using goat anti-mouseIgG (1:100; Chemicon, Temecula, CA) or goat anti-rabbitIg peroxidase conjugate (1:100; Sigma). Peroxidase activ-ity was demonstrated by incubation for 50–120 in a solu-tion of 0.015% 3-30-diaminobenzidine in 0.01 M Trisbuffer (pH 7.6), that contained 0.005% H2O2.
Immunofluorescence Labeling
After several rinses in PBS, sections were incubatedfor double immunofluorescence labeling with antisera
TABLE 1. Primary antibodies used
Antigen Animal source Distributor Dilution
Tyrosine hydroxylase (TH) Mouse Sigma, St. Louis (U.S.A.) 1:100neuronal nitric oxide synthase (nNOS) Rabbit Biomol, Milan (Italy) 1:100Acetylcholinesterase (AchE) Mouse Sigma, St. Louis (USA) 1:50P2X2 receptor Rabbit Alomone Labs, Jerusalem, Israel 1:1005-Hydroxytryptamine (5-HT) Mouse DakoCytomation, Milano 1:50Substance P (SP) Rabbit Sigma, St. Louis (USA) 1:50Vasoactive intestinal polypeptide (VIP) Rabbit Biomeda, Milan (Italy) PredilutedAdenylatecyclase/activatingPolypeptide 38 (PACAP)
Rabbit Peninsula Labs. (USA) 1:300
S100 Protein Rabbit Sigma, St. Louis (USA) 1:1005-HT3 receptor Rabbit Sigma, St. Louis (USA) 1:100
1167NEUROTRANSMITTERS IN LUNG OF Bichir P. bichir bichir
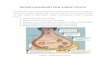
Diagram 1. A,B: Schematic illustration of the bichir lungs and the glot-tis as viewed from the dorsal side (A) and ventral side (B), respectively.A: Disposition of pulmonary branches of vagus rami intestinalis nervesand anatomical location of pulmonary artery are shown. RL, large right
lung; LL, l small left lung; RV, right vagus; LV, left vagus; RP, right pul-monary artery; LP, left pulmonary artery; RPN, right pulmonary nerve;G, glottis; OE, esophagus; S, stomach, I, IntestineB. Opening (OP) ofthe glottis into the right lung is shown. Adapted from Kerr (1907).
1168 ZACCONE ET AL.
against nNOS/AchE, nNOS/5-HT, TH/nNOS, SP/AchE,PACAP/AchE, VIP/AchE, AchE/S100 protein, P2X2/5-HT,5-HT/5-HT3 (concentrations and suppliers as indicatedin Table 1). The distribution of neuronal cell bodies wasevaluated after colabeling with antibodies to both pep-tide and nonpeptide transmitters and the general neuro-nal marker S100. After four rinses in PBS, binding sitesof primary antibodies were visualized by correspondingfluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma) and tetramethylrodamine isothiocya-nate (TRITC)-goat anti-rabbit IgG (Sigma), diluted 1:100for 2 hr at RT.
Fluorescence Microscopy
To document immunofluorescence double labeling, aZeiss Axio Imager Z1 microscope was used. It is inte-grated with AxioVision 4.5 and an AxioCam digital cam-era (Zeiss) was used for image processing. Sections wereimaged using the appropriate filter settings for the exci-tation of FITC (480–525 nm; channel 1) and TRITC(515–590; channel 2). Channel 1 and 2 were coded greenand red, respectively. The colocalization of two markersin the same structure, or an overlap of closely apposedstructures in the same focal plane, resulted in a yellowmixed color. Sections were then taken at the same planeand the two channels were merged. Image processingincluding merging was carried out using AxioVision 4.5(Zeiss) software.
Control Experiments
Negative controls for all immunohistochemical label-ing were performed by substitution of nonimmune serafor the primary or secondary antisera. Specificity of thelabeling of some peptides was verified by incubating sec-tions with antiserum preabsorbed with the respective
antigen (10–100 mg/ml). The preabsorption procedureswere carried out overnight at 48C. The purified antigenswere obtained from Bachem (VIP, PACAP), Biomol-DBA(nNOS), and Sigma (5-HT, SP).
RESULTSAnatomy
The paired lungs of Polypterus bichir bichir extend aslong hollow unchambered sacs. The larger right lungruns along the whole length of the body cavity, whereasthe smaller left lung extends to the stomach (Diagram1). The two lungs arise from a ventral glottis whichopens into the right lung (Fig. 1A,B); the left lung beingconnected to the right by a separate opening. A muscle-ridged slit arranged as a sphincter is observed aroundthe glottis (Diagram 1). Each lung is innervated by abranch of the ramus intestinalis from the vagus nerve.These pulmonary branches reach the lungs on the dorsalsurface and run to the distal end of each lung (Diagram1). The right lung is innervated by the pulmonarybranch of the left ramus intestinalis, whereas the leftlung receives an innervation from a branch of the rightramus intestinalis (Lechleuthner et al., 1989). Pulmo-nary arteries and veins run along the length of the wallof the lungs and branch perpendicular to the axis.A description of sympathetic chains and postgan-
glionic sympathetic fibers connecting with the outflow ofvagus nerve has not been reported in a bichir species.The following account provides evidence for a sympa-thetic adrenergic innervation (see below) by immunohis-tochemical methods.The glottis is lined by ciliated, goblet cells and neuroe-
pithelial cells (NECs), including areas adjacent to thelung opening, whereas the lung itself is characterizedonly by small patches of ciliated cells (see below). The
Fig. 1. A,B: A view of the glottis muscle and the lung ciliated fur-row. A: The glottis-a muscle-ridged slit consisting of smooth musclecells (SM) is seen opening into the right lung. B: A furrow in the lung
epithelium showing presence of mucous goblet cells (g) and ciliatedcells. Flat cells of the respiratory epithelium (Re) are richly vascular-ized. Scale bar 5 20 mm.
