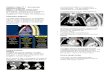
Introduction This paper reviews the common spectrum of disorders of the neonatal chest. Emphasis is on radiographic changes that have been produced by the introduction of new ther- apeutic maneuvers, particularly the use of artificial sur- factant in treating hyaline membrane disease and the sur- vival of profoundly premature newborns (less than 650 grams). A discussion of meconium aspiration syndrome, neonatal pneumonia, transient tachypnea of the newborn, congenital lymphangiectasia and congenital heart disease is also included. The effect on the neonatal chest radi- ograph of extracorporeal membrane oxygenation and high frequency ventilation are also mentioned [1]. A re- view of ‘surgical’ lesions is also included [2]. Although cross-sectional and ultrasound imaging have specific but limited roles in assessing the newborn chest, the standard chest radiograph remains the most common imaging tool. Diseases that affect the neonate’s chest sig- nificantly overlap in their radiographic and clinical ap- pearances. Therefore, an open exchange of information between the neonatologist and the radiologist is critical to the intelligent interpretation of these images. The main disease entities of concern are hyaline mem- brane disease (HMD), chronic lung disease (BPD), meco- nium aspiration syndrome (MAS), neonatal pneumonia, and transient tachypnea of the newborn (TTN). Congenital lymphangiectasia may mimic the findings of MAS or neonatal pneumonia. Congenital heart disease may be pre- sent in a fashion that is easily confused with a pulmonary parenchymal process. This is most common when the heart remains normal in size, but there is pulmonary venous hy- pertension. Specific infective organisms, such as Chlamydia trachomatis or viruses, that are acquired during or follow- ing birth may be present within the neonatal period, with clinical and radiographic findings that are easily confused with one or several of the above-mentioned entities. Hyaline Membrane Disease Hyaline membrane disease is a manifestation of pul- monary immaturity, seen predominantly in children less than 36-38 weeks of gestational age and weighing less than 2.5 kg. HMD remains the leading cause of death in live-born infants. The death rate is highest in the most premature infants, with few deaths occurring in infants weighing more than 1.5 kg. Males are affected almost twice as often as females. HMD is more common in whites than in blacks. The disease results from a defi- ciency of surfactant in the lungs, which leads to the in- ability to maintain acinar distention. Exposure to air rapidly leads to the development of hyaline membranes containing fibrin and cellular debris. There frequently is epithelial necrosis beneath the hyaline membranes. By the second day of life, repair has begun as evidenced by the proliferation of type 2 pneumocytes (which produce surfactant) and increased secretions. The lungs are almost impossible to inflate and on gross inspection appear sim- ilar to liver. Clinically, the infants are usually sympto- matic within minutes of birth, with grunting, nasal flar- ing, retractions, tachypnea, and cyanosis; however, it may be a few hours before symptoms are recognized. Although the initial radiographic findings may be noted minutes after birth, occasionally the maximum radi- ographic findings are not present until 12-24 h of life. The rigid, noncompliant lungs and the associated hy- poxia and acidosis often result in persistent pulmonary hypertension. As pulmonary resistance decreases, there may be the onset of left to right shunting across a patent ductus arteriosus. This may be recognized radiographi- cally before clinical symptoms or a murmur may devel- op. Shunting is heralded by the development of pul- monary edema and a suddenly enlarging heart size. The ductus arteriosus can only respond to stimuli to close if it is term or near term in development. These stimuli in- clude a normal oxygen level, a normal pH, and normal levels of prostaglandin E1 and E2. In the premature new- born with HMD, the ductus is open, but the pulmonary and systemic pressures may be equal or the pulmonary pressure may exceed the systemic pressure, precluding left to right shunting. These babies are usually hypoxic and acidotic with high levels of prostaglandin E1 and E2. By several days of life, as the pulmonary resistance drops, if the ductus is not responsive or the stimuli are not present, the ductus arteriosus will remain open allowing IDKD 2007 Imaging of the Newborn Chest R. Cleveland 1 , V. Donoghue 2 1 Radiology Department, Children’s Hospital, Harvard Medical School, Boston, MA, USA 2 Radiology Department, Children’s University Hospital, Dublin, Ireland

Imaging of the Newborn Chest
Feb 09, 2023
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Introduction
This paper reviews the common spectrum of disorders of the neonatal chest. Emphasis is on radiographic changes that have been produced by the introduction of new ther- apeutic maneuvers, particularly the use of artificial sur- factant in treating hyaline membrane disease and the sur- vival of profoundly premature newborns (less than 650 grams). A discussion of meconium aspiration syndrome, neonatal pneumonia, transient tachypnea of the newborn, congenital lymphangiectasia and congenital heart disease is also included. The effect on the neonatal chest radi- ograph of extracorporeal membrane oxygenation and high frequency ventilation are also mentioned [1]. A re- view of ‘surgical’ lesions is also included [2].
Although cross-sectional and ultrasound imaging have specific but limited roles in assessing the newborn chest, the standard chest radiograph remains the most common imaging tool. Diseases that affect the neonate’s chest sig- nificantly overlap in their radiographic and clinical ap- pearances. Therefore, an open exchange of information between the neonatologist and the radiologist is critical to the intelligent interpretation of these images.
