This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
This article was published in an Elsevier journal. The attached copyis furnished to the author for non-commercial research and
education use, including for instruction at the author’s institution,sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling orlicensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of thearticle (e.g. in Word or Tex form) to their personal website orinstitutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies areencouraged to visit:
http://www.elsevier.com/copyright
Author's personal copy
Journal of Membrane Science 302 (2007) 27–35
Human hepatocyte functions in a galactosylated membrane bioreactor
Sabrina Morelli a, Simona Salerno a, Maria Rende a,c, Linda C. Lopez b, Pietro Favia b,Alfredo Procino d, Bruno Memoli d, Vittorio E. Andreucci d, Riccardo d’Agostino b,
Enrico Drioli a,c, Loredana De Bartolo a,∗a Institute on Membrane Technology, National Research Council of Italy, ITM-CNR,
c/o University of Calabria, via P. Bucci, Cubo 17/C, I-87030 Rende (CS), Italyb Department of Chemistry, University of Bari, Bari, Italy
c Department of Chemical Engineering and Materials of University of Calabria,via P. Bucci, Cubo 45/A, 87030 Rende (CS), Italy
d Department of Nephrology, University Federico II of Naples, Italy
Received 20 October 2006; received in revised form 5 June 2007; accepted 10 June 2007Available online 19 June 2007
Abstract
This paper reports on the performance of galactosylated polyethersulphone (PES) membrane bioreactor that enables the long-term maintenanceof liver-specific functions of human hepatocytes under continuous perfusion. Galactose derivate was immobilized on PES membranes modifiedby plasma deposited acrylic acid coating. Galactosylated membranes had specific interactions with hepatocytes because of binding between thegalactose moiety and the asyaloglycoprotein receptor present on the hepatocyte cytoplasmatic membrane.
The liver-specific functions of human hepatocytes cultured in the galactosylated membrane bioreactor were explored with respect to ureasynthesis, albumin production and secretion of total proteins.
Human hepatocytes were cultured in the membrane bioreactor under continuous perfusion for 21 days. Morphological examinations of hep-atocytes in the bioreactor showed that cells developed aggregation and formed tight junctions. Vinculin was distributed into the cytoplasm andfocal adhesions were visible. Human hepatocytes maintained their liver-specific functions for the whole culture time in terms of urea synthesis andalbumin production as well as protein secretion. The gene expression of albumin and C-reactive proteins confirmed the maintenance at the genelevel of the specific functions of cells in the bioreactor.
This study demonstrated that the galactosylated membrane bioreactor is able to support extended in vitro hepatocyte functions.© 2007 Elsevier B.V. All rights reserved.
Keywords: Bioreactor; Galactosylated membrane; Human hepatocytes; Liver-specific functions; Morphology
1. Introduction
Engineered liver tissue constructs may provide an inexpen-sive and reliable in vitro physiological model with great controlof variables for studying disease, drug, infection and moleculartherapeutics.
Isolated hepatocytes may be able to undertake the full rangeof known in vivo biotransformation and liver-specific functions[1,2]. Thus, the in vitro maintenance of viable hepatocytes isdesirable so that the liver functions can be studied in a controlled
∗ Corresponding author. Tel.: +39 0984 492036; fax: +39 0984 402103.E-mail address: [email protected] (L. De Bartolo).
environment. Hepatocytes cultured in conventional systemsquickly lose both viability and many of their liver-specificfunctions, including the ability to metabolise drugs. Traditionalstatic culture methods are characterized by an unstirred mediumlayer overlying cells attached to a gas impermeable substra-tum and are exposed to changes of nutrient concentration andcatabolite accumulation with time. In these conditions, quan-titative reliable information on cell metabolism is difficult toobtain, because the concentration changes of a given measuredmetabolite cannot be related completely to cell metabolism: infact, other factors, such as the development of concentrationgradients in the culture medium resulting from the diffusiveresistances to mass transport or of poor mixing, affect reac-tion rates. Furthermore, for liver cells which are highly perfused
0376-7388/$ – see front matter © 2007 Elsevier B.V. All rights reserved.doi:10.1016/j.memsci.2007.06.027
Author's personal copy
28 S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35
in vivo, such conditions are susceptible to oxygen and nutri-ent limitations with consequent reduction of cell viability andfunctionality.