1169NEUROTRANSMITTERS IN LUNG OF Bichir P. bichir bichir
internal surface of the lungs is smooth except for a pat-tern of longitudinal striations, called furrows (Fig. 1B).The furrowed epithelium contains a rich concentrationof ciliated cells, Type II pneumocytes (Zaccone et al.,1989), mucous cells, and NECs. Type II pneumocytesoverlie the surface capillary bed and, together with theendothelial cells, form the blood–air barrier. Two layersof striated muscle line the lung walls.Paraganglia within the trunk of the pulmonary vagus
nerves are present. Local ganglion cells are sometimesfound next to large vessels. Nerve cell bodies and nervefibers are also found in submucous localizations in theglottic region and form ganglionic plexuses.
AchE and S100 Protein
Blood vessels in the submucosa were supplied bydense plexuses of AchE- and S100-positive nerve fibers.Nearly all nerve fibers running within the layers of stri-ated muscle lining the lung wall displayed strong AchEimmunoreactivity. Most of these fibers did not co-labelwith S100 (Fig. 2A,B). Many AchE-positive nerve fiberswere seen penetrating the muscle layers of the glottis.Neurons immunoreactive for both AchE and S100 werealso found in nerve fiber bundles within the glottis mus-cle layer (Fig. 2C). Associated with the soma of someneurons were AchE- and S100-positive nerve fibers.
AchE and nNOS
The AchE-nNOS double labeling method does notdemonstrate different nerve fiber populations. Details ofthe double label method revealed that a population ofnerve fibers that approached paraganglion cells showedimmunoreactivity for AchE, but colocalization withnNOS could not be demonstrated. Figure 2D shows thatintravagal paraganglionic cells were surrounded by abasket of AchE-immunopositive nerve fibers. A smallnumber of cells were reactive to AchE and nNOS, andoccasionally found within the outer part of the musclecoat. Thick nerve trunks were located on the externalsurface of the muscle coat exhibiting double immunolab-eling of nNOS and AchE, thus showing that these nervefibers belong to the same population (Fig. 2E). NECswere localized with antibodies against AchE and nNOS(Fig. 2F,I) within the epithelial lining (mucociliated epi-thelium) of the lung where they formed a thin apicalprocess that extended to the airway lumen. AchE-nNOSmerged images show an association of nNOS nerve ter-minals running between the NECs toward their apicalpart (Fig. 2G). There were sparse submucosal nervefibers displaying immunoreactivity for AchE. Some ofthese fibers were in contact with blood vessels (Fig. 2H).A rich supply of AchE-immunoreactive nerve fibersformed a dense network with a circular orientationalong the long axis of the pulmonary artery. These fibersfollowed a conspicuously similar course as the nNOS-immunoreactive nerve fibers, as demonstrated by thecombination of the two channels.
nNOS and 5-HT
Single or small groups of NECs showing nNOS and 5-HT immunoreactivity (Fig. 2I,J) were present in themucociliated epithelium of the lung. NECs were associ-ated with a dense network of nNOS-immunopositive var-
icose nerve fibers running in an apical direction. Doublelabeling with antibodies against nNOS and 5-HT charac-terized the presence of ganglionic plexuses showingnerve cell bodies both in the submucosal and musclelayers of the glottis. The immunohistochemical analysisrevealed the presence of 5-HT in these cells. Paragan-glionic cells were nNOS-immunopositive and surroundedby nerve fibers with a vesicular morphology showing 5-HT immunoreactivity (Fig. 2K). Combinations of bothchannels revealed that few nNOS-immunopositive nervefibers ran in proximity to 5-HT–positive fibers, thusbelonging to separate populations. Thick nerve trunkswere located on the external surface of lung muscle coat.They contain nerve fibers as evidenced by 5-HT immu-noreactivity. 5-HT–immunoreactive nerve fibers werefound in the longitudinal and circular muscle of the lungwall. Numerous 5-HT–immunoreactive nerve fibers werepresent in the adventitia of pulmonary artery wall thatformed dense nerve plexuses.
nNOS and TH
In double-label experiments with TH and nNOS, com-plex neural plexuses in striated muscle are shown in theexternal surface of lung muscle coat. All the immunore-active nerve terminals in between muscle cells label forTH (Fig. 2L). Small paraganglionic cells are seen withinvagal nerves. These cells stain sometimes for TH (Fig.2M,N) and are surrounded by TH-immunoreactive nervefibers which also supply blood vessels associated withthe paraganglia. Thicker nerves showing only immunor-eactivity for TH are found in the muscular layers of theglottis (Fig. 2O) and the submucosal vasculature of thelung epithelium.Double-label immunohistochemistry revealed that NECs
in the mucociliated lung epithelium coexpressed TH andnNOS. Most single NECs formed a thin apical process thatgave a positive immunoreaction for TH and nNOS (Fig.3B). A dense TH-positive perivascular plexus was foundthat surrounded the pulmonary artery (Fig. 3A).
AchE and SP
Both green and red channels revealed that bundles ofnerve fibers both in the outer and inner layers of the mus-cle coat in the lung wall contain SP- and AchE-immuno-positive nerve terminals (Fig. 3C,D). Merged imagesshowed that only few nerve fibers were labeled only withAchE antibodies. Immunocytochemical double labelingdemonstrated plexus of AchE- and SP-immunopositivenerve fibers at the wall of the pulmonary artery. Severalendothelial cells were also found to express SP. NECs inthe furrowed epithelium showed SP immunoreactivity.