The main disease entities of concern are hyaline mem- brane disease (HMD), chronic lung disease (BPD), meco- nium aspiration syndrome (MAS), neonatal pneumonia, and transient tachypnea of the newborn (TTN). Congenital lymphangiectasia may mimic the findings of MAS or neonatal pneumonia. Congenital heart disease may be pre- sent in a fashion that is easily confused with a pulmonary parenchymal process. This is most common when the heart remains normal in size, but there is pulmonary venous hy- pertension. Specific infective organisms, such as Chlamydia trachomatis or viruses, that are acquired during or follow- ing birth may be present within the neonatal period, with clinical and radiographic findings that are easily confused with one or several of the above-mentioned entities.
Hyaline Membrane Disease
Hyaline membrane disease is a manifestation of pul- monary immaturity, seen predominantly in children less
than 36-38 weeks of gestational age and weighing less than 2.5 kg. HMD remains the leading cause of death in live-born infants. The death rate is highest in the most premature infants, with few deaths occurring in infants weighing more than 1.5 kg. Males are affected almost twice as often as females. HMD is more common in whites than in blacks. The disease results from a defi- ciency of surfactant in the lungs, which leads to the in- ability to maintain acinar distention. Exposure to air rapidly leads to the development of hyaline membranes containing fibrin and cellular debris. There frequently is epithelial necrosis beneath the hyaline membranes. By the second day of life, repair has begun as evidenced by the proliferation of type 2 pneumocytes (which produce surfactant) and increased secretions. The lungs are almost impossible to inflate and on gross inspection appear sim- ilar to liver. Clinically, the infants are usually sympto- matic within minutes of birth, with grunting, nasal flar- ing, retractions, tachypnea, and cyanosis; however, it may be a few hours before symptoms are recognized. Although the initial radiographic findings may be noted minutes after birth, occasionally the maximum radi- ographic findings are not present until 12-24 h of life.
The rigid, noncompliant lungs and the associated hy- poxia and acidosis often result in persistent pulmonary hypertension. As pulmonary resistance decreases, there may be the onset of left to right shunting across a patent ductus arteriosus. This may be recognized radiographi- cally before clinical symptoms or a murmur may devel- op. Shunting is heralded by the development of pul- monary edema and a suddenly enlarging heart size. The ductus arteriosus can only respond to stimuli to close if it is term or near term in development. These stimuli in- clude a normal oxygen level, a normal pH, and normal levels of prostaglandin E1 and E2. In the premature new- born with HMD, the ductus is open, but the pulmonary and systemic pressures may be equal or the pulmonary pressure may exceed the systemic pressure, precluding left to right shunting. These babies are usually hypoxic and acidotic with high levels of prostaglandin E1 and E2. By several days of life, as the pulmonary resistance drops, if the ductus is not responsive or the stimuli are not present, the ductus arteriosus will remain open allowing
IDKD 2007
R. Cleveland1, V. Donoghue2
1 Radiology Department, Children’s Hospital, Harvard Medical School, Boston, MA, USA 2 Radiology Department, Children’s University Hospital, Dublin, Ireland
055_062_Donoghue_Cleveland 28-02-2007 07:16 Pagina 55
left to right shunting and the development of congestive heart failure. Treatment with indomethacin (by inhibiting production of prostaglandin) may produce ductal closure but at the risk of inducing gastrointestinal perforation and/or osteoarthropathy. Surgery may be required to close a persistent patent ductus arteriosus.
Sudden diffuse opacification of the lungs in HMD may be seen with other conditions. A frequent cause is de- creasing ventilatory settings, including inspiratory pres- sure, PEEP, or rate. Less commonly, diffuse pulmonary hemorrhage is a cause. Sudden catastrophic intracranial hemorrhage may produce central nervous system (CNS)- mediated edema.
Since Northway’s original description of bronchopul- monary dysplasia (BPD), in 1967 [3], many changes have occurred in the management and outcome of children with HMD. Much has been learned about the specific toxic effects of oxygen and assisted ventilation. Babies of much lower gestational age and birth weight are surviv- ing with BPD, particularly since the advent of high-fre- quency ventilation and the use of artificial surfactant. The likelihood of developing BPD or retinopathy of prematu- rity and the severity of these conditions in any particular baby has diminished due to the successful attempts at keeping inspired oxygen at or below 60% (FiO2 <0.6), keeping ventilatory pressures and rate as low as possible, and extubating as soon as possible. Infants at greatest risk for chronic lung disease (BPD) are the profoundly pre- mature who require longer courses of assisted ventilation at greater pressures and rate and a higher inspired oxygen concentration (FiO2 of 0.6-1.0). Infants with air-leak phe- nomenon (pneumothorax, pneumomediastinum, or pneu- mopericardium) also usually require prolonged treatment with a higher oxygen concentration. With the exception of an occasional baby, usually in one of these two latter groups, it is rare to see the development of the classic four stages of BPD described by Northway [3]. In chil- dren successfully managed with short courses of assisted ventilation and FiO2 <0.4-0.6, the usual radiographic course is to evolve from HMD to a hazy diffuse opacifi- cation of the lungs to normal over a period of several days to 2-3 weeks.