A variety of culture methods have been developed to amelio-rate the retention of hepatocyte functions including modificationof the culture medium, co-culture with non-parenchymal cells[3,4] the influence of the attachment substrata [5–8] and thedevelopment of bioreactors using different materials, config-uration and size [9–17]. Among these systems membranebioreactors are particularly attractive because membranes allowthe selective transport of metabolites and nutrients to cells andthe removal of catabolites and specific products from cells[18,19] and also play a role of mechanical and chemical sup-port for adhesion and growth of cells. However, not all of theimportant liver functions can be replicated yet at desired levels.Hepatocyte culture systems that appear to offer longevity alsoadd a layer of complexity to the interpretation of metabolic stud-ies where outcomes can depend on the nature of the matrix, fluiddynamics, mass transport, membrane interaction, etc., prompt-ing the continuation of the development of dynamic systems[20].
Several previous reactor designs for 3D liver cell culture haveaddressed mass transfer constraints and the homogeneous dis-tribution of flow [21–25]. In the case of membrane bioreactorimprovements can be obtained by solving the issues relatedto the membrane properties. Most of the membranes weredesigned to be inert with respect to blood proteins and cells.Hepatocytes are anchorage-dependent cells that require adhe-sive substrates for their functional and phenotypic maintenance[5–8].
Previously we developed a strategy to modify polyether-sulphone membranes with galactose derivatives because theyelicit specific interactions with hepatocyte asyaloglycoproteinreceptors [26,27] thereby improving cell adhesion and the main-tenance of differentiated functions.
This functionalized membrane was incorporated into a biore-actor, which was designed for liver cell culture to be used in invitro evaluation of hepatic metabolic transformation of com-pounds and metabolites. A high-density hepatocyte culture wasobtained under sufficient oxygenation, comparable to an in vivomicroenvironment. Hepatocytes in the bioreactor were grownon the surface of polyethersulphone (PES) membranes withcovalently immobilized galactose oxidized to galactonic acid(GAL). In this system, fresh medium containing nutrients andmetabolites is continuously fed to the cells and catabolites andspent medium continuously removed from them, as occurs inthe liver. A distinguishing feature of the developed bioreactoris that it has a modelled fluid dynamics that ensures a uniformconcentration of metabolites at the cell site owing to thoroughmixing. The performance of this bioreactor was evaluated byassessing the maintenance of liver-specific functions of hep-atocytes in terms of urea synthesis, albumin production andsecretion of total proteins. The gene expression of two impor-tant plasma proteins such as albumin and C-reactive protein(CRP), produced by liver was evaluated for the first time inhuman hepatocytes cultured in the galactosylated membranebioreactor.
2. Experimental
2.1. Membranes for cell culture
Flat PES membranes (7 cm diameter, 0.1 �m pore size: PALLCorp., Michigan, USA) were plasma deposited acrylic acid(pdAA) coated in a stainless-steel parallel plate radiofrequency(RF, 13.56 MHz) plasma reactor in order to achieve a stable sur-faces dense enough of –COOH functionalities to perform theimmobilization of Gal moieties. Details of the reactor and of thepdAA deposition process are given in Ref. [28].
Homogeneous pdAA coverage of the PES membranes wasattested with XPS. Scanning electron microscopy (SEM) anal-ysis ensured that morphology and porosity of the membraneswere not altered.
Galactonic acid was immobilized at the PES–pdAA surfacethrough a hydrophilic spacer arm (SA) to ensure confor-mational freedom and unaltered biological activity to thetethered biomolecule. A linear bis-amine SA molecule (O,O′-bis-(2-aminopropyl)-polyethylene glycol 500) was covalentlyimmobilized at the PES–pdAA surfaces through amide bonds.To activate the pdAA COOH groups, PES–pdAA substrateswere immersed in a 1-ethyl-3-(3-dimethylamino-propyl) car-bodiimide 0.05 M solution in morpholine ethane sulphonatebuffer (pH 5.5; 1 h, 4 ◦C; mild stirring). Substrates were thenimmersed in a 0.2 mM SA solution (pH 10.0; 24 h, 4 ◦C; mildstirring) to react and the resulting PES–pdAA–SA substrateswere sonicated three times in distilled water. The immobilizationof the SA molecule was attested with XPS, since N atoms wererevealed. GAL moieties in their acid oxidized form [29], wereimmobilized on PES–pdAA–SA substrates through the forma-tion of an amide bond as described for the SA molecule, with asimilar chemical procedure; the resulting PES–PdAA–SA–GALsubstrates were rinsed three times in distilled water.