AchE and PACAP
Combination of both channels clearly demonstratesthat PACAP and AchE immunoreactivity is in the sub-mucous neurons in the glottic region (Fig. 3E). Globularor ellipsoid-shaped neurons in a nerve fiber bundle inthe smooth muscle layer of the glottis were immunoreac-tive for AchE (Fig. 3G) and PACAP (Fig. 3F). The redchannel (PACAP) shows a perivascular plexus aroundsubmucosal blood vessels and pulmonary artery in thelung and the glottis, but combination of the two channelsrevealed no colocalization with AchE. Intraepithelial
1170 ZACCONE ET AL.
Fig. 2. A–C: Immunohistochemical double staining for acetylcholines-terase (AchE) and S-100 protein in the glottis and lung muscle. A,B: Combi-nation of both channels showing the immunostained cholinergic innervationin the striated lung muscle (arrowheads).C:Combination of the two channelsreveal that nerve terminals in glottic muscle are AChE-immunopositive(arrows) and a group of neurons aligned along the nerve fibers in the musclelayer show the overlap signal (yellow orange). D–I: Immunohistochemicaldouble staining for AchE and neuronal nitric oxide synthase (nNOS) in thelung muscle submucosa, neuroepithelial cells (NECs), and paraganglia. D:Paraganglion cells are surrounded by dense networks of AchE-immunoreac-tive axons (arrowhead). Only a few nitrergic nerve terminals running alongthe cells are seen (arrow). E:Overview of a large nerve trunk (arrows) runningalong outer wall of the lung. A delicate neural network (arrow) is visible show-ing colocalization of AchE and nNOS in the nerves. F: AchE immunoreactivityof a NEC in the mucociliated epithelium with a thin apical process (arrow)facing the airway lumen. G: Combination of green and red channel showingthe nerve fibers running between two NECs in an apical direction. Thesefibers are nNOS-immunoreactive (arrows). The asterisk indicates the slenderprocess of a NEC touching the lumen (lu). H: Green channel showing a sub-
mucosal blood vessel (bv) contacted by a nerve bundle (arrow) composed ofAchE-immunopositive nerve fibers. I: nNOS immunopositivity of a NEC inthe furrowed lung epithelium with an apical process reaching the airwaylumen (lu). J,K: Immunohistochemical double staining for nNOS and 5-HT inthe lung epithelium and paraganglia. J: A 5-HT–immunopositive NEC with aalong process (arrow) facing the airway lumen and 5-HT–positive nervefibers (arrowhead) at the base of the epithelium.K: Combination of the greenand red channel showing the nNOS immunoreactivity of the paraganglioncells (arrowhead), which are surrounded by a basket of 5-HT–immunoposi-tive axons. L–N: Immunohistochemical double staining for nNOS and tyro-sine hydroxylase (TH) in lung and glottis muscle and paraganglia. L: Greenchannel showing a more extensive TH-immunopositive neural plexuses(arrowheads) surrounding the striated muscle (m). No nNOS immunoreactiv-ity is found in these plexuses.M: Green and red channel showing TH-immu-nopositive nerve fibers (arrow) surrounding paraganglion cells (arrowhead)showing nNOS immunoreactivity. N: Green channel of same structuresshowing presence of TH-immunopositive nerve fibers (arrow) around theparaganglion cells. O: A rich network of TH-immunopositive nerve plexuses(arrowheads) in the muscle layers of the glottis. Scale bars5 20 mm.
Fig. 3. A,B: Immunohistochemical staining for neuronal nitric oxidesynthase (nNOS) and tyrosine hydroxylase (TH) in lung epithelium andpulmonary artery. A: Cross-section of pulmonary artery stained withthe double-labeling method nNOS-TH. The TH-immunopositive nervefascicles and fibers form a dense network in the wall of the artery. B:Combination of both channels revealing that a neuroepithelial cell(NEC) in the mucociliated epithelium expresses both nNOS and TH,although the staining intensity varies along the cell cytoplasm; g, mu-cous goblet cells. The arrow indicates its apical process. C,D: Doublelabeling for Acetylcholinesterase (AchE)/substance P (SP) in lung mus-cle. C,D: Green and red channel showing the outer muscle layer con-tacted by AchE (arrowhead) and AchE/SP-expressing nerve fibers. E–H: Double labeling for AchE-adenylatecyclase/activating polypeptide38 (PACAP38) in the nerve cell bodies of the glottis. E: The overlapsignal (yellow orange) reflects colocalization of AchE and PACAP38 inthe nerve cell bodies on the submucosal aspect. ep, epithelium. F:
PACAP38 immunoreactivity of the same neurons. G: AchE immunor-eactivity of the neurons on the submucosal aspect. ep, epithelium.H: Immunoreactivity for AchE showing nerve cell bodies in a nervefiber bundle that is entering the smooth muscle (sm) layer of the glot-tis. I–K: Double labeling for AchE-vasoactive intestinal polypeptide(VIP) in outer muscle and lung submucosa. I: Overview of the lungwith more extensive neural plexuses surrounding outer muscle layer(m), immunostained with antibodies to AchE and VIP. Combination ofboth channels revealing that AchE and VIP immunoreactivities areexpressed by the same nerve fiber populations (arrow). J,K: The greenchannel and the red channels show AchE- and VIP-immunoreactivenerve fibers are observed in contact with submucosal vasculature(bv). L: Immunostaining for ATP receptor P2X2 in the lung epithelium.Red channel reveals P2X2-immunoreactive nerve fibers entering thelung epithelium (arrows) to form intraepithelial terminals facing the air-way lumen (a). Scale bars 5 20 mm.
1172 ZACCONE ET AL.
complexes of PACAP-immunopositive nerve fibers werealso found.
AchE and VIP
VIP immunoreactivity was found in the external mus-cle coat (Fig. 3I), longitudinal and circular musclelayers, and submucosal vasculature. In the striated mus-cle layer, the VIP innervation was pronounced on theexternal surface where nerve trunks were associatedwith lung muscle. Combination of the red and greenchannel showed a direct relationship between the VIPand AchE innervation of this muscle region. AchE- andVIP-immunoreactivity of nerve fibers associated withsubmucosal vessels are shown in Figure 3J,K.
5-HT and P2X2
Labeling of sections processed for P2X2 receptor local-ization with antibodies to 5-HT showed the coexistenceof immunopositive nerve fibers expressing P2X2 purinor-eceptors and 5-HT. These fibers approach the lung epi-thelium, protrude between the epithelial cells, and formintraepithelial terminals (Fig. 3L). Many terminals were
seen close to the epithelial surface. P2X2 and 5-HTimmunoreactivity was demonstrated in submucosalnerves and in nerve fibers in contact with blood vessels.Immunoreactivity against P2X2 and 5-HT was found inthe NECs (Fig. 4A) in the mucociliated lung epithelium,in several monopolar neurons and nerve fibers occurringin the submucosal layers of the glottis (Fig. 4B). Onoccasion, cell bodies located along nerve strands wereseen in these layers (Fig. 4D–F). A high number of neu-rons containing immunoreactivity to P2X2 and 5-HT wasalso present between nerve bundles that enter thesmooth muscle layer of the glottis, and the submucosalplexus but ganglia were usually absent. Serotonergicneurons were seen surrounding pulmonary artery (Fig.4C), but were not shown by P2X2 staining. The 5-HTimmunoreactivity was also demonstrated in nerveplexus around the pulmonary artery.