Since glucocorticoids accelerate lung maturation, the incidence of RDS in babies born at under 32 weeks ges- tations can be significantly reduced by administering betamethasone to the mother during the 2 days prior to delivery.
Several emerging technologies have greatly altered the clinical and radiographic evaluation of children with HMD. Two modes of treatment are now being used in an attempt to reduce the incidence of pneumothorax, tradi- tionally seen in up to 25% of ventilated babies with HMD. Artificial surfactant is being given at birth, with up to three or four additional doses in the first 48 h in selected cases. This treatment modality has significantly improved the early management and survival of these children, al- though the long-term consequences of their disease, such as BPD, have not been clearly altered [4-6]. The use of ar-
56
tificial surfactant has produced several significant changes in the radiographic configurations. The surfactant is given as liquid boluses via an ET tube. Usually, the sur- factant is not evenly distributed throughout the lungs; therefore, it is common to see areas of lung that rapidly improve in aeration alternating with areas of unchanged HMD. The uneven distribution produces a radiograph that may simulate other entities, such as neonatal pneumonia or MAS. In addition, the surfactant may reach the level of the acinae, causing sudden and effective distention of multiple acinar units and thereby producing a radiograph- ic configuration quite suggestive of pulmonary interstitial emphysema (PIE) [7]. In these situations, close commu- nication with the neonatologist to ascertain clinical status is mandatory to the intelligent interpretation of the radi- ograph. Babies with surfactant effect generally improve at this point, those with PIE deteriorate.
High-frequency ventilation (HiFi) (10-15 hertz, 600- 900 cycles/min) has also been employed to reduce the in- cidence of barotrauma [8]. For both of these modalities, the overall improvement in the long-term prognosis has yet to be determined. The radiographs of babies receiving HiFi are not significantly altered from those of babies treated with conventional ventilator therapy. However, the degree of pulmonary inflation is used to adjust mean air- way pressure (MAP). Ideally the dome of the diaphragm should project over the eighth to tenth posterior rib if MAP is appropriately adjusted.
A limited number of institutions have successfully managed most of their premature infants with nasal CPAP, and thus have avoided many of the complications of intubation.
With advances in medical management, more pro- foundly premature babies are being maintained and res- cued. It is not uncommon today for babies weighing as lit- tle as 650 grams to be successfully treated and to survive their prematurity. The radiographic presentation of these babies may differ from that of the more gestationally ma- ture infant. In this profoundly premature age group, it is common that initial radiographs performed within the first few hours to 2 days of life are normal or nearly nor- mal with only minimal evidence of HMD. Then, sudden- ly, over a period of 2-3 days, the radiographic pattern may evolve into one reflecting much coarser and somewhat ir- regularly distributed lung disease, similar to that seen in stage three BPD (a situation originally described as oc- curring at several weeks of age) [3].
Meconium Aspiration Syndrome
Meconium aspiration syndrome is the result of intra- partum or intrauterine aspiration of meconium. It most commonly occurs in babies who are post mature (the mean gestational age for MAS has been reported as 290 days or 10 days past the expected day of delivery), small for gestational age, or when there has been intrauterine stress causing hypoxemia. Although there is meconium
R. Cleveland, V. Donoghue
055_062_Donoghue_Cleveland 28-02-2007 07:16 Pagina 56
staining of the amniotic fluid in approximately 10-15% of live births, MAS, which is diagnosed by the presence of meconium in the airway below the vocal cords, is seen in 1-5% of newborns.
The radiographic findings in MAS vary, in part sec- ondary to the severity of the aspiration. The tenacious meconium often will cause both medium and small-air- way obstruction, even after vigorous endobronchial suc- tioning. This typically will cause areas of atelectasis al- ternating with areas of overinflation. The meconium is an irritant to bronchial mucosa and may cause a chemical pneumonia. This increases the risk for subsequent gram- negative bacterial infection. Hypoxia usually results, with all factors combining to produce pulmonary hyperten- sion, referred to as persistent fetal circulation (PFC). As vigorous suctioning and aggressive therapy with endotra- cheal intubation and ventilatory support are often re- quired, it is not surprising that air-leak phenomenon is en- countered, with pneumothorax present in 25-40% of cas- es. Occasionally, however, babies with MAS requiring ventilatory support will have a normal chest X-ray or possibly only a pneumothorax. Small pleural effusions are also seen in approximately 10% of cases. The chest X-rays of infants with MAS may be indistinguishable from those of children with neonatal pneumonia. Due to the difficulty in excluding neonatal pneumonia and the possibility of developing a superimposed pneumonia in MAS, most of these infants are treated with antibiotics.
In spite of optimal care, there is a persistent mortality rate of up to 25% in babies with MAS. Recently, this was addressed by the use of extracorporeal membrane oxy- genation (ECMO). Since ECMO has potentially life- threatening side effects, including hemorrhage, frequent- ly into the brain, its use is limited to babies who have failed conventional therapy. Although selection criteria are evolving, the usual practice is to attempt convention- al therapy for 1-2 days before resorting to ECMO. Survival rates with ECMO have been excellent. The most critical factor in weaning from ECMO is not simply im- proved pulmonary mechanics, but a reduction of pul- monary vascular pressure to below systemic pressure. Some investigators have postulated a primary abnormal- ity of the pulmonary microcirculation in MAS, while oth- ers feel that the increased pressures within the airways, produced by assisted ventilation, is the cause of PFC in these babies. ECMO, which bypasses the lungs, allows pulmonary inflation to be at a minimum while maintain- ing physiologic levels of oxygen tension and saturation [9-11]. Therefore, the lungs of infants on ECMO are usu- ally nearly or completely airless.
