Olson, Rhonda Bassel-Duby and R. Sanders Williams John Yang, Beverly Rothermel, Rick B. Vega, Norbert Frey, Timothy A. McKinsey, Eric N. and MCIP2 in Striated Muscles Independent Signals Control Expression of the Calcineurin Inhibitory Proteins MCIP1 Print ISSN: 0009-7330. Online ISSN: 1524-4571 Copyright © 2000 American Heart Association, Inc. All rights reserved. is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Circulation Research doi: 10.1161/01.RES.87.12.e61 2000;87:e61-e68 Circ Res. http://circres.ahajournals.org/content/87/12/e61 World Wide Web at: The online version of this article, along with updated information and services, is located on the http://circres.ahajournals.org//subscriptions/ is online at: Circulation Research Information about subscribing to Subscriptions: http://www.lww.com/reprints Information about reprints can be found online at: Reprints: document. Permissions and Rights Question and Answer about this process is available in the located, click Request Permissions in the middle column of the Web page under Services. Further information Editorial Office. Once the online version of the published article for which permission is being requested is can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Circulation Research in Requests for permissions to reproduce figures, tables, or portions of articles originally published Permissions: by guest on July 22, 2014 http://circres.ahajournals.org/ Downloaded from by guest on July 22, 2014 http://circres.ahajournals.org/ Downloaded from

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Olson, Rhonda Bassel-Duby and R. Sanders WilliamsJohn Yang, Beverly Rothermel, Rick B. Vega, Norbert Frey, Timothy A. McKinsey, Eric N.
and MCIP2 in Striated MusclesIndependent Signals Control Expression of the Calcineurin Inhibitory Proteins MCIP1
Print ISSN: 0009-7330. Online ISSN: 1524-4571 Copyright © 2000 American Heart Association, Inc. All rights reserved.is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231Circulation Research
doi: 10.1161/01.RES.87.12.e612000;87:e61-e68Circ Res.
http://circres.ahajournals.org/content/87/12/e61World Wide Web at:
The online version of this article, along with updated information and services, is located on the
http://circres.ahajournals.org//subscriptions/
is online at: Circulation Research Information about subscribing to Subscriptions:
http://www.lww.com/reprints Information about reprints can be found online at: Reprints:
document. Permissions and Rights Question and Answer about this process is available in the
located, click Request Permissions in the middle column of the Web page under Services. Further informationEditorial Office. Once the online version of the published article for which permission is being requested is
can be obtained via RightsLink, a service of the Copyright Clearance Center, not theCirculation Researchin Requests for permissions to reproduce figures, tables, or portions of articles originally publishedPermissions:
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
Independent Signals Control Expression of the CalcineurinInhibitory Proteins MCIP1 and MCIP2 in Striated MusclesJohn Yang, Beverly Rothermel, Rick B. Vega, Norbert Frey, Timothy A. McKinsey, Eric N. Olson,
Rhonda Bassel-Duby, R. Sanders Williams
Abstract—Calcineurin, a calcium/calmodulin-regulated protein phosphatase, modulates gene expression in cardiac andskeletal muscles during development and in remodeling responses such as cardiac hypertrophy that are evoked byenvironmental stresses or disease. Recently, we identified two genes encoding proteins (MCIP1 and MCIP2) that areenriched in striated muscles and that interact with calcineurin to inhibit its enzymatic activity. In the present study, weshow that expression of MCIP1 is regulated by calcineurin activity in hearts of mice with cardiac hypertrophy, as wellas in cultured skeletal myotubes. In contrast, expression of MCIP2 in the heart is not altered by activated calcineurinbut responds to thyroid hormone, which has no effect on MCIP1. A'900-bp intragenic segment located between exons3 and 4 of the MCIP1 gene functions as an alternative promoter that responds to calcineurin. This region includes adense cluster of 15 consensus binding sites for NF-AT transcription factors. Because MCIP proteins can inhibitcalcineurin, these results suggest that MCIP1 participates in a negative feedback circuit to diminish potentiallydeleterious effects of unrestrained calcineurin activity in cardiac and skeletal myocytes. Inhibitory effects of MCIP2 oncalcineurin activity may be pertinent to gene switching events driven by thyroid hormone in striated muscles. The fulltext of this article is available at http://www.circresaha.org.(Circ Res. 2000;87:e61-e68.)
Key Words: calcineurinn hypertrophyn gene transcriptionn thyroid hormone
Changes in intracellular calcium concentrations controlgene expression in many cell types by calmodulin-
dependent activation of calcineurin, a serine-threonine pro-tein phosphatase.1,2 Signaling pathways controlled by cal-cineurin have been most intensively characterized inlymphocytes, where binding of antigen to cell surface recep-tors triggers calcium entry in a pattern that activates cal-cineurin, which then removes phosphate groups from tran-scriptional regulatory proteins of the NF-AT family.Dephosphorylated NF-AT proteins translocate from the cy-toplasm to the nucleus, where they bind cognate recognitionelements within target genes, in association with other tran-scription factors such as AP-1. Downstream genes requiredfor T-cell activation (eg, interleukin-2) are induced in thismanner. The pharmacological actions of immunosuppressivedrugs such as cyclosporin A and FK-506 are based oninhibition of calcineurin activity in immune effector cells.