5-HT and 5-HT3
After double labeling with antibodies against the 5-HT3 receptor and 5-HT, immunoreactivity was observedin the monopolar neurons in glottic submucosal layers
Fig. 4. A–F: Immunohistochemical double staining for 5-hydroxy-tryptamine (5-HT) and P2X2 receptor in the lung epithelium and thenerve cell bodies in the glottis. A: The green channel reveals a 5-HT–immunopositive NEC with a long process reaching the airway lumen.The red channel showing the P2X2 receptor immunoreactivity is uni-form throughout the cell cytoplasm. B: Green and red channels show-ing the presence of two 5-HT and P2X2 receptor-immunopositive glot-tic monopolar neurons in the submucosal layer. C: Neurons (arrows)containing immunoreactivity to 5-HT projecting to pulmonary artery
(a). D,E: Green and red channel demonstrating the localization of 5-HTand P2X2 receptor respectively in some nerve cell bodies in internodalstrands. F: Overlap signal showing 5-HT/P2X2 receptor colocalization.G–I: Immunohistochemical staining for 5-HT and 5-HT3 receptor in thenerve cell bodies of the glottis. G: Monopolar neurons imaged by im-munostaining for 5-HT3 receptor in submucosal plexus adjacent tosmooth muscle. E, epithelium. H,I: Green and red channel demonstrat-ing the presence of 5-HT and 5-HT3 receptor respectively in ellipsoid-shaped neurons underneath the glottic epithelium. Scale bars 5 20 mm.
1173NEUROTRANSMITTERS IN LUNG OF Bichir P. bichir bichir
and at junctions between fiber bundles in the smoothmuscle (Fig. 4G). More aggregated spindle-shapedneurons also occurred underneath ciliated mucous epi-thelium of the glottis (Fig. 4H,I). NECs in the liningmucosa were 5-HT positive, but did not express 5-HT3
receptor immunoreactivity.
DISCUSSION
A general account of the fish autonomic nervous sys-tem (ANS) has been given by Campbell (1970). However,have been no structural or functional studies of theinnervation of visceral and vascular muscles in the lungof the bichirs, which comprise both Polypterus and Erpe-toichthys extant forms. These fishes are non-teleosteanfishes that are among a group of brachiopterygians andare considered to be more primitive than teleosts. How-ever, their systematic position has been questioned, lead-ing to a proposal to include these fishes in a group of tel-eosts of its own (Bjerring, 1985). The present study pro-vides, for the first time, a framework for interpretingthe structure of the ANS in the lung of the Nile bichir,Polypterus bichir bichir. In comparison to the lungs ofdipnoan fishes, there appears to be a complexity of theANS of lung tissue in bichirs associated with a highlydeveloped sympathetic adrenergic system. A vagal inner-vation of the lungs and gill arches in bichirs has onlybeen inferred from anatomical studies (Lechleuthneret al., 1989; Piotrowski and Northcutt, 1996), and thereis no information on the spinal autonomic system orfunctional studies on the role of innervation in polypter-ids. The lungs of the bichirs receive output from bothcranial and spinal autonomic sources, but the location ofthese nerves and their functional significance awaitpharmacological testing. Numerous nerve terminalswere seen running in the striated muscle, whereas theouter muscle was innervated by vagal nerve trunks. Asubmucosal plexus was present and often associatedwith the vasculature, but lacked nerve cell bodies in thelung region. By contrast, numerous globular or ellipsoidmonopolar neurons were present in the submucous andmuscle regions limited to the glottis and pulmonaryartery. A diffuse neuroepithelial cell system was foundin mucociliated epithelium of the furrows in the lungmucosa.Despite physiological studies showing the locations
and effects of neurotransmitters in some fish tissues(Donald, 1998), the role of the ANS in lung tissue ismore difficult to elucidate. Based on immunohistochemi-cal evidence, cholinergic and adrenergic componentscharacterized the lung and glottis intramural innerva-tion and primary systemic vasculature. The pulmonaryartery was innervated by adrenergic, serotonergic,nitrergic and cholinergic nerves. PACAP-, VIP-, 5-HT-,and P2X2-containing nerves were also present near ves-sels in the submucosa. These observations are consistentwith previous studies which have shown in fish vascula-ture the presence of peptidergic nerves (Nilsson andHolmgren, 1992a; Donald, 1998; Zaccone et al., 2006a)and purinoreceptors (Burnstock, 1996). Fish gill vascula-ture is innervated by axons of spinal autonomic originfacilitating adrenergic regulation (Wahlqvist and Nilsson,1981). Furthermore, catecholamine-containing nerves arepresent on the gill afferent filament arteries and venoussinus (Donald, 1998). These nerves are not found on the
pulmonary artery of the lung of dipnoan fishes, but adre-nergic regulation may occur by means of circulating cate-cholamines (Axelsson et al., 1989) released from the chro-maffin tissues in the wall of intercostal arteries and theposterior cardinal veins and the atrium of the heart(Nilsson and Holmgren, 1992b). The dipnoan lung doesnot receive any innervation from spinal autonomic fibers(Parker, 1892; Holmgren et al., 1994). Sympathetic nervesrun to the posterior cardinal veins, while the parasympa-thetic nervous system is limited to the vagal nervesinnervating the heart, gut and lung (Nilsson and Holmg-ren, 1992b).There is no evidence for cholinergic innervation of the
fish systemic vasculature. The lung visceral muscle ofdipnoans is contracted by Ach and vagal nerve stimula-tion (Abrahamsson et al., 1979) and is innervated bycholinergic nerves. A vagal cholinergic, sympathetic, ad-renergic and NANC component, including the serotoner-gic neurons, may be used by pulmonary arteries to con-trol lung blood flow in our bichir species. Future work isneeded to clarify the physiological control of the sympa-thetic and neuropeptide innervation of the pulmonaryartery and changes associated with physiological eventsdue to air breathing. It is possible that the cholinergicinnervation arose in this ancient air-breathing fish wheninitial selective forces for buoyancy, respiration, or both,led to the appearance of the primitive lung-like organ(Graham, 1997), a beautiful example of the plasticity ofthe nervous system (Campbell and McLean, 1994).In a few teleosts and holosteans, including the bichirs
(Lechleuthner et al., 1989), the swimbladder is primarilya respiratory organ analogous to the lung. In most acti-nopterygians with a swimbladder its function is to regu-late buoyancy.No information exists on the control of visceral muscle