Neonatal Pneumonia
Neonatal pneumonia is seen in less than 1% of live-born infants; premature infants are at increased risk. It may be acquired in utero, during labor, at delivery, or shortly af- ter birth. The major risk factor for intrauterine develop-
ment of pneumonia is prolonged rupture of the maternal membranes, particularly if labor is active during this pe- riod. Some organisms, particularly viruses, may cross the placenta. Group B streptococcus infections are currently the most common cause of pneumonia in the newborn, with an incidence of 3/1000 live births, and are acquired in utero or during labor and delivery. Both clinically and radiographically, it may be difficult to distinguish neona- tal pneumonia from HMD. Chest radiographs may reveal lung disease identical to that of HMD; however, pleural effusions are associated with pneumonia (in up to 67% of cases) and essentially never with uncomplicated HMD. In neonatal pneumonia, the lungs may reveal irregular patchy infiltrates or occasionally be normal. There may be mild cardiac enlargement with pneumonia but it is un- usual with uncomplicated HMD [12]. Infections with other bacterial organisms may produce radiographic find- ings identical to those described above in group B strep- tococcal infections. HMD and neonatal pneumonia fre- quently co-exist.
Several infants with late-onset (at several days of life) right-sided diaphragmatic hernia in association with neonatal pneumonia were described [13]. At surgery, there were no distinguishing characteristics of the hernia to suggest a specific effect of the pneumonia or of sep- sis. These cases presumably represent a late onset of her- niation of the liver and intestine through a pre-existing, congenital diaphragmatic defect.
Transient Tachypnea of the Newborn
Transient tachypnea of the newborn is also referred to as retained fetal lung liquid. Fetal lung liquid is an ultrafil- trate of fetal serum. Under normal circumstances, it is cleared from the lungs at or shortly after birth via the tra- cheobronchial system (30%), the interstitial lymphatics (30%), and the capillaries (40%). TTN is most common- ly seen in infants born by cesarean section but may also be identified in children who are quite small or who have experienced a precipitous delivery. The lungs usually are diffusely affected, with a variable characterization rang- ing from an appearance similar or identical to HMD, a coarse interstitial pattern similar to pulmonary edema, or an irregular opacification as may be seen with meconium aspiration or neonatal pneumonia. A pleural effusion is a common accompaniment. A transient slight cardiac en- largement may occur. The hallmark of this process is a relatively benign clinical course (i.e., tachypnea) com- pared to the overall severity of disease suggested by chest X-ray and rapid clearing (1-2 days). In particularly severe cases, where clearing may require up to 3 days, there should be a rapid improvement on each successive image.
Focal retention of fetal lung liquid within congenital lobar emphysema has been recognized for several years. A recent publication suggested that the retention occurs only, or mainly, within polyalveolar lobes, in contrast to classic congenital lobar emphysema [14].
Imaging of the Newborn Chest 57
055_062_Donoghue_Cleveland 28-02-2007 07:16 Pagina 57
Congenital Lymphangiectasia
Babies with congenital lymphangiectasia may present with symptoms similar to those of HMD. The lungs may be normal or reveal a course interstitial infiltrate sec- ondary to the distended and abnormally draining lym- phatics. There may be generalized overinflation. Pleural effusions may be present. A particularly large pleural ef- fusion should suggest this diagnosis or it may be related to a traumatic chylous or hemorrhagic effusion. The pres- ence of a chylous effusion without a history of trauma is suggestive of congenital lymphangiectasia, although iso- lated chylous effusions do occur. This diagnosis is best made by sampling of the pleural effusion following the administration of fatty foods.
Congenital Heart Disease
Cases of congenital heart disease may closely mimic sev- eral of the entities mentioned previously. The presence of pulmonary interstitial edema may be difficult to distin- guish from HMD, neonatal pneumonia or TTN.
A particularly confusing constellation of chest find- ings may occur in those congenital heart lesions pro- ducing obstruction to pulmonary venous return but without significant cardiac enlargement. This includes congenital obstructing lesions that occur at the level of the mitral valve or between the mitral valve and the pul- monary venous system. Specifically, these lesions in- clude mitral valve stenosis, supravalvular mitral steno- sis, cor triatriatum, stenosis of a common pulmonary vein, or total anomalous pulmonary venous connection (TAPVC) with obstruction. Physiologically, TAPVC may present with or without obstruction. If obstruction is sufficient, children will present in the first week of life with cyanosis (in severe cases) or with signs of pul- monary edema, dyspnea, and feeding difficulties (in less severe cases). A chest radiograph will show evi- dence of pulmonary venous hypertension but without an enlarged heart. If there is little or no obstruction to ve- nous return, the children usually present with high out- put failure at 6-12 months of age and have a radiograph suggestive of congestive heart failure. Several recent re- views have estimated an overall incidence of obstruction in TAPVC of 50-65%. With supradiaphragmatic drain- ing veins, obstruction has been noted in up to 53% of patients. Essentially, all subdiaphragmatic draining veins are obstructed [15].