The presence of GAL onto PES–pdAA–SA–GAL sur-faces was confirmed by fluorescence microscopy after bindingFITC-lectin to GAL moieties. Modified and unmodified PESmembranes (surface area 25 mm2) were incubated in a flu-orescein isothiocyanate (FITC)-lectin solution (from Arachishypogaea, 5 mg/ml, Sigma, St. Louis, MO, USA, 100 �l, 1 hat 37 ◦C). The samples were extensively rinsed with water andobserved under a Zeiss Fluorescence microscope (Eex 488 nm)[30].
The wettability of the membrane was characterized by meansof static and dynamic water contact angle (WCA) measurements.The contact angle of the water droplets was measured at tem-perature of 20 ◦C with a CAM 200 contact angle meter (KSVInstruments Ltd., Helsinki, Finland). WCA measurements wereperformed in standardised conditions, which take into accountvarious parameters (e.g., temperature, clean of sample, drop vol-ume). The instrument supported by video camera and softwarepermits precise drop measurements and evolution with time.
At least 30 measurements on different regions of the mem-brane sample were averaged for each WCA value measured inthe time. The static contact angles values measured at t = 0 ondifferent regions of the membrane ranging from 57.6◦ to 63.4◦.The water sorption of the membrane surfaces with time was
Author's personal copy
S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35 29
Fig. 1. Scheme of the bioreactor and perfusion system.
evaluated by following the decrease of drop volume in contactwith the surface at temperature of 20 ◦C. In all measurements thepercentage of water sorption ranging from 65% to 72% after 5 s.
2.2. Human hepatocytes culture
Primary human hepatocytes (Cambrex Bio Science) iso-lated from the non-transplantable tissue of young single donorswere used for cell culture experiments. The purity of isolatedhepatocytes is 95% and non-parenchymal cells are present ina very low percentage (5%). Cryopreserved human hepato-cytes were quickly thawed in a 37 ◦C water bath with gentleshaking. Then, the cell suspension was transferred slowly intoa tube containing 25 ml of cold hepatocyte culture medium(HCMTM, Cambrex Bio Science), and centrifuged at 50 × g at4 ◦C for 3 min. The HCMTM is constituted of hepatocyte basalmedium (HBMTM) together with all the components providedin HCMTM bulletkit® (Cambrex Bio Science, Milan, Italy):epidermal growth factors, insulin, ascorbic acid, transferrin,hydrocortisone 21-hemosuccinate, bovine serum albumin–fatacid free 2% (BSA–FAF) and gentamicin sulphate 50 �g/mlamphotericin B 50 ng/ml. The cell pellet was suspended inHCMTM and tested for the cell viability by trypan blue exclusion.
Human hepatocytes were seeded in the bioreactor cham-ber on galactosylated membrane, previously conditioned withHCMTM containing BSA–FAF, to give a concentration of1.3 × 105 cells/cm2 and incubated for the first 24 h at 37 ◦C in a5% CO2; 20% O2 atmosphere (v/v) with 95% relative humid-ity in HCMTM containing 2% BSA–FAF. Thereafter, the culturewas continued under serum-free conditions for the whole culturetime (21 days). The liver-specific functions of the human hepato-cytes cultured in the bioreactor were investigated in terms of ureasynthesis, albumin production and secretion of total proteins.Proteins secreted by cells and released in the medium were iden-tified by gel electrophoresis. The metabolic data reported in thegraphs are the mean of six experiments from different cell isola-tion ± standard deviation. For each experiment medium samples
were collected from the bioreactor at each time and analysed intriplicate.
2.3. Membrane bioreactor
The membrane bioreactor inserted in the perfusion systemused for liver cell culture is schematically shown in Fig. 1. Thebioreactor consists of a circular acrylic housing (volume 65 cm3)fitted with inlet and outlet ports. At the bottom of chamber putup the galactosylated PES membrane with an active area of41.8 cm2. The bioreactor was connected to the perfusion sys-tem consisting of a glass medium reservoir, tubing, oxygenatorand two microperistaltic pumps. The medium from the reservoirenters before the oxygenator where it is warmed and oxygenated,and then the medium flows into the membrane bioreactor. Theoxygenated medium was continuously fed to the cells adheredon the membrane in the bioreactor with flow rate Qin, whichwas set on the basis of average retention time; a fraction of thestream leaving the bioreactor Qr was recycled back to mix withthe stream of fresh medium, Qin, before entering the bioreac-tor. The investigation was aimed at establishing the operatingconditions that ensure uniform metabolite concentration in thebioreactor whose fluid dynamics resemble that of a continuousstirred tank reactor (CSTR). The bioreactor fluid dynamics werecharacterized by tracer experiments in terms of the cumulativeresidence time distribution to a step input [31].