of the lung of bichirs in comparison to that in dipnoanfishes. The lung of these fishes is innervated by vagalautonomic nerves, and intrinsic nerve cell bodies arerecognizable within the vagus and on the pulmonary ar-tery of some species.In particular, the lung wall showed a parasympathetic
and sensorimotor innervation (Holmgren et al., 1994). ANANC and cholinergic innervation was not studied,although there is information about the contraction oflung visceral muscle of Protopterus by Ach and vagalnerve stimulation (Nilsson and Holmgren, 1992b). Inthis study, it was found that the lung musculature wasinnervated by cholinergic, NANC, and sensorimotornerve fibers. There is, however, no physiological evi-dence to verify these innervation patterns and to deter-mine whether parasympathetic and sympathetic nervesupplies follow separate paths to the lung. The origin ofadrenergic innervation of lung muscle awaits furtherinvestigation. Further experiments will clarify if thearrangement of sympathetic chains in the bichirs resem-bles that of teleost and ganoid fishes, and if there aresubstantial contributions of spinal autonomic neurons tothe vagi, as reported in the tetrapods. Interestingly,lungfishes lack vagosympathetic nerve trunks, and it issurprising that non-teleostean fishes (for instance, thebichirs) which comprise an extremely heterogeneousgroup and are considered primitive, show relationshipsto the tetrapods. As explained above, we are not surethat the innervation of the bichir lung muscle is homolo-gous with the smooth muscle of fish swimbladder,
1174 ZACCONE ET AL.
although both are primarily concerned with adrenergicand cholinergic nerves (McLean and Nilsson, 1981;Zaccone et al., 2006c) and also show a peptidergic inner-vation (Lundin and Holmgren, 1989; Zaccone et al.,2006a). This finding may be related to the dependence ofteleosts on alternative air-breathing structures, whichare strongly correlated with their adaptative radiationand with evolutionary canalization of gas bladder struc-ture. Therefore, some fish species have respiratory gasbladders differing in complexity or retaining a primitiveorgan trait to support buoyancy (Graham, 1997).Although the ANS in teleosts is more sophisticated
than in elasmobranchs and cyclostomes, there is anincreased level of organization of the branchiomericpathways in mammals compared with fish, and the neu-rochemistry of ganglion cells within the cranial nervesof lower vertebrate species has been overlooked (Gibbins,1994). The present study demonstrates the presence ofparaganglionic cells in the vagal nerve trunks or withinthe lung wall, and sympathetic cholinergic postgan-glionic fibers terminating on the lung muscle. Paragan-glionic cells are also surrounded by a basket of both cho-linergic and adrenergic nerve terminals, suggesting apossible extent of a cephalic sympathetic chain and acontribution of spinal autonomic neurons to the vagusnerve. These cells contain acetylcholine, tyrosine hydrox-ylase as well as substance P, nNOS, and PACAP. It ispossible that adrenergic components in the lung muscleuse NO, 5-HT, or PACAP as possible neuromodulators.These compounds may be, in turn, modulated in theirrelease by the cholinergic innervation whose presence ishistochemically demonstrated in relation with the neuro-peptide-immunoreactive neurons showing a different an-atomical distribution. On the other hand, a further rela-tionship is suggested between NO and the adrenergicand cholinergic components of the intramural innerva-tion because nNOS is shown in those submucous neu-rons of the glottis and paraganglion cells within thetrunk of vagus nerve in which AchE and TH immunor-eactivity is present. It is surprising that there is no evi-dence for adrenergic innervation of the dipnoan lungs(Nilsson and Holmgren, 1992b). Dipnoi may be closelyrelated to ancestors of amphibians (Glass, 1992), whichshow adrenergic nerves in lung muscle (Campbell andMcLean, 1994).A prominent feature of the autonomic nervous system
division of the lung in bichir is the large collection ofmonopolar neurons located in the submucosa and mus-cular layers of the glottis. These neurons express AchE,5-HT, VIP, PACAP 38, SP and 5-HT3 and P2X2 receptors.The coexistence of many peptide and nonpeptide trans-mitters, including receptors, may be species-specific, butthe more complex functional consequence of the combi-nations of these transmitters is not known. Nerve cellbodies are also encountered in the pneumatic duct inthe swimbladder and the gill arch of some teleost species(Lundin and Holmgren, 1989; Bruning et al., 1996; DeGirolamo et al., 1998; Finney et al., 2006), includinglungfishes (see Holmgren et al., 1994). But the precisearrangement and function of the above neurons of vari-ous shapes and sizes, varies within a single species, andin the same region in different species (Gibbins, 1994),thus indicating their involvement in the regulation ofseveral functions, i.e., smooth muscle motility, secretion,and ciliary activity in the lung epithelium and local
blood flow. It is of interest to note that there exists ahomology between vagal innervation of the tetrapodlungs and the various branches of the vagus in fish.Based on the ventral origin of the lungs from the bran-chial arch region of the pharynx, it is suggested that theganglia in the lung are likely to be homologous with thesmall ganglia in the branchial and pharyngeal rami onthe vagus in fish (see for review, Gibbins, 1994). Theganglion cells in the pulmonary rami of the vagus inanurans are monopolar and morphologically identical toother cranial postganglionic neurons. Ganglion cells areusually scattered in the pulmonary rami of the vagus inmammals. Therefore, both vagal paraganglion cells andglottic neurons found in Polypterus may bear a morpho-logical resemblance to genuine postganglionic vagal neu-rons, some of which become incorporated into the entericplexuses of the foregut (Gibbins, 1994). Glottic neurons,both in submucous and muscle localizations, do not showany association with PACAP-, SP-, and 5-HT-immuno-reactive axons and lie in the nerve branches in the glotticregion. Some AchE/S100-positive nerve cell bodies showan array of AchE/S100-imunoreactive axons arrangedaround them. Submucousal neurons express P2X2 and 5-HT3 receptors. This may suggest a possible interrelation-ship between the serotonin secretion and purinergicmechanisms in chemosensory signalling in the foregut ofthe bichir.In addition to the lung muscles of bichir, extensive