If the heart is significantly enlarged, the likelihood that the baby has true congestive heart failure is suggested. Within the first week of life, before the pulmonary vas- cular resistance drops sufficiently to allow left to right shunting, congestive heart failure is usually secondary to pressure overload, obligatory volume overload, or my- ocardial dysfunction. The lesions most commonly pro- ducing this phenomenon include critical aortic stenosis,
58
hypoplastic left heart syndrome, coarctation of the aorta (interruption of the aortic arch), myocarditis, dysrhyth- mias, myocardial ischemia, and arterial venous fistula. Significant arterial shunting outside the heart, such as vein of Galen aneurysm, hepatic hemangioma, or he- mangioendothelioma, may also produce congestive fail- ure in this time period. By the second week of life, after pulmonary resistance has dropped sufficiently, volume overload lesions are the predominant cause for congestive failure. This includes ventricular septal defect, endocar- dial cushion defect, patent ductus arteriosus, aortopul- monic window, as well as milder forms of those lesions potentially presenting within the first week of life. Children with untreated hypoplastic left-heart syndrome may live beyond the first week and are therefore a po- tential category of children having congestive heart fail- ure in the older age group [16].
Chlamydia Pneumonia
Chlamydia pneumonia is caused by a bacterial-like in- tracellular parasite, Chlamydia trachomatis [17-19], which is acquired from the mother by direct contact dur- ing vaginal delivery. This organism is widely distrib- uted, having been identified in the vagina of up to 13% of women tested. Up to 50% of babies infected become symptomatic with conjunctivitis, the most common manifestation, which usually appears around 5-14 days of life. Pneumonia develops in 10-20% of infected ba- bies, appearing between 2 weeks and 3 months of age. However, since the average age of onset is 6 weeks, Chlamydia infection is somewhat unusual in the neona- tal age group. Cough, sometimes paroxysmal and asso- ciated with tachypnea, is…
This paper reviews the common spectrum of disorders of the neonatal chest. Emphasis is on radiographic changes that have been produced by the introduction of new ther- apeutic maneuvers, particularly the use of artificial sur- factant in treating hyaline membrane disease and the sur- vival of profoundly premature newborns (less than 650 grams). A discussion of meconium aspiration syndrome, neonatal pneumonia, transient tachypnea of the newborn, congenital lymphangiectasia and congenital heart disease is also included. The effect on the neonatal chest radi- ograph of extracorporeal membrane oxygenation and high frequency ventilation are also mentioned [1]. A re- view of ‘surgical’ lesions is also included [2].
Although cross-sectional and ultrasound imaging have specific but limited roles in assessing the newborn chest, the standard chest radiograph remains the most common imaging tool. Diseases that affect the neonate’s chest sig- nificantly overlap in their radiographic and clinical ap- pearances. Therefore, an open exchange of information between the neonatologist and the radiologist is critical to the intelligent interpretation of these images.
The main disease entities of concern are hyaline mem- brane disease (HMD), chronic lung disease (BPD), meco- nium aspiration syndrome (MAS), neonatal pneumonia, and transient tachypnea of the newborn (TTN). Congenital lymphangiectasia may mimic the findings of MAS or neonatal pneumonia. Congenital heart disease may be pre- sent in a fashion that is easily confused with a pulmonary parenchymal process. This is most common when the heart remains normal in size, but there is pulmonary venous hy- pertension. Specific infective organisms, such as Chlamydia trachomatis or viruses, that are acquired during or follow- ing birth may be present within the neonatal period, with clinical and radiographic findings that are easily confused with one or several of the above-mentioned entities.
Hyaline Membrane Disease
Hyaline membrane disease is a manifestation of pul- monary immaturity, seen predominantly in children less
than 36-38 weeks of gestational age and weighing less than 2.5 kg. HMD remains the leading cause of death in live-born infants. The death rate is highest in the most premature infants, with few deaths occurring in infants weighing more than 1.5 kg. Males are affected almost twice as often as females. HMD is more common in whites than in blacks. The disease results from a defi- ciency of surfactant in the lungs, which leads to the in- ability to maintain acinar distention. Exposure to air rapidly leads to the development of hyaline membranes containing fibrin and cellular debris. There frequently is epithelial necrosis beneath the hyaline membranes. By the second day of life, repair has begun as evidenced by the proliferation of type 2 pneumocytes (which produce surfactant) and increased secretions. The lungs are almost impossible to inflate and on gross inspection appear sim- ilar to liver. Clinically, the infants are usually sympto- matic within minutes of birth, with grunting, nasal flar- ing, retractions, tachypnea, and cyanosis; however, it may be a few hours before symptoms are recognized. Although the initial radiographic findings may be noted minutes after birth, occasionally the maximum radi- ographic findings are not present until 12-24 h of life.