Under the chosen operating conditions (Qin = 0.58 ml/minand Qr = 4.5 ml/min) the bioreactor contents are well mixed: asa result the metabolite concentration in the bioreactor is uniformand equal to that in the stream leaving the bioreactor.
2.4. Biochemical assays
Samples of the culture medium were collected from the biore-actor in pre-chilled tubes and stored at −20 ◦C until assayed. Theurea concentration was assayed by the enzymatic urease method(Sentinel, Milan, Italy).
Author's personal copy
30 S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35
Table 1Selected sense and anti-sense primers used in the identification of albumin and C-reactive protein gene expression
Primers Sense Anti-sense
�-Actin CACCATGGATGATGATATCG TGGATAGCAACGTACATGGCRP TTTCTTCGTCTTGACCAGCC TTCTTCAGACTCTTCCTCACCCAlbumin CTTGAATGTGCTGATGACAGG GCAAGTCAGCAGGCATCTCAT
The protein content in the samples was determined by proteinassay using bicinchoninic acid solution (Sigma, Milan, Italy) byspectrophotometer analysis.
Albumin production was measured in the samples by theimmunometric method (ELISA) [21] with the modification thatantibodies against human albumin and human albumin wereused (Sigma, St. Louis, MO, USA). ELISAs were done fromcells of six different isolations. Chromatographically purifiedhuman albumin and the monoclonal antibody for human albu-min were Bethyl (Bethyl Laboratories, Inc., USA). Ninety-sixwell plates were coated with 50 �g/ml of albumin and leftovernight at 4 ◦C. After washing the plate 4 times, 100 �l ofcell culture supernatant was added to the wells and incubatedwith 100 �l of anti-human albumin antibody conjugated withhorseradish peroxidase (Bethyl Laboratories, Inc.). After 24 h at4 ◦C, the substrate buffer containing tetramethylbenzidine andH2O2 (Sigma, St. Louis, MO, USA) was added for 7 min. Thereaction was stopped with 50 �l of 8N H2SO4. Absorbance wasmeasured at 450 nm using a Multiskan Ex (Thermo Lab Systems,Vantaa, Finland).
The statistical significance of the experimental results wasestablished according to the unpaired statistical Student’s t-test(p < 0.05) and ANOVA test.
2.5. Gel electrophoresis of proteins
Proteins from medium collected by samples were separatedby one-dimensional native-PAGE on an 8–25% PhastGelTM gra-dient using buffer strips. The 8/1 �l sample applicator was used(Amersham Biosciences, Italy). The gel has a continuous 8–25%gradient gel zone with 2% crosslinking. The buffer system inPhastGel Native Buffer Strip is of 0.88 M l-alanine and 0.25 MTris, pH 8.8. Each sample was loaded onto a separate lane ofthe gel containing 1 �l of sample. The gels were stained withCoomassie blue and then destained with 30% methanol and10% acetic acid in distilled water. The solution for preserv-ing the gels contained 10% glycerol and 10% acetic acid inwater.
2.6. Hepatocyte gene expression
2.6.1. Cell detachmentFor genomic analysis the cells were detached from the mem-
brane, after experiments, by the addition of 0.05% collagenasein PBS (Sigma, Milan, Italy) followed by incubation at 37 ◦Cfor 30 min. The cells were then centrifuged for 5 min at 800 rpm.The cell pellet was washed with 10 ml PBS and then stored at−80 ◦C until assay.
2.6.2. Identification of primers for albumin and C-reactiveprotein (CRP)
The genome sequences corresponding to both albuminand CRP were obtained from the GeneBank (www.ncbi.nlm.nih.gov) in order to identify specific primers. We analysedthe exon sequences in order to establish, for each gene, a pair ofprimers (sense and anti-sense) able to generate amplified frag-ments measuring between 150 and 500 bp in length and withannealing temperature between 55 and 62 ◦C. The sense andanti-sense primers were selected to include at least one intron toprevent genomic DNA contamination during amplification. Theselected primers are shown in Table 1.