innervation of the submucosa is present, and severalnerves contain a variety of neurotransmitters and pro-ject to an epithelium that is endowed with specializedNECs. PACAP-, SP-, P2X2-, and 5-HT-immunopositivefibers are in contact with submucosal arteries. TheNECs are regarded as O2-sensitive cells that have beendescribed in the mammalian carotid body and pulmo-nary epithelium, and in the fish gill (Zaccone et al.,2003, 2006a; Jonz and Nurse, 2006; Saltys et al., 2006).Associated with the apical cytoplasm of NECs are axonsthat form varicosities. The majority contains nNOSalone or in combination with TH or ACh. The origin ofthese distinct populations of axons is unknown. A simi-lar innervation pattern of NECs in the zebrafish gill wasobserved by Jonz and Nurse (2003) and by Saltys et al.(2006) in the NECs of gill tufts of Xenopus larvae, usingother antibodies. Several endocrine systems and epithe-lial tissues are regulated by the activity of ANS in fishes(Donald, 1998), but we do not know the functional roleof NO and adrenalin found in the nerves associated withNECs. The results of the present study may be paral-leled with those from the previous descriptions of NEBsand nitrergic innervation of the rat lung (Brouns et al.,2002). These authors suggest that NO, released from thenerve terminals, might result in a direct inhibition ofthe sensory discharge of NEBs in response to hypoxia.Clearly, further investigations of neural mechanismscontrolling the bichir lung, and oxygen sensing in thelung of bichirs, are warranted. However, we demon-strated for the first time, intraepithelial nitrergic termi-nals selectively related to NECs in the lung of bichir,including the expression of nNOS, SP, P2X2 purinorecep-tors, and nonpeptide transmitters (AchE and TH) bythese cells. These findings may be consistent with thehypothesis of NO, Ach, and adrenalin being used forcommunication between NECs and afferent nerve end-ings. A conspicuous feature is the terminal arborization
1175NEUROTRANSMITTERS IN LUNG OF Bichir P. bichir bichir
of P2X2 receptor-positive nerve fibers in the lung epithe-lial layer. It was, however, seen that these fibers did notapproach NECs. Rat pulmonary NEBs receive a supplyof P2X3 receptor-immunopositive nerve fibers and sen-sory fibers showing a different origin (Brouns et al.,2000). The expression of P2X2 receptors on the lung epi-thelial nerves and NECs in bichir may be intimately cor-related with the vagal afferent ATP-mediated mecha-nisms that have been postulated by Burnstock (1999)and Brouns et al. (2000), indicating NECs as source ofsecretable ATP with local effects on the lung function.We may say that the structural morphology of the
lung tissue of the primitive bichir is very similar to thatof the lungs in tetrapod vertebrates. The saclike lungs,however, do not contain orders of septa like in amphibia(Goniakowska-Witalinska, 2001; Zaccone et al., 2004).Lungs in these fishes play a hydrostatic role in additionto a respiratory function. Bichirs also have the capacityto breathe air using lung wall muscles that activelyforce expiration, which is then followed by passive lungaspiration (Graham, 1997). Both lungs and swimblad-ders have a common evolutionary origin. It is generallyagreed that a respiratory lung, perhaps appearing firstin early jawed vertebrates, was the ancestral organ(Liem, 1988), and Denison (1941) described in the placo-derm Bothriolepsis. The lung was thus regarded as aprimitive fish characteristic present in both sarcoptery-gians and actinopterygians (Liem, 1988). Another theoryholds the evolutionary transition of lung from gas blad-ders (Kerr, 1919) showing a more complex structure dueto the reacquisition of the aerial respiration by theseorgans (Graham, 1997). It seems that the evolutionaryhistory of the actinopterygian lung followed a differentcourse by its gradual transformation from a respiratoryglass bladder into a nonrespiratory physostomous gasbladder, and finally to a physoclistous gas bladder pri-marily specialized for sound reception, buoyancy control,or both (Graham, 1997). In the bichir lung, the gas-exchange area is located on very flat folds containingthe respiratory cells (pneumocytes I), which constitutesthe very thin blood–air barrier (Lechleuthner et al.,1989). The airway cells in the mucociliated epithelium ofthe furrowed epithelium are both ciliated cells, pneumo-cytes II (Lechleuthner et al., 1989), and NECs. All ofthese epithelial cell types are encountered in the lung oftetrapods, but rare in the lung of the polypterids. Lung-fishes contain an elaborate network of septa in theirlungs and have innervated NECs limited to the muco-ciliated epithelium in the pneumatic duct region(Adriaensen et al., 1990). The physiological role of theinnervation patterns of the bichir lungs remains obscureto us, as well as the function of neuroepithelial O2-sensi-tive chemoreceptor cells and regulation of respiration.The bichirs rely predominantly on gill ventilation, but inanoxic water they depend exclusively on pulmonary ven-tilation, unlike dipnoan fishes, which have proper lungs.The origin of respiratory control is in these fishes notknown, but is driven primarily by changes in O2 in tele-ost fishes. Respiratory control in bichirs also raises thequestion of the importance of the modulation of bicar-bonate levels. Recent studies have confirmed commonfeatures of respiratory control in lungfishes to that oftetrapods (Sanchez et al., 2006).We conclude that the different patterns of innervation
and populations of neurons including paraganglia in the
bichir lung are unique features among autonomic path-ways in air-breathing organs of fishes. In contrast todipnoan fishes, several neurotransmitters are involved.The arrangement of the neurons, and their distinctionby their contents of transmitters, may be a rule in theautonomic neurotransmission in fish tissues, althoughautonomic control of the regulation of circulation andrespiration is not fully investigated. In the transitionfrom gill breathing to predominantly air breathing tetra-pods, the neuroepithelial oxygen-sensitive chemoreceptorpathways have undergone extensive changes. It is prob-ably due to the considerable fluctuations in atmosphericoxygen during the geological eras leading to the develop-ment of the lungs of sarcopterygian lungfishes and thebichirs, although a uniform evolutionary pattern couldbe not expected due to their phylogenetic diversity. Itseems, however, that the diversification of bioactive com-pounds and nerve control of the lungs are far less vari-able than the distribution of respiratory chemoreceptorsin air-breathing fishes and tetrapods.