Recent studies also have revealed roles for calcineurin-dependent signaling pathways in cardiac and skeletal mus-cles. Transgenic mice that express constitutively active formsof calcineurin or NF-AT3 in the heart develop massivecardiac hypertrophy that progresses to dilated cardiomyopa-thy.3 Administration of calcineurin antagonist drugs preventscardiac hypertrophy induced by a calcineurin transgene andalso blocks hypertrophic responses to other stimuli in some,
but not all, models.4–7 In skeletal muscles, calcineurin signal-ing has been implicated in both the hypertrophic response toinsulin-like growth factor-18,9 and the remodeling of myofi-ber phenotypes in response to motor neuron activity.10–13
Calcineurin also stimulates differentiation of myogenic pre-cursor cells,14 and animals lacking NF-AT2 fail to developnormal cardiac valves,15 indicating that calcineurin signalingis pertinent to development of heart and skeletal muscles aswell.
A number of different proteins have been shown to bindto the catalytic subunit of calcineurin (calcineurin A) andto regulate its enzymatic activity. The holoenzyme in-cludes calcineurin A and a regulatory subunit, calcineurinB. This complex is activated upon binding of calcium/calmodulin.16 CHP is a calcineurin B homologue thatinhibits calcineurin activity by hindering the formation ofthe calcineurin/calmodulin/calcineurin B heterotrimer.17
Cyclosporin A and FK506 when bound to their respectivebinding proteins, cyclophilin A and FKBP12, form oligo-meric complexes with calcineurin and inhibit its activity.18
Other proteins act to localize calcineurin within the cell.FKBP12, in the absence of FK506 ligand, anchors cal-cineurin to IP3 and ryanodine receptors,19 and AKAP79 isa scaffolding protein that binds calcineurin, protein kinaseA, and protein kinase C.20 Forced expression of a ubiqui-
Received October 24, 2000; accepted November 9, 2000.From the Departments of Internal Medicine and Molecular Biology, University of Texas Southwestern Medical Center, Dallas, Tex.Correspondence to R. Sanders Williams, University of Texas Southwestern Medical Center, 6000 Harry Hines Blvd, NB11.200, Dallas, TX
75390-8573. E-mail [email protected]© 2000 American Heart Association, Inc.
Circulation Researchis available at http://www.circresaha.org
1
UltraRapid Communication
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
tously expressed calcineurin-binding protein called Cabin/Cain inhibits calcineurin signaling in cultured cells and canprevent hypertrophic responses in rat cardiomyocytes.21–23
We have recently described a family of proteins—MCIP1and MCIP2—that are highly expressed in striated musclesand that inhibit calcineurin through a direct physical interac-tion.24 The human gene encoding MCIP1 resides on chromo-some 21 within the Down syndrome critical region (termedDSCR1) and was shown independently by other laboratoriesto function as an inhibitor of calcineurin.25,26 Two otherhuman genes annotated as ZAKI-4/DSCR1L1 and DSCR1L2encode closely related proteins that we term MCIP2 andMCIP3, respectively.27,28 The MCIP gene family includes ayeast protein Rcn1p capable of inhibiting calcineurin.25 MCIPproteins differ from previously described inhibitors of cal-cineurin in several important respects. Unlike immunophilinand FKBP, no exogenous molecules are required for theability of MCIPs to inhibit calcineurin. MCIP proteins binddirectly to calcineurin A using different binding surfacescompared with the larger AKAP79 or Cabin/Cain proteins.Finally, MCIP1 and MCIP2 are expressed most abundantly instriated muscles, compared with the ubiquitous expression ofAKAP79, Cabin/Cain, and CHP.
In the present study, we report that the genes encodingMCIP1 and MCIP2 are subject to distinctive mechanisms ofregulation. Specifically, expression of MCIP1 is induced bycalcineurin activity, whereas the MCIP2 gene fails to respondto this stimulus. Conversely, MCIP2 expression is regulatedby thyroid hormone, which has no discernible effects onMCIP1. An intragenic region of the MCIP1 gene located 59 toexon 4 contains a dense cluster of 15 NF-AT binding motifswithin a '900-bp segment and functions as an alternativecalcineurin-responsive promoter. These results identify inde-pendent mechanisms by which different MCIP proteins areinduced, presumably to protect the cell from otherwisedeleterious effects of unrestrained calcineurin activity indifferent contexts.
Materials and MethodsPlasmid ConstructionsThe segment of intron 3 from the human MCIP1 (DSCR1) gene wasisolated by polymerase chain reaction (PCR) using human genomicDNA as template and primers based on sequence information fromthe human chromosome 21 databank.29 This '900-bp fragment wassubcloned into a pGL3 luciferase reporter vector (Promega). Otherplasmids were previously described.10,24
Tissue Culture, Cell Transfection or Infection, andReporter Gene AssaysC2C12 myoblasts and myotubes were cultured as previously de-scribed.30 Ionomycin (2 mmol/L) and cyclosporin A (50 to 200nmol/L) were added 4 hours before harvesting the cells. Whenincluded, cycloheximide (25mmol/L) was added 15 minutes beforeionomycin. Transient transfection with plasmids or infection withrecombinant, replication-defective adenoviruses and luciferase as-says were performed as previously reported.10,24,31
Animal ExperimentsLines of transgenic mice in which thea-myosin heavy chainpromoter is used to drive expression of a constitutively active formof calcineurin selectively in the heart were generated and describedpreviously.3 Wild-type male C57Bl/6 mice were injected intraperi-
toneally with 3,5,39-triiodothyromine (T3) (0.1mg/g body weight) oran equal volume of 0.9% saline once a day for 10 days.32 Allexperiments involving animals were conducted using IACRAC-approved protocols.