2.6.3. RNA extraction and expression analysis by means ofreverse transcriptase polymerase chain reaction (RT-PCR)
Hepatocytes from the bioreactor were pulverized with ablender and lysed in guanidinium isothiocyanate. Total RNAwas extracted by the single-step method, using phenol andchloroform/isoamylalcohol. Four micrograms of total RNAwere subjected to cDNA synthesis for 1 h at 37 ◦C using the“Ready to go You-Primer First-Stand Beads” kit (AmershamPharmacia Biotech Little Chalfont Buckinghamshire, UK) in areaction mixture containing 0.5 �g oligo-dT (Amersham Phar-macia Biotech Little Chalfont Buckinghamshire, UK).
PCR amplification of cDNA was performed in a reactionmixture containing 5 �l of cDNA sample and different primersets (20 pmol each). The amplification of both albumin and CRPand human �-actin gene, as housekeeping, was achieved usingone pair of primers in a single reaction. We selected a pair of �-actin primers to obtain amplified fragments measuring 450 bp.PCR products were separated by ethidium 1.2% agarose gelelectrophoresis.
2.7. Cell morphology
2.7.1. Sample preparation for SEMSpecimens of cell cultures were prepared for scanning elec-
tron microscopy (SEM) by fixation in 2.5% glutaraldehyde, pH7.4 phosphate buffer, followed by post-fixation in 1% osmiumtetroxide and by progressive dehydration in ethanol. The spec-imens were examined by SEM (ESEM FEG Quanta 200, FEICompany, Oregon, USA) after plating with gold under vacuum.
2.7.2. Hepatocyte staining for laser confocal scanningmicroscopy
The morphological behaviour of human hepatocytes culturedin a galactosylated PES membrane bioreactor was investigatedafter 21 days of culture by laser confocal scanning microscopy
Author's personal copy
S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35 31
(LCSM). Hepatocytes cultured on galactosylated membraneswere washed with PBS, fixed for 15 min in 3% paraformalde-hyde in PBS at room temperature, permeabilized for 5 min with0.5% Triton-X100 and saturated for 15 min with 2% normalgoat serum (NGS). To visualize vinculin, a specific monoclonalmouse anti-human vinculin antibody (Sigma, Milan, Italy),diluted 1:50 in 1% NGS, was incubated for 30 min at room tem-perature [32]. Then samples were washed twice in PBS andincubated for 30 min with goat anti-mouse IgG TRITC conju-gated (Sigma, Milan, Italy), diluted 1:100 in PBS. Then sampleswere washed twice in PBS and incubated for 20 min with DAPI(Sigma, Milan, Italy) to visualize nucleic acid. Finally, the sam-ples were washed, mounted and viewed with Laser ConfocalScanning Biological Microscope (Fluoview FV300, Olympus,Milan, Italy).
3. Results
Flat PES membranes were coated with a thin (30 ± 5 nm),functional, pdAA layer, very stable in water media and furthermodified with GAL moieties. After every modification steps thesurfaces were analysed with X-ray photoelectron spectroscopy(XPS). The atomic composition of the PES–pdAA surface (C78.9%, O 21.1%) did not reveal any signal of the underlyingPES substrate (e.g. from sulphur). The stability of the pdAAcoating was tested by means of XPS and WCA (water contactangle) before and after prolonged water soaking; no composi-tional changes were found after up to 1 month [28]. The pdAAlayer is characterized by a 4% density (relative to all surfaceC atoms) of COOH groups that were utilized for the covalentimmobilization of a diamine SA molecule through the synthesisof an amide bond. The resulting PES–pdAA–SA surface wasfurther modified with the covalent immobilization of GAL moi-eties through another amide bond. The PES–pdAA–SA–GALsurface exhibited a C 75.7%; O 22.9%; N 1.3% XPS atomiccomposition.
The presence of GAL derivatives immobilized onto the mem-brane surface was demonstrated by fluorescence microscopyafter binding fluorescein isothiocyanate (FITC)-lectin to GALmoieties. Galactosylated PES membranes (Fig. 2a) exhibited amarked high level of FITC fluorescence signal with respect tounmodified PES (Fig. 2b).