ACKNOWLEDGMENTS
The authors thank Professor Geoffrey Burnstock forencouragement given for the inclusion of purinergic sig-nalling in the present investigation, and ProfessorWolfgang Kummer for helpful discussion on structureand the chemical nature of the paraganglionic cells.
LITERATURE CITED
Abrahamsson T, Holmgren S, Nilsson S, Petterson K. 1979. Adre-nergic and cholinergic effects on the heart, the lung and thespleen of the African lungfish, Protopterus aethiopicus. Acta Phys-iol Scand 107:141–147.
Adriaensen D, Scheuermann DW, Timmermans JP, De-Groodt-Lasseel MHA. 1990. Neuroepithelial endocrine cells in the lung ofthe lungfish Protopterus aethiopicus: an electron and fluores-cente-microscopical investigation. Acta Anat (Basel) 139:70–77.
Adriaensen D, Scheuermann DW, Timmermans JP, Gomi T, De-Groodt-Lasseel MHA. 1994. Neuroepithelial endocrine and nerv-ous system in the respiratory Tract of Cynops pyrrhogaster withspecial reference to the distribution of nitric Oxide synthase andserotonin. Microsc Res Tech 29:79–89.
Axelsson M, Abe MA, Bicudo JEPW, Nilsson S. 1989. On the cardiaccontrol in the South American lungfish, Lepidosiren paradoxa.Comp Biochem Physiol 93:561–565.
Bjerring HC. 1985. Facts and thoughts on piscine phylogeny. In:Foreman RE, Gorbman A, Dodd JM, Olsson R, editors. Evolution-ary biology of primitive fishes. New York: Plenum PublishingCorporation. p 31–57.
Black JL. 1997. Innervation of airway smooth muscle. In: BarnesPJ, editor. Autonomic control of the respiratory system. Amster-dam: Harwood Academic Publishers. p 201–227.
Brouns I, Adriaensen D, Burnstock G, Timmermans JP. 2000. Intra-epithelial vagal sensory nerve terminals in rat pulmonary neuroe-pithelial bodies express P2X3 receptors. Am J Respir Cell Mol Biol23:52–61.
Brouns I, Genechten JV, Scheuermann DW, Timmermans JP,Adriaensen D. 2002. Neuroepithelial bodies: a morphologic sub-strate for the link between neuronal nitric oxide and sensitivityto airway hypoxia. J Comp Neurol 449:343–354.
Burnstock G. 1996. Purinoreceptors: ontogeny and phylogeny. DrugDev Res 39:204–242.
Burnstock G. 1999. Release of vasoactive substances from endothe-lial cells By shear stress and purinergic mechano-sensory trans-duction. J Anat 194:335–343.
1176 ZACCONE ET AL.
Bruning G, Hattwig K, Mayer B. 1996. Nitric oxide synthase in theperipheral nervous system of the goldfish, Carassius auratus.Cell Tissue Res 284:87–98.
Campbell G. 1970. Autonomic nervous system. In: Hoar WS, Ran-dall DJ, editors.Fish physiology. Vol. 4. New York: AcademicPress. p 109–132.
Campbell G, McLean JR. 1994. Lungs and swimbladders. In: Nils-son S, Holmgren S, editors. Comparative physiology and evolutionof the autonomic nervous system. Chur: Harwood Academic Pub-lishers. p 257–309.
Denison RH. 1941. The soft anatomy of Botriolepsis. J Paleontol15:553–561.
De Girolamo P, Arcamone N, Rosica A, Gargiulo G. 1998. PACAP(pituitary adenylate-cyclase activating peptide)- like immunoreac-tivity in the gill arch of the goldfish, Carassis auratus: distribu-tion and comparison with VIP. Cell Tissue Res 293:567–571.
Diaz de Rada O, Villaro AC, Montuenga LM, Martinez A, SpringallDR, Polak JM. 1993. Nitric oxide synthase-immunoreactive neu-rons in human and porcine respiratory tract. Neurosci Lett 162:121–124.
Donald JA. 1998. Autonomic nervous system. In: Evans DH, editor.The physiology of fishes. 2nd ed. Boca Raton: CRC Press. p 407–439.
Finney JL, Robertson GN, McGee CA, Smith FM, Croll RP. 2006.Structure and Autonomic innervation of the swim bladder in thezebrafish (Danio rerio). J Comp Neurol 495:587–606.
Gibbins I. 1994. Comparative anatomy and evolution of the auto-nomic nervous system. In: Nilsson S, Holmgren S, editors. Com-parative physiology and evolution of the autonomic nervous sys-tem. Chur: Harwood Academic Publishers. p 1–67.
Glass ML. 1992. Ventilatory responses to by ipoxia in ectothermicvertebrates. In: Woods SC, Weber RE, Hargens AR, Millard RW,editors. Physiological adaptations in vertebrates. New York:Marcel Dekker, Inc. p 97–113.