RNA Isolation and Northern Blot AnalysisTotal RNA was prepared from mouse tissues or C2C12 cells usingTripure (Boehringer Mannheim, Inc) following the manufacturer’sprotocol. Northern blot analysis was performed with 20mg of totalRNA in each lane and probed in Ultrahyb (Ambion) with comple-mentary sequences representing the 39untranslated region (UTR) ofMCIP1 (common to all known splicing variants), exon 1 of MCIP1,exon 4 of MCIP1, or ORF segments of MCIP2 or GAPDH cDNA.Probes were generated by PCR and labeled as described previous-ly.24 Signals from Northern blots were detected on a Storm Phos-phorImager (Molecular Dynamics) and quantified using ImageQuant(version 1.2).
cDNA Microarray Analysis ofCalcineurin-Transgenic MiceRNA was isolated from two calcineurin-transgenic mice3 at 10weeks of age and from a wild-type littermate. One of the transgenicmice was determined to be in heart failure, on the basis of anasarcawith massive ascites, whereas the other appeared grossly normal.Both transgenic mice showed.100% increase in heart weightrelative to the wild-type control (250 and 240 mg versus 96 mg,respectively). Total body weights of the wild-type (26 g) andnonfailing calcineurin transgenic mice (27.5 g) were comparable,whereas the calcineurin-transgenic mouse in heart failure had agreater body weight (35.6 g), reflecting the edematous state. Mi-croarray analyses were conducted by Incyte Genomics as describedelsewhere.33 Briefly, polyA1 RNA was labeled with Cy3/Cy5fluorescent dyes and hybridized with a mouse cDNA microarray(mouse GEM 1.14) containing 8734 elements each (7832 uniquegenes: 3336 annotated/4496 unannotated) genes. Differential expres-sion was calculated as the ratio of fluorescent signals after subtrac-tion of background.
ResultsCalcineurin Induces Expression of MCIP1 butNot MCIP2Gene expression profiling by microarray analysis was con-ducted to identify genes that are differentially regulated inhearts of transgenic mice engineered to express a constitu-tively active form of calcineurin compared with normalcontrols. Calcineurin-transgenic animals (aMHC-CnA*) de-velop massive cardiac hypertrophy that progresses to dilatedcardiomyopathy.3 This analysis identified MCIP1 as a genethat is potently upregulated in this model (Figure 1A). Othergenes known to be controlled by hypertrophic signals (eg,atrial natriuretic factor) also were identified by this analysisand were induced to a comparable extent ('3-fold) as MCIP1in hypertrophic, nonfailing hearts of animals at 10 weeks ofage compared with a wild-type littermate (Figure 1A, top).Both MCIP1 and calcineurin A transcripts were elevatedfurther in hearts of age-matched animals that had progressedto overt heart failure compared with levels noted in hyper-trophic, nonfailing hearts (Figure 1A, bottom). This inductionof MCIP1 gene expression within the intact myocardium byactivated calcineurin was confirmed by Northern blot analy-sis (Figure 1B). Quantitative estimates of the extent ofMCIP1 induction inaMHC-CnA* hearts (Table 1A) variedamong individual animals of comparable age (3- to 17-fold),perhaps reflecting different stages in disease progression.
2 Circulation Research December 8/22, 2000
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
Northern blots (Figure 1B) also revealed that the MCIP2 genewas not similarly regulated. In contrast to the upregulation ofMCIP1 mRNA in this model, there was no detectable changein MCIP2 mRNA (Figure 1B and Table 1A).
We also assessed the ability of calcineurin to stimulateexpression of MCIP1 in C2C12 cells that differentiate intoskeletal myotubes in cell culture. Increased intracellularcalcium concentrations evoked by administration of thecalcium ionophore ionomycin led rapidly (#4 hours) to anincreased abundance of MCIP1 mRNA in C2C12 cells(Figure 2A). MCIP1 mRNA is increased during differentia-tion of C2C12 cells (reported previously,24 not shown here),but a 2-fold induction of MCIP1 by ionomycin was evident
irrespective of the stage of differentiation (myoblasts ormyotubes) and the correspondingly lower or higher initiallevels of MCIP1 mRNA. In contrast, expression of MCIP2mRNA was unaffected by ionomycin (Figure 2A and Table1A). To determine whether the induction of MCIP1 geneexpression by calcium ionophore was attributable to cal-cineurin activity, as opposed to other calcium-regulatedsignaling events, we assessed the effects of cyclosporin A onthis response. In a dose-dependent manner, cyclosporin Ablocked the ability of ionomycin to upregulate MCIP1 tran-script levels in C2C12 cells (Figure 2B) and even reducedMCIP1 transcripts below the levels observed in cells un-treated with either drug, presumably by blocking basal as wellas ionophore-stimulated calcineurin activity. Compared withcells treated with the highest dose of cyclosporin A, MCIP1mRNA was increased 4-fold by ionomycin in the absence ofcyclosporin A (Figure 2B). In addition, MCIP1 mRNA wasincreased 2-fold in cultured myocytes by infection with arecombinant adenoviral vector encoding a constitutively ac-tivated form of calcineurin, compared with the effects ofinfection by a control virus encoding green fluorescent
Figure 1. Differential regulation of MCIP1 and MCIP2 mRNA bycalcineurin in hearts from wild-type and transgenic miceexpressing constitutively active calcineurin (aMHC-CnA*). A,Gene expression profiling by microarray analysis (Incyte Genom-ics). Listed are the 8 genes found to be potently upregulatedduring compensated hypertrophy (top) and in the transition toovert heart failure (bottom) that are characteristic of this model,3along with accession numbers and estimated fold change inmRNA abundance. Calcineurin A is the transgene product inthis model, and upregulation of atrial natriuretic factor geneexpression is a well-defined marker of cardiac hypertrophy.MCIP1 (arrows) is one of the genes most strongly activated inhypertrophic, nonfailing hearts of aMHC-CnA* animals and isupregulated further in hearts of animals that have progressed toovert cardiac failure. B, Northern blot analysis of RNA extractedfrom hearts of wild-type and transgenic mice. Each lane wasloaded with 20 mg total RNA from a single mouse heart, andblots were hybridized with probes complementary to MCIP1,MCIP2, and GAPDH (loading control). This experiment wasrepeated 3 times with comparable results.