The rigid, noncompliant lungs and the associated hy- poxia and acidosis often result in persistent pulmonary hypertension. As pulmonary resistance decreases, there may be the onset of left to right shunting across a patent ductus arteriosus. This may be recognized radiographi- cally before clinical symptoms or a murmur may devel- op. Shunting is heralded by the development of pul- monary edema and a suddenly enlarging heart size. The ductus arteriosus can only respond to stimuli to close if it is term or near term in development. These stimuli in- clude a normal oxygen level, a normal pH, and normal levels of prostaglandin E1 and E2. In the premature new- born with HMD, the ductus is open, but the pulmonary and systemic pressures may be equal or the pulmonary pressure may exceed the systemic pressure, precluding left to right shunting. These babies are usually hypoxic and acidotic with high levels of prostaglandin E1 and E2. By several days of life, as the pulmonary resistance drops, if the ductus is not responsive or the stimuli are not present, the ductus arteriosus will remain open allowing
IDKD 2007
R. Cleveland1, V. Donoghue2
1 Radiology Department, Children’s Hospital, Harvard Medical School, Boston, MA, USA 2 Radiology Department, Children’s University Hospital, Dublin, Ireland
055_062_Donoghue_Cleveland 28-02-2007 07:16 Pagina 55
left to right shunting and the development of congestive heart failure. Treatment with indomethacin (by inhibiting production of prostaglandin) may produce ductal closure but at the risk of inducing gastrointestinal perforation and/or osteoarthropathy. Surgery may be required to close a persistent patent ductus arteriosus.
Sudden diffuse opacification of the lungs in HMD may be seen with other conditions. A frequent cause is de- creasing ventilatory settings, including inspiratory pres- sure, PEEP, or rate. Less commonly, diffuse pulmonary hemorrhage is a cause. Sudden catastrophic intracranial hemorrhage may produce central nervous system (CNS)- mediated edema.
Since Northway’s original description of bronchopul- monary dysplasia (BPD), in 1967 [3], many changes have occurred in the management and outcome of children with HMD. Much has been learned about the specific toxic effects of oxygen and assisted ventilation. Babies of much lower gestational age and birth weight are surviv- ing with BPD, particularly since the advent of high-fre- quency ventilation and the use of artificial surfactant. The likelihood of developing BPD or retinopathy of prematu- rity and the severity of these conditions in any particular baby has diminished due to the successful attempts at keeping inspired oxygen at or below 60% (FiO2 <0.6), keeping ventilatory pressures and rate as low as possible, and extubating as soon as possible. Infants at greatest risk for chronic lung disease (BPD) are the profoundly pre- mature who require longer courses of assisted ventilation at greater pressures and rate and a higher inspired oxygen concentration (FiO2 of 0.6-1.0). Infants with air-leak phe- nomenon (pneumothorax, pneumomediastinum, or pneu- mopericardium) also usually require prolonged treatment with a higher oxygen concentration. With the exception of an occasional baby, usually in one of these two latter groups, it is rare to see the development of the classic four stages of BPD described by Northway [3]. In chil- dren successfully managed with short courses of assisted ventilation and FiO2 <0.4-0.6, the usual radiographic course is to evolve from HMD to a hazy diffuse opacifi- cation of the lungs to normal over a period of several days to 2-3 weeks.
Since glucocorticoids accelerate lung maturation, the incidence of RDS in babies born at under 32 weeks ges- tations can be significantly reduced by administering betamethasone to the mother during the 2 days prior to delivery.
Several emerging technologies have greatly altered the clinical and radiographic evaluation of children with HMD. Two modes of treatment are now being used in an attempt to reduce the incidence of pneumothorax, tradi- tionally seen in up to 25% of ventilated babies with HMD. Artificial surfactant is being given at birth, with up to three or four additional doses in the first 48 h in selected cases. This treatment modality has significantly improved the early management and survival of these children, al- though the long-term consequences of their disease, such as BPD, have not been clearly altered [4-6]. The use of ar-
56
tificial surfactant has produced several significant changes in the radiographic configurations. The surfactant is given as liquid boluses via an ET tube. Usually, the sur- factant is not evenly distributed throughout the lungs; therefore, it is common to see areas of lung that rapidly improve in aeration alternating with areas of unchanged HMD. The uneven distribution produces a radiograph that may simulate other entities, such as neonatal pneumonia or MAS. In addition, the surfactant may reach the level of the acinae, causing sudden and effective distention of multiple acinar units and thereby producing a radiograph- ic configuration quite suggestive of pulmonary interstitial emphysema (PIE) [7]. In these situations, close commu- nication with the neonatologist to ascertain clinical status is mandatory to the intelligent interpretation of the radi- ograph. Babies with surfactant effect generally improve at this point, those with PIE deteriorate.
High-frequency ventilation (HiFi) (10-15 hertz, 600- 900 cycles/min) has also been employed to reduce the in- cidence of barotrauma [8]. For both of these modalities, the overall improvement in the long-term prognosis has yet to be determined. The radiographs of babies receiving HiFi are not significantly altered from those of babies treated with conventional ventilator therapy. However, the degree of pulmonary inflation is used to adjust mean air- way pressure (MAP). Ideally the dome of the diaphragm should project over the eighth to tenth posterior rib if MAP is appropriately adjusted.
A limited number of institutions have successfully managed most of their premature infants with nasal CPAP, and thus have avoided many of the complications of intubation.