All the morphological and physico-chemical properties ofthe galactosylated PES membrane are reported in Table 2. Themodification process did not change the pore size (0.1 �m) ofthe membranes; the modified membranes have a thickness of190 �m with a porosity of 72%. The membranes displayed anadvancing (�adv) and receding (�rec) contact angle value, respec-
Table 2Morphological and physico-chemical properties of galactosylated PESmembranes
Pore size (�m) 0.1Thickness (�m) 190 ± 15Porosity (%) 72.5 ± 0.19Water sorption (%) 68.5 ± 4Water CA degree θadv = 60 ± 2, θrec = 37 ± 3
Fig. 2. Fluorescence microscopy images of (a) galactosylated PES membrane,after incubation with FITC-lectin at 37 ◦C for 1 h; (b) unmodified PES mem-brane.
tively of 60 ± 2◦ and 37 ± 3◦. The hydrophilic character of themembrane surface was confirmed also by the evolution withtime of the water surface sorption that was 68.5 ± 4% after5.12 s.
The bioreactor fluid dynamics was optimised in order tohave an average residence time of the fluid inside the biore-actor that allows an appreciable cell metabolic conversion tobe obtained by using the available analytic instruments. To aresidence time of 1.25 h corresponded a significantly low inletflow rate that provided an inadequate degree of mixing insidethe bioreactor. For this reason part of the stream leaving thebioreactor back to the inlet was recycled where it was mixedwith fresh medium before entering the bioreactor. The operat-ing conditions chosen with a value of recycle ratio (Qr/Qin) of5.5 gave a good mixing without any channelling or stagnantzone.
Human hepatocytes in the galactosylated membrane bioreac-tor were cultured up to 21 days with a good expression of theirdifferentiated functions.
Author's personal copy
32 S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35
Fig. 3. Urea synthesis of human hepatocytes cultured in the galactosylatedmembrane bioreactor for 21 days. The values are the mean of six experi-ments ± standard deviation.
In particular, liver-specific functions of human hepatocyteswere explored with respect to urea synthesis and albumin pro-duction. The changes in urea synthesis of hepatocytes withtime were evaluated and are reported in Fig. 3. These resultsevidenced that human hepatocytes cultured in galactosylatedmembrane bioreactor synthesized urea for the first 11 days atconstant values of about 44–50 �g/ml; a slight decrease wasmeasured on days 12 and 13 and then an increment of urea syn-thesis was observed reaching the highest value of 60 �g/ml onthe 18th day.
The albumin production increased at the beginning of theculture reaching values of 88.8 ng/ml on the 5th day (Fig. 4);then the concentrations remained at constant levels until the 10thday followed by a decrease to values of about 9–14.6 ng/ml.However, the expression of albumin synthesis was maintainedfor 21 days of culture in the galactosylated membrane bioreactor.
The gene expression of albumin and C-reactive protein wasinvestigated and reported in Fig. 5. Human hepatocytes culturedin the bioreactor expressed the genes of albumin and C-reactiveprotein like in the liver, as the mRNA evidenced.
The performance of the bioreactor in the maintenance of dif-ferentiated liver functions was also evaluated in terms of totalproteins secreted by human hepatocytes cultured in the mem-brane bioreactor. Fig. 6 evidences that also the ability to secrete
Fig. 4. Albumin production of human hepatocytes cultured in the galacto-sylated membrane bioreactor for 21 days. The values are the mean of sixexperiments ± standard deviation.
Fig. 5. (a) �-Actin (as housekeeping, top left) and albumin (bottom right) geneexpression in human hepatocytes cultured in the bioreactor. Image is representa-tive of five experiments. (b) �-Actin (as housekeeping, top left) and CRP (bottomright) gene expression in human hepatocytes cultured in the bioreactor. Imageis representative of five experiments.
proteins was maintained for whole period of culture and in arange of 47.5–540 �g/ml.
Moreover, the molecular weight (MW) of proteins containedin the culture medium was identified by native-PAGE gel elec-trophoresis (Fig. 7). The gel evidenced the presence of two verymarked bands containing proteins with MW of 66 and 140 kDa.Other bands with minor intensity corresponding to moleculeswith higher MWs were also identified.
The morphological behaviour of human hepatocytes culturedin the galactosylated membrane bioreactor was evaluated bySEM and LCSM in order to have information about the adhe-sion of cells to the surface of the modified membranes in thebioreactor. As is shown in Fig. 8, human hepatocytes adheredon the membrane exhibiting a polygonal shape and establishedcell to cell contacts with nearby cells forming aggregates. Theseobservations were confirmed by confocal microscopy inves-tigation that gives information about the distribution of thecytoskeleton proteins involved in the adhesion of the cells tothe membrane. Confocal microscopy images (Fig. 9a–c) evi-dence the 3D structure of hepatocytes after 21 days of culture ingalactosylated membrane bioreactor; cells reorganized in smallaggregates maintaining a round shape, so many of the features ofthe liver in vivo are reconstituted. In particular, the distributionof vinculin was investigated, which is a multidomain protein that
Fig. 6. Protein secretion of human hepatocytes cultured in the galactosylatedmembrane bioreactor for 21 days. The values are the mean of six experi-ments ± standard deviation.