Figure 2. Calcineurin-dependent regulation of MCIP1 but notMCIP2 in cultured C2C12 myogenic cells. A, Induction of MCIP1mRNA by a calcium-dependent mechanism. Northern blot anal-ysis of RNA extracted from proliferating, undifferentiated C2C12myoblasts or differentiated myotubes in the presence orabsence of ionomycin (2 mmol/L). B, Calcium-dependent induc-tion of MCIP1 mRNA is blocked by cyclosporin A (CsA), aninhibitor of calcineurin. Northern blot analysis of RNA extractedfrom differentiated C2C12 myotubes in the presence or absenceof ionomycin (2 mmol/L) and increasing doses of CsA (0, 50,100, or 200 nmol/L). C, Calcineurin increases expression ofMCIP1 mRNA. Northern blot analysis of RNA extracted fromdifferentiated C2C12 myotubes after infection with adenoviralvectors expressing a control protein (2) or a constitutively activeform of calcineurin A (Adeno-CnA* [1]). D, Induction of MCIP1mRNA by calcineurin in the presence of cycloheximide (25mmol/L). Each lane was loaded with 20 mg total RNA from a sin-gle culture dish, and blots were hybridized with probes comple-mentary to MCIP1, MCIP2, and GAPDH (loading control). Cyclo-heximide control (lanes 4 and 5) was loaded on a separate gel.Each experiment was repeated 2 to 4 times with comparableresults.
Yang et al Control of MCIP1 and MCIP2 Expression 3
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
protein (Figure 2C). Thus, experimental strategies based onloss-of-function and gain-of-function approaches in culturedmyocytes support the conclusion that MCIP1 gene expres-sion, but not that of MCIP2, is regulated by calcineurinsignaling.
Induction of MCIP1 Expression by CalcineurinDoes Not Require New Protein SynthesisIn the presence of cycloheximide, an inhibitor of proteinsynthesis, activation of calcineurin by ionomycin continuedto upregulate MCIP1 mRNA in C2C12 cells, indicating thatthis induction is not dependent on the generation of newproteins (Figure 2D). The magnitude of ionomycin-stimulated upregulation of MCIP1 in the presence of cyclo-heximide ('10-fold) was greater than that observed whencycloheximide was absent ('2-fold). We interpret these datato support the hypothesis that calcineurin stimulates MCIP1gene transcription by posttranslational modification of apreexisting pool of NF-AT proteins (and possibly othertranscription factors). The greater magnitude of MCIP1induction by calcineurin when new protein synthesis isblocked is potentially attributable to abrogation ofcalcineurin-dependent induction of endogenous MCIP1 syn-thesis, thereby eliminating negative feedback that otherwisewould restrain calcineurin activity.
Thyroid Hormone Induces Expression of MCIP2but Not MCIP1The gene encoding MCIP2 was identified originally in asubtractive cloning experiment designed to identify genesthat are upregulated by thyroid hormone in cultured humanfibroblasts.28 To determine whether MCIP genes are regu-lated by thyroid hormone in hearts of intact animals, hyper-thyroidism was induced in wild-type mice by intraperitonealinjection of T3 for 10 days. As noted previously,32 T3-treatedhearts were uniformly hypertrophic (mean heart weight5180mg versus 130 mg; mean heart weight/body weight ratio57.2
mg/g versus 4.9 mg/g; n54 animals in each group). Incontrast to the effects of activated calcineurin in the murineheart (Figure 1), the expression of MCIP1 was unaltered inhyperthyroid hearts (Figure 3). However, MCIP2 transcriptlevels were increased'2-fold in both heart and soleusskeletal muscles of T3-treated mice (Figure 3 and Table 1B).It remains to be determined whether the effects of T3 are adirect consequence of nuclear receptor binding to regulatoryelements of the MCIP2 gene or a result of indirectmechanisms.