With advances in medical management, more pro- foundly premature babies are being maintained and res- cued. It is not uncommon today for babies weighing as lit- tle as 650 grams to be successfully treated and to survive their prematurity. The radiographic presentation of these babies may differ from that of the more gestationally ma- ture infant. In this profoundly premature age group, it is common that initial radiographs performed within the first few hours to 2 days of life are normal or nearly nor- mal with only minimal evidence of HMD. Then, sudden- ly, over a period of 2-3 days, the radiographic pattern may evolve into one reflecting much coarser and somewhat ir- regularly distributed lung disease, similar to that seen in stage three BPD (a situation originally described as oc- curring at several weeks of age) [3].
Meconium Aspiration Syndrome
Meconium aspiration syndrome is the result of intra- partum or intrauterine aspiration of meconium. It most commonly occurs in babies who are post mature (the mean gestational age for MAS has been reported as 290 days or 10 days past the expected day of delivery), small for gestational age, or when there has been intrauterine stress causing hypoxemia. Although there is meconium
R. Cleveland, V. Donoghue
055_062_Donoghue_Cleveland 28-02-2007 07:16 Pagina 56
staining of the amniotic fluid in approximately 10-15% of live births, MAS, which is diagnosed by the presence of meconium in the airway below the vocal cords, is seen in 1-5% of newborns.
The radiographic findings in MAS vary, in part sec- ondary to the severity of the aspiration. The tenacious meconium often will cause both medium and small-air- way obstruction, even after vigorous endobronchial suc- tioning. This typically will cause areas of atelectasis al- ternating with areas of overinflation. The meconium is an irritant to bronchial mucosa and may cause a chemical pneumonia. This increases the risk for subsequent gram- negative bacterial infection. Hypoxia usually results, with all factors combining to produce pulmonary hyperten- sion, referred to as persistent fetal circulation (PFC). As vigorous suctioning and aggressive therapy with endotra- cheal intubation and ventilatory support are often re- quired, it is not surprising that air-leak phenomenon is en- countered, with pneumothorax present in 25-40% of cas- es. Occasionally, however, babies with MAS requiring ventilatory support will have a normal chest X-ray or possibly only a pneumothorax. Small pleural effusions are also seen in approximately 10% of cases. The chest X-rays of infants with MAS may be indistinguishable from those of children with neonatal pneumonia. Due to the difficulty in excluding neonatal pneumonia and the possibility of developing a superimposed pneumonia in MAS, most of these infants are treated with antibiotics.
In spite of optimal care, there is a persistent mortality rate of up to 25% in babies with MAS. Recently, this was addressed by the use of extracorporeal membrane oxy- genation (ECMO). Since ECMO has potentially life- threatening side effects, including hemorrhage, frequent- ly into the brain, its use is limited to babies who have failed conventional therapy. Although selection criteria are evolving, the usual practice is to attempt convention- al therapy for 1-2 days before resorting to ECMO. Survival rates with ECMO have been excellent. The most critical factor in weaning from ECMO is not simply im- proved pulmonary mechanics, but a reduction of pul- monary vascular pressure to below systemic pressure. Some investigators have postulated a primary abnormal- ity of the pulmonary microcirculation in MAS, while oth- ers feel that the increased pressures within the airways, produced by assisted ventilation, is the cause of PFC in these babies. ECMO, which bypasses the lungs, allows pulmonary inflation to be at a minimum while maintain- ing physiologic levels of oxygen tension and saturation [9-11]. Therefore, the lungs of infants on ECMO are usu- ally nearly or completely airless.
Neonatal Pneumonia
Neonatal pneumonia is seen in less than 1% of live-born infants; premature infants are at increased risk. It may be acquired in utero, during labor, at delivery, or shortly af- ter birth. The major risk factor for intrauterine develop-
ment of pneumonia is prolonged rupture of the maternal membranes, particularly if labor is active during this pe- riod. Some organisms, particularly viruses, may cross the placenta. Group B streptococcus infections are currently the most common cause of pneumonia in the newborn, with an incidence of 3/1000 live births, and are acquired in utero or during labor and delivery. Both clinically and radiographically, it may be difficult to distinguish neona- tal pneumonia from HMD. Chest radiographs may reveal lung disease identical to that of HMD; however, pleural effusions are associated with pneumonia (in up to 67% of cases) and essentially never with uncomplicated HMD. In neonatal pneumonia, the lungs may reveal irregular patchy infiltrates or occasionally be normal. There may be mild cardiac enlargement with pneumonia but it is un- usual with uncomplicated HMD [12]. Infections with other bacterial organisms may produce radiographic find- ings identical to those described above in group B strep- tococcal infections. HMD and neonatal pneumonia fre- quently co-exist.
Several infants with late-onset (at several days of life) right-sided diaphragmatic hernia in association with neonatal pneumonia were described [13]. At surgery, there were no distinguishing characteristics of the hernia to suggest a specific effect of the pneumonia or of sep- sis. These cases presumably represent a late onset of her- niation of the liver and intestine through a pre-existing, congenital diaphragmatic defect.