Goniakowska-Witalinska L. 2001. Development and ultrastructureof the amphibian lungs. Scanning and transmission electron mi-croscopy study. In: Datta HM, Munshi JSD, editors. Vertebratefunctional morphology. Enfield: Science Publishers. p 241–265.
Graham JB. 1997. Air-breathing fishes: evolution, diversity andadaptation. San Diego: Academic Press. p 1–288.
Holmgren S, Fritsche R, Karila P, Gibbins I, Axelsson M, FranklinC, Grigg G, Nilsson S. 1994. Neuropeptides in the australianlungfish Neoceratodus forsteri: effects in vivo and presence in au-tonomic nerves; Am J Physiol 266:R1568–R1577.
Jonz MG, Nurse CA. 2003. Neuroepithelial cells and associatedinnervation of the zebrafish gill: a confocal immunofluorescencestudy. J Comp Neurol 461:1–17.
Jonz MG, Nurse CA. 2006. Ontogenesis of oxygen chemoreceptionin aquatic vertebrates. Respir Physiol Neurobiol 154:139–152.
Kerr JG. 1907. The development of Polypterus senegalus Cuv. In:Kerr JG, editor. The work of John Samuel Budgett. Cambridge:Cambridge University Press. p 195–290.
Kerr JG. 1919. Textbook of embryology, with exception of mamma-lia. London: Macmillan.
Kummer W, Fischer A, Mundel P, Mayer B, Hoba B, Philippin B,Preissler U. 1992. Nitric oxide synthase in VIP-containing vasodi-lator nerve fibers in the guinea-pig. Neuro Report 3:653–655.
Lechleuthner U, Schumacher U, Negele RD, Welsch U. 1989. Lungsof Polypterus and Erpetoichthys. J Morphol 201:161–178.
Liem KF. 1988. Form and function of lungs: the evolution of air-breathing mechanisms. Am Zool 28:739–759.
Lundin K, Holmgren S. 1989. The occurrence and distribution ofpeptide- or 5-HT-Containing nerves in the swimbladder of fourdifferent species of teleosts (Gadus morhua, Stenolabrus rupest-ris, Anguilla anguilla, Salmo gairdneri). Cell Tissue Res 257:641–647.
McLean JR, Nilsson S. 1981. A histochemical study of the gas glandinnervation of The Atlantic cod, Gadus morhua. Acta Zool(Stockh) 62:187–194.
Nilsson S, Holmgren S. 1992a. Cardiovascular control by purines,5-hydroxy-tryptamine and neuropeptides. In: Randall DJ, FarrellAP, editors. Fish physiology. Vol. 12B.The cardiovascular system.New York: Academic Press. p 301–341.
Nilsson S, Holmgren S. 1992b. Autonomic nerve function and cardi-ovascular control in lungfish. In: Wood SC, Weber RE, HargensAR, Millard RW, editors. Physiological adaptations in vertebrates.New York: Marcel Dekker, Inc. p 377–395.
Noack K, Zardoya R, Meyer A. 1996. The complete mitochondrialDNA sequence of the bichir (Polypterus ornatipinnis), a basal ray-finned fish: ancient establishment of the consensus vertebrategene order. Genetics 144:1165–1180.
Olson KR. 1998. The cardiovascular system. In: Evans DH, editor. Thephysiology of fishes. 2nd ed. Boca Raton: CRC Press. p 129–154.
Parker WN. 1892. On the anatomy and physiology of Protopterusannectens. Trans R Ir Acad 30:109–230.
Piotrowski T, Northcutt RG. 1996. The cranial nerves of the Sene-gal bichir, Polypterus senegalus (Osteichthyes: Actinopterygii:Clastidia). Brain Behav Evol 47:55–102.
Saltys HA, Jonz MG, Nurse CA. 2006. Comparative study of gillneuroepithelial cells and their innervation in teleosts and Xeno-pus tadpoles. Cell Tissue Res 323:1–10.
Sanchez AP, Bassi M, Giusti H, Glass ML. 2006.Respiratory controlin lungfish. Proceedings of the ICRB. Bonn, Germany, August 13–16, 29 p.
Undem BJ, Myers AC. 1997. Autonomic ganglia. In: Barnes PJ, edi-tor. Autonomic control of the respiratory system. Amsterdam:Harwood Academic Publishers. p 87–118.
Wahlqvist I, Nilsson S. 1981. Sympathetic nervous control of thevasculature in the tail of the Atlantic cod, Gadus morhua.J Comp Physiol 144B:153–156.
Zaccone G, Goniakowska-Witalinska L, Lauweryns JM, Fasulo S,Tagliafierro G. 1989. Fine structure and serotonin immunohisto-chemistry of the neuroendocrine cells in the lungs of Polypterusdelhezi and P. ornatipinnis. Basic Appl Histochem 33:277–287.
Zaccone G, Ainis L, Mauceri A, Lo Cascio P, Lo Giudice F, Fasulo S.2003. NANC nerves in the respiratory air sac and branchial vas-culature of the Indian catfish, Heteropneustes fossilis. Acta Histo-chem 105:151–163.
Zaccone G, Mauceri A, Lo Cascio P, Minniti F, Parrino V, Fasulo S.2004. Immunohistochemical study of the innervation of pulmo-nary vessels and Smooth muscles in the respiratory tract of twofrog species. Acta Histochem 106:179–193.
Zaccone G, Mauceri A, Fasulo S. 2006a. Neuropeptides and nitricoxide synthase in the gill and air-breathing organs of fishes. JExp Zool 305A:428–439.
Zaccone G, Mauceri A, Maisano M, Fasulo S. 2006b. Immunolocalisa-tion of nitric oxide synthase isoforms in the epidermis of the tigersalamander, Ambystoma tigrinum. Acta Histochem 108:407–410.
Zaccone G, Mauceri A, Fasulo S. 2006c. Neuropeptides and nitricoxide synthase in the respiratory organs of air-breathing fishes.In: Munshi JSD, Singh HR, editors. Advances in fish research.Vol. 4. New Delhi: Akhil Books Pvt. Ltd. p 111–124.
1177NEUROTRANSMITTERS IN LUNG OF Bichir P. bichir bichir
Related Documents