An Intragenic Region Located 5* to Exon 4 of theMCIP1 Gene Is Sufficient to Promote aTranscriptional Response to CalcineurinThe human MCIP1 gene (annotated initially as DSCR1) wasreported to express four variant mRNAs with each of fouralternative exons incorporated selectively at the 59 terminusof the expressed transcripts.34 The majority of these tran-scripts were identified to represent isoforms that include
Quantitative Estimates of Changes in MCIP1 and MCIP2 mRNA in Response to Calcineurin andThyroid Hormone
A, Response to Calcineurin Activation B, Response to Thyroid Hormone
Stimulus Tissue MCIP1 MCIP2 Stimulus Tissue MCIP1 MCIP2
aMHC-CnA* Heart 6.03 1.31 T3 Heart 1.05 1.59
Heart 3.70 0.79 T3 Heart 0.87 1.81
Heart 16.77 1.32 T3 Heart 1.08 1.71
CMV-CnA* C2C12 1.48 ND T3 Soleus 0.78 1.72
C2C12 1.54 0.91 T3 Soleus 1.05 2.21
Ionomycin C2C12 2.42 0.94 T3 Soleus 1.24 1.62
C2C12 1.94 1.00
C2C12 1.26 1.12
CnA Effect z z z 4.8061.30 1.0660.11 T3 Effect z z z 1.0160.06 1.7860.01
Each line presents the fold change induced by the stimulus within a single experimental animal (heart), pooledmuscles from two animals (soleus), or a single-cell culture dish (C2C12), quantified by PhosphorImager analysis ofNorthern blots in duplicate or triplicate, after background subtraction and normalization to a loading control (GAPDHmRNA). The aggregate effects of calcineurin (CnA) and thyroid hormone (T3) are presented as mean values (6SE) ofthese 14 experiments.
ND indicates not determined.
Figure 3. Differential regulation of MCIP1 and MCIP2 mRNA bycalcineurin in striated muscles from hyperthyroid mice. Northernblot analysis of RNA extracted from heart and soleus skeletalmuscle of control and T3-treated mice. Each lane was loadedwith 20 mg total RNA from a single mouse heart or from a poolof both soleus muscles of 2 animals, and blots were hybridizedwith probes complementary to MCIP1, MCIP2, and GAPDH(loading control). This experiment was repeated 2 times withcomparable results.
4 Circulation Research December 8/22, 2000
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
sequences encoded either by exon 1 or exon 4.34 Thesevariants have unique 59UTR regions and encode proteins thatdiffer within the first 29 amino acids. The remaining 168residues of MCIP1, encoded by exons 5 to 7, are identical inall MCIP1 variants (Figure 4A). In our experiments on heartsof transgenic mice, we determined that expression of the exon4 variant of MCIP1 mRNA was particularly sensitive tocalcineurin activity. The increased abundance of MCIP1mRNA detected by a probe complementary to the 39 UTR,which is included within all variants of MCIP1 (Figure 4A),was mirrored by the increase detected with a probe comple-mentary only to unique exon 4 sequences (Figure 4B). Incontrast, MCIP1 transcripts that include exon 1 sequenceswere present only at the limit of detection in wild-type murinehearts and were not induced by the activated calcineurintransgene (not shown).
The selectively increased expression of the exon 4 variantof MCIP1 mRNA suggested the possibility of alternativepromoter use as a function of calcineurin activation, and wesought to determine whether transcriptional regulatory ele-ments involved in transducing this signal reside in proximityto exon 4 of the MCIP1 gene. Accordingly, we isolated a'900-bp genomic segment from this position (2874 to130relative to the first nucleotide of exon 4). This region wasfound to contain a remarkably dense cluster of consensusNF-AT binding motifs (T/AGGAAANA/T/C)35 (Figure 4A).A reporter plasmid was constructed to link this MCIP1genomic region to a luciferase reporter gene (Figure 4C), andthis construct was tested for its ability to respond to cal-cineurin after transfection into C2C12 cells. Like the endog-enous MCIP1 gene, expression of this transgene is increasedby activated calcineurin (Figure 4D), which has no effect on
Figure 4. An intragenic calcineurin response element from the MCIP1 gene. A, Sche-matic representation of the organization of the human MCIP1 (DSCR1) gene, indicat-ing 4 alternative initial exons (E1 through E4) and 3 exons common to all forms ofMCIP1 mRNA (E5 through E7).34 The nucleotide sequence flanking exon 4 is shown toillustrate the presence of 15 consensus binding sites for NF-AT transcription factors(boxes). The first nucleotide of exon 4 is designated as 11. B, Northern blot analysisof RNA extracted from hearts of wild-type and transgenic mice expressing constitu-tively active calcineurin. Each lane was loaded with 20 mg total RNA from a singlemouse heart, and blots were hybridized with probes complementary to exon 4 of theMCIP1 gene or to GAPDH (loading control). A probe complementary to exon 1 of theMCIP1 gene was also tested but yielded a signal of low and equal intensity in wild-type and transgenic hearts (not shown). This experiment was repeated 2 times withcomparable results. C, MCIP1-luciferase reporter plasmids. Plasmids were con-structed to link-defined genomic segments proximal to exon 4 of the human MCIP1gene to a luciferase reporter gene. The numbers of NF-AT consensus binding sitescontained within each segment are shown in parentheses. D, Transient transfectionassays of MCIP1-luciferase reporter plasmids. Results were corrected for variations intransfection efficiency by normalization to expression of a cotransfected pCMV-lacZplasmid. Fold activation was determined relative to the basal activity of the 2874 to130 MCIP1-luciferase reporter construct. Histograms represent mean6SEM val-ues of 3 independent transfections of C2C12 myogenic cells.