Transient Tachypnea of the Newborn
Transient tachypnea of the newborn is also referred to as retained fetal lung liquid. Fetal lung liquid is an ultrafil- trate of fetal serum. Under normal circumstances, it is cleared from the lungs at or shortly after birth via the tra- cheobronchial system (30%), the interstitial lymphatics (30%), and the capillaries (40%). TTN is most common- ly seen in infants born by cesarean section but may also be identified in children who are quite small or who have experienced a precipitous delivery. The lungs usually are diffusely affected, with a variable characterization rang- ing from an appearance similar or identical to HMD, a coarse interstitial pattern similar to pulmonary edema, or an irregular opacification as may be seen with meconium aspiration or neonatal pneumonia. A pleural effusion is a common accompaniment. A transient slight cardiac en- largement may occur. The hallmark of this process is a relatively benign clinical course (i.e., tachypnea) com- pared to the overall severity of disease suggested by chest X-ray and rapid clearing (1-2 days). In particularly severe cases, where clearing may require up to 3 days, there should be a rapid improvement on each successive image.
Focal retention of fetal lung liquid within congenital lobar emphysema has been recognized for several years. A recent publication suggested that the retention occurs only, or mainly, within polyalveolar lobes, in contrast to classic congenital lobar emphysema [14].
Imaging of the Newborn Chest 57
055_062_Donoghue_Cleveland 28-02-2007 07:16 Pagina 57
Congenital Lymphangiectasia
Babies with congenital lymphangiectasia may present with symptoms similar to those of HMD. The lungs may be normal or reveal a course interstitial infiltrate sec- ondary to the distended and abnormally draining lym- phatics. There may be generalized overinflation. Pleural effusions may be present. A particularly large pleural ef- fusion should suggest this diagnosis or it may be related to a traumatic chylous or hemorrhagic effusion. The pres- ence of a chylous effusion without a history of trauma is suggestive of congenital lymphangiectasia, although iso- lated chylous effusions do occur. This diagnosis is best made by sampling of the pleural effusion following the administration of fatty foods.
Congenital Heart Disease
Cases of congenital heart disease may closely mimic sev- eral of the entities mentioned previously. The presence of pulmonary interstitial edema may be difficult to distin- guish from HMD, neonatal pneumonia or TTN.
A particularly confusing constellation of chest find- ings may occur in those congenital heart lesions pro- ducing obstruction to pulmonary venous return but without significant cardiac enlargement. This includes congenital obstructing lesions that occur at the level of the mitral valve or between the mitral valve and the pul- monary venous system. Specifically, these lesions in- clude mitral valve stenosis, supravalvular mitral steno- sis, cor triatriatum, stenosis of a common pulmonary vein, or total anomalous pulmonary venous connection (TAPVC) with obstruction. Physiologically, TAPVC may present with or without obstruction. If obstruction is sufficient, children will present in the first week of life with cyanosis (in severe cases) or with signs of pul- monary edema, dyspnea, and feeding difficulties (in less severe cases). A chest radiograph will show evi- dence of pulmonary venous hypertension but without an enlarged heart. If there is little or no obstruction to ve- nous return, the children usually present with high out- put failure at 6-12 months of age and have a radiograph suggestive of congestive heart failure. Several recent re- views have estimated an overall incidence of obstruction in TAPVC of 50-65%. With supradiaphragmatic drain- ing veins, obstruction has been noted in up to 53% of patients. Essentially, all subdiaphragmatic draining veins are obstructed [15].
If the heart is significantly enlarged, the likelihood that the baby has true congestive heart failure is suggested. Within the first week of life, before the pulmonary vas- cular resistance drops sufficiently to allow left to right shunting, congestive heart failure is usually secondary to pressure overload, obligatory volume overload, or my- ocardial dysfunction. The lesions most commonly pro- ducing this phenomenon include critical aortic stenosis,
58
hypoplastic left heart syndrome, coarctation of the aorta (interruption of the aortic arch), myocarditis, dysrhyth- mias, myocardial ischemia, and arterial venous fistula. Significant arterial shunting outside the heart, such as vein of Galen aneurysm, hepatic hemangioma, or he- mangioendothelioma, may also produce congestive fail- ure in this time period. By the second week of life, after pulmonary resistance has dropped sufficiently, volume overload lesions are the predominant cause for congestive failure. This includes ventricular septal defect, endocar- dial cushion defect, patent ductus arteriosus, aortopul- monic window, as well as milder forms of those lesions potentially presenting within the first week of life. Children with untreated hypoplastic left-heart syndrome may live beyond the first week and are therefore a po- tential category of children having congestive heart fail- ure in the older age group [16].
Chlamydia Pneumonia
Chlamydia pneumonia is caused by a bacterial-like in- tracellular parasite, Chlamydia trachomatis [17-19], which is acquired from the mother by direct contact dur- ing vaginal delivery. This organism is widely distrib- uted, having been identified in the vagina of up to 13% of women tested. Up to 50% of babies infected become symptomatic with conjunctivitis, the most common manifestation, which usually appears around 5-14 days of life. Pneumonia develops in 10-20% of infected ba- bies, appearing between 2 weeks and 3 months of age. However, since the average age of onset is 6 weeks, Chlamydia infection is somewhat unusual in the neona- tal age group. Cough, sometimes paroxysmal and asso- ciated with tachypnea, is…
Related Documents