Yang et al Control of MCIP1 and MCIP2 Expression 5
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
a control plasmid (minimal TATA plus luciferase; notshown). Inhibition of calcineurin activity by concomitantoverexpression of MCIP1 represses this response (notshown). Luciferase reporter plasmids controlled by shortersegments of this genomic region 59to exon 4 (2231 to130or 2163 to130; Figure 4C) retain basal activity equivalentto the 2874 to 130 segment but progressively lose cal-cineurin responsiveness as the number of NF-AT bindingsites is reduced (Figure 4D).
DiscussionA major finding of this study is that the genes encoding thecalcineurin-interacting proteins MCIP1 and MCIP2 are reg-ulated selectively in skeletal and cardiac myocytes by cal-cineurin and thyroid hormone, respectively. Both genes areexpressed in striated myocytes, and both proteins are capableof inhibiting the enzymatic activity of calcineurin. However,only MCIP1 is induced by calcineurin and only MCIP2 bythyroid hormone. Regulatory responses of this nature havenot been reported for genes encoding other proteins (Cabin/Cain, CHP) that function as endogenous inhibitors ofcalcineurin.
The induction of MCIP1 expression by calcineurin instriated myocytes is rapid and robust. An increased abun-dance of MCIP1 mRNA is detected within 4 hours afteractivation of calcineurin by calcium influx into culturedC2C12 myoblasts or myotubes. A microarray analysis capa-ble of screening 7832 independent genes identified MCIP1 asone of the genes most markedly upregulated in hearts oftransgenic mice engineered to express a constitutively activeform of calcineurin. Among several variants of MCIP1mRNA that arise by alternative promoter use and/or alterna-tive splicing, transcripts including sequences encoded byexon 4 were found to be induced by calcineurin. Thisresponse was recapitulated by a plasmid construct that linkeda '900-bp intragenic region located 59 to exon 4 to aluciferase reporter gene. It is possible that other regions of theMCIP1 gene contribute to its transcriptional regulation, butthe exceptionally dense clustering of NF-AT binding motifsupstream of exon 4 is likely to mediate the potent response tocalcineurin signaling. Serial deletions of this promoter regionlose responsiveness to calcineurin in proportion to the num-ber of NF-AT binding sites that are removed.
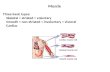
These findings with respect to MCIP1 gene regulation bycalcineurin, in concert with the ability of MCIP1 to inhibit theenzymatic activity of calcineurin,24 support the concept thatMCIP1 functions in a negative feedback circuit (Figure 5) tolimit potentially deleterious consequences of otherwise un-hindered calcineurin signaling, such as apoptosis.36 Ourexperiments using cycloheximide show that new proteinsynthesis is not required for induction of MCIP1 transcriptionby calcineurin, in keeping with a mechanism based onposttranslational modification of NF-AT (Figure 5). More-over, the magnitude of MCIP1 induction by calcineurin isincreased in the presence of cycloheximide, as would bepredicted if newly synthesized MCIP1 negatively regulatescalcineurin activity. Findings recently reported by otherlaboratories25,26also are consistent with the notion of negativefeedback on calcineurin by MCIP proteins. It remains to be
determined, however, whether inhibition of calcineurin is thesole function of MCIP1 in mammalian cells. The MCIPorthologue of Saccharomyces cerevisiae(Rcn1p) inhibitscalcineurin signaling at high concentrations, but calcineurinsignaling is impaired if Rcn1p is absent. This latter observa-tion suggests that basal levels of Rcn1p function in somemanner to facilitate calcineurin signaling. Loss-of-functionexperiments based on targeted disruption of each of the threeMCIP genes will be critical to determine whether this is alsotrue for MCIP proteins in mammalian cells.
The responsiveness of endogenous MCIP1 gene expressionto calcineurin activity in cultured cells and in tissues of intactanimals, and the ability of the exon 4 flanking region torecapitulate this response when placed into a reporter plas-mid, potentially can be exploited as sensitive indicators ofcalcineurin activity. As noted in recent reviews,37 currentmethods used to assess the activation state of calcineurin invivo are subject to problematical artifacts. It may be possibleto redress this deficiency by designing assays based on thepeculiar responsiveness of MCIP1 to calcineurin activation.Such assays could aid in the interpretation of experiments thatseek to modify calcineurin signaling in animal models or beused in high-throughput screens for discovery of new chem-ical agents that alter calcineurin signaling.
The ability of T3 to induce expression of MCIP2 inmammalian striated muscles was anticipated on the basis ofexperiments in which a cDNA encoded by the humanZAKI-4 gene (here termed MCIP2) was identified in a screenfor transcripts upregulated by treatment of human fibroblastswith thyroid hormone.28 In the present study, we confirm that
Figure 5. Negative feedback loop for regulation of calcineurinactivity. Evidence presented in the present study identifiesMCIP1 as a target for positive transcriptional regulation by cal-cineurin in heart and skeletal muscles, probably as a direct con-sequence of NF-AT binding to a calcineurin-responsive region inproximity to exon 4 of the MCIP1 gene. Because MCIP1 iscapable of inhibiting the enzymatic activity of calcineurin,24 it islikely that this regulatory circuit serves to inhibit calcineurinactivity under conditions of sustained stimulation by calcium/calmodulin, thereby to protect cells from deleterious effects ofunrestrained calcineurin activation.
6 Circulation Research December 8/22, 2000
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
this response occurs in skeletal and cardiac muscles, and weprovide new information to show that such regulation by T3does not extend to the MCIP1 gene. In addition, we demon-strate that, unlike MCIP1, MCIP2 is not subject to regulationby calcineurin signaling. It will be important in future studiesto ascertain whether the induction of MCIP2 by thyroidhormone and the ensuing inhibition of calcineurin activitythat should result from this response are pertinent to any ofthe consequences of hyperthyroidism that affect skeletal andcardiac muscles. For example, inhibition of calcineurin ac-tivity in skeletal muscles by cyclosporin A promotes trans-formation of slow myofibers to the fast fiber phenotype.10,13
Excess T3 can induce a similar transformation of myofibersubtypes, as well as myosin isoform switching in theheart.38,39 It is plausible to propose that T3-induced accumu-lation of the calcineurin inhibitory protein MCIP2 maycontribute to these effects.
Our observation that, unlike MCIP2, expression of MCIP1is not induced in hearts of thyrotoxic mice has additionalimplications. Unchanged levels of MCIP1 mRNA in hyper-trophic hearts of T3-treated animals suggests that the induc-tion of MCIP1 produced by expression of an activatedcalcineurin transgene is a direct consequence of calcineurinactivity, rather than a uniform feature of all forms of cardiachypertrophy. Moreover, on the premise that expression ofMCIP1 provides an indicator of the state of activation of thecalcineurin signaling pathway, normal levels of MCIP1 inhypertrophic hearts of T3-treated animals can be taken asevidence that calcineurin is less pertinent to the mechanismsof cardiac hypertrophy driven by T3, compared with otherhypertrophic stimuli.40 In future studies, measurements ofMCIP1 mRNA concentrations and studies of the effects offorced expression of MCIP1 in many different models ofcardiac hypertrophy may help to distinguish calcineurin-dependent from calcineurin-independent pathways that con-trol cardiac mass.
Calcineurin plays an important biological role in manydifferent types of cells and tissues. Activating signals arisingfrom calcineurin are directed to a large and diverse set oftarget genes, distinctive subsets of which are selected indifferent cell types and on the basis of the parallel activationof other signaling pathways. It is not surprising that such apotent signaling molecule as calcineurin, subject to activationby a great diversity of primary stimuli that generate appro-priate waveforms of intracellular calcium, is also subject tonegative regulation by a diversity of inhibitory processes.Because of their prominent expression in cardiac and skeletalmuscles, MCIP proteins are of particular interest in thisregard. The distinctive responses of genes encoding MCIP1and MCIP2 to different regulatory stimuli reveal an addi-tional level of complexity with respect to our understandingof calcineurin-dependent signaling in mammalian cells. Fur-ther studies of MCIP proteins may lead to the development ofnew measures to modulate calcineurin activity selectively incardiac or skeletal muscles for experimental, and possiblyclinical, purposes.
AcknowledgmentsThis work was supported by grants from the National Institutes of
Health (NIH) (to E.N.O and R.S.W.), the D.W. Reynolds Foundation
(to E.N.O and R.S.W.), the Robert A. Welch Foundation (to E.N.O.),and Myogen, Inc (to E.N.O.). N.F. was supported by a fellowship ofthe Deutsche Forschungsgemeinschaft. T.A.M. is a Pfizer Fellow ofthe Life Sciences Research Foundation. J.Y. and R.V. were sup-ported by an NIH training grant. We are grateful to John Shelton forpreparing images of primary data for publication.
References1. Olson EN, Williams RS. Calcineurin signaling and muscle remodeling.
Cell. 2000;101:689–692.2. Crabtree GR. Generic signals and specific outcomes: signaling through
Ca21, calcineurin, and NF-AT.Cell. 1999;96:611–614.3. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J,
Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway forcardiac hypertrophy.Cell. 1998;93:215–228.
4. Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, ColbertMC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiachypertrophy in mice by calcineurin inhibition.Science. 1998;281:1690–1693.
5. Zhang W, Kowal RC, Rusnak F, Sikkink RA, Olson EN, Victor RG.Failure of calcineurin inhibitors to prevent pressure-overload left ven-tricular hypertrophy in rats.Circ Res. 1999;84:722–728.
6. Ding B, Price RL, Borg TK, Weinberg EO, Halloran PF, Lorell BH.Pressure overload induces severe hypertrophy in mice treated with cyclo-sporine, an inhibitor of calcineurin.Circ Res. 1999;84:729–734.
7. Chien KR. Meeting Koch’s postulates for calcium signaling in cardiachypertrophy.J Clin Invest. 2000;105:1339–1342.
8. Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1induces skeletal myocyte hypertrophy through calcineurin in associationwith GATA-2 and NF-ATc1.Nature. 1999;400:581–585.
9. Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, HarveyRP, Graham RM. Skeletal muscle hypertrophy is mediated by a Ca21-dependent calcineurin signalling pathway.Nature. 1999;400:576–581.
10. Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM,Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependenttranscriptional pathway controls skeletal muscle fiber type.Genes Dev.1998;12:2499–2509.
11. Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN.Stimulation of slow skeletal muscle fiber gene expression by calcineurinin vivo. J Biol Chem. 2000;275:4545–4548.
12. Dunn SE, Burns JL, Michel RN. Calcineurin is required for skeletalmuscle hypertrophy.J Biol Chem. 1999;274:21908–21912.
13. Bigard X, Sanchez H, Zoll J, Mateo P, Rousseau V, Veksler V, Ventura-Clapier R. Calcineurin co-regulates contractile and metabolic componentsof slow muscle phenotype.J Biol Chem. 2000;275:19653–19660.
14. Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required forthe initiation of skeletal muscle differentiation.J Cell Biol. 2000;149:657–666.
15. Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC,Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factorNF-ATc is essential for cardiac valve formation.Nature. 1998;392:186–190.
16. Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulatedprotein phosphatase, calcineurin.J Biol Chem. 1998;273:13367–13370.
17. Lin X, Sikkink RA, Rusnak F, Barber DL. Inhibition of calcineurinphosphatase activity by a calcineurin B homologous protein.J Biol Chem.1999;274:36125–36131.
18. Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belshaw PJ, CohenP, MacKintosh C, Klee CB, Schreiber SL. Inhibition of T cell signalingby immunophilin-ligand complexes correlates with loss of calcineurinphosphatase activity.Biochemistry. 1992;31:3896–38901.
19. Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH.Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca21 flux. Cell. 1995;83:463–472.
20. Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD.Coordination of three signaling enzymes by AKAP79, a mammalianscaffold protein.Science. 1996;271:1589–1592.
21. Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition ofcalcineurin prevents agonist-induced cardiomyocyte hypertrophy.ProcNatl Acad Sci U S A. 2000;97:1196–1201.
22. Lai MM, Burnett PE, Wolosker H, Blackshaw S, Snyder SH. Cain, anovel physiologic protein inhibitor of calcineurin.J Biol Chem. 1998;273:18325–18331.
Yang et al Control of MCIP1 and MCIP2 Expression 7
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
23. Sun L, Youn HD, Loh C, Stolow M, He W, Liu JO. Cabin 1, a negativeregulator for calcineurin signaling in T lymphocytes.Immunity. 1998;8:703–711.
24. Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. Aprotein encoded within the Down syndrome critical region is enriched instriated muscles and inhibits calcineurin signaling.J Biol Chem. 2000;275:8719–8725.
25. Kingsbury TJ, Cunningham KW. A conserved family of calcineurinregulators.Genes Dev. 2000;14:1595–1604.
26. Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M,Estivill X, Luna S. DSCR1, overexpressed in Down syndrome, is aninhibitor of calcineurin-mediated signaling pathways.Hum Mol Genet.2000;9:1681–1690.
27. Strippoli P, Lenzi L, Petrini M, Carinci P, Zannotti M. A new gene familyincluding DSCR1 (Down Syndrome Candidate Region 1) and ZAKI-4:characterization from yeast to human and identification of DSCR1-like 2,a novel human member (DSCR1L2).Genomics. 2000;64:252–263.
28. Miyazaki T, Kanou Y, Murata Y, Ohmori S, Niwa T, Maeda K,Yamamura H, Seo H. Molecular cloning of a novel thyroid hormone-responsive gene, ZAKI-4, in human skin fibroblasts.J Biol Chem. 1996;271:14567–14571.
29. Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS, ToyodaA, Ishii K, Totoki Y, Choi DK, Soeda E, Ohki M, Takagi T, Sakaki Y,Taudien S, Blechschmidt K, Polley A, Menzel U, Delabar J, Kumpf K,Lehmann R, Patterson D, Reichwald K, Rump A, Schillhabel M, Schudy A.The DNA sequence of human chromosome 21. The chromosome 21mapping and sequencing consortium.Nature. 2000;405:311–319.
30. Grayson J, Williams RS, Yu YT, Bassel-Duby R. Synergistic interactionsbetween heterologous upstream activation elements and specific TATAsequences in a muscle-specific promoter.Mol Cell Biol. 1995;15:1870–1878.
31. Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, SimardAR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2responds to multiple calcium-regulated signals in the control of skeletalmuscle fiber type.EMBO J. 2000;19:1963–1973.
32. Robbins RJ, Swain JL. C-myc protooncogene modulates cardiachypertrophic growth in transgenic mice.Am J Physiol. 1992;262:H590 –H597.
33. Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallelhuman genome analysis: microarray-based expression monitoring of1000 genes.Proc Natl Acad Sci U S A. 1996;93:10614–10619.
34. Fuentes JJ, Pritchard MA, Estivill X. Genomic organization, alternativesplicing, and expression patterns of the DSCR1 (Down syndrome can-didate region 1) gene.Genomics. 1997;44:358–361.
35. Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family:regulation and function.Annu Rev Immunol. 1997;15:707–747.
36. Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F,McKeon F, Bobo T, Franke TF, Reed JC. Ca21-induced apoptosis throughcalcineurin dephosphorylation of BAD.Science. 1999;284:339–343.
37. Aramburu J, Rao A, Klee CB. Calcineurin, from structure to function.Curr Top Cell Regul. 2000;36:237–295.
38. Caiozzo VJ, Baker MJ, McCue SA, Baldwin KM. Single-fiber and wholemuscle analyses of MHC isoform plasticity: interaction between T3 andunloading.Am J Physiol. 1997;273:C944–C952.
39. Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC mul-tigene family respond to thyroid hormone in a highly tissue-specificmanner.Science. 1986;231:597–600.
40. Bugaisky L, Zak R. Biological mechanisms of hypertrophy. In: FozzardH, Haber E, Jennings R, Katz A, Morgan H, eds.The Heart and Car-diovascular System-Scientific Foundation. New York, NY: Raven Press;1986:1412–1506.
8 Circulation Research December 8/22, 2000
by guest on July 22, 2014http://circres.ahajournals.org/Downloaded from
Related Documents