Author's personal copy
S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35 33
Fig. 7. Qualitative analysis of proteins secreted by cells in the culture medium.Native-PAGE electrophoresis with PhastGel gradient 10–15% of proteinssecreted by human hepatocytes after 21 days of culture in the galactosylatedmembrane bioreactor. Std: standard proteins.
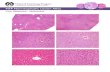
localizes to the cytoplasmic face of cell–cell and cell–membraneadhesion sites. In Fig. 9 the staining for vinculin (red), which isa protein associated with adherens junctions and focal adhesion,shows a localization of the protein at the sites of cell–cell contactand cell–membrane adhesion (white arrows) and the staining fornucleic acid (blue) evidences a central position of nuclei.
Fig. 8. SEM image of human hepatocytes after 21 days of culture in PESgalactosylated membrane bioreactor.
4. Discussion
The design of a bioreactor for tissue engineering must addressthe creation of a physiological environment for the growth ofliving tissue taking into account the material properties aimed atreconstructing the extracellular matrix (ECM) and stimulatingspecific functions, the continuous perfusion of the cells avoidingshear stress and limitation to mass transport.
In this paper the ability of galactosylated PES membranebioreactor to support the long-term maintenance of liver-specificfunctions of human hepatocytes has been explored.
The bioreactor was designed to culture hepatocytes adheredto galactosylated PES membrane under well-defined fluiddynamics conditions which allows the metabolic date unaffectedby mass transport to be obtained.
The galactosylated membrane surface represents an alterna-tive to ECM-coated substrates because of the specific interactionbetween the galactose ligand and asyaloglycoprotein receptorspresent on the hepatocytes surface. Previous studies have shownthat galactose-immobilized substrates could improve hepato-cytes adhesion and functions [33–36]. We used galactosylatedmembranes in a bioreactor that permits a constant perfusion ofthe cells and the long-term maintenance of hepatic functions.
The modification of the PES membrane surface by the immo-bilization of galactose was demonstrated by the FITC-lectinbinding experiments. Studies have revealed that the lectin used inour experiments could specifically bind galactose or N-acetyl-d-galactosamine [37]. FITC fluorescence proved to be relativelyhigh on galactosylated PES membranes, while on native PESno fluorescence signal was detected, so the fluorescence imagesclearly displayed the real immobilization of the galactose deriva-tives.
Hepatocytes in the membrane bioreactor displayed anenhanced metabolic activity, which was maintained in the cul-ture time at significantly higher levels with respect to the batchsystem [27].
In particular, the cell metabolic functions of urea synthesis,albumin and protein secretion were maintained for 21 days. Inour experiments the ability of human hepatocytes to synthesizeurea, as reported in Fig. 3, was measured in a range value of28–60 �g/ml with a peak on 18th day.
The albumin was produced at high levels in the galactosylatedmembrane bioreactor reaching the best value of 88.8 ng/ml onthe 5th day (Fig. 4). The gene expression of albumin and C-reactive protein (Fig. 5a and b) confirmed the maintenance atthe gene level of the specific activities of cells in the bioreactor.
Also the metabolic activity of human hepatocytes in termsof protein secretion was sustained for the whole investigatedexperimental period.
A characteristic feature is the formation of hepatocyte aggre-gates as SEM analysis evidenced. Hepatocytes formed tightcell–cell contact, reminiscent of native liver and showed a roundshape as the vinculin localization demonstrated (Fig. 9).
The distribution of the vinculin indicates a balanced cell–celland cell–membrane interactions. No spreading of cells wasobserved as a result of controlled interaction with membrane sur-face. In the literature it was discovered that the lack of vinculin
Author's personal copy
34 S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35
Fig. 9. Confocal images at different magnification (a–c) of human hepatocytes on PES galactosylated membrane bioreactor after 21 days of culture by vinculinstaining with primary anti-vinculin antibody and secondary antibody TRITC conjugated (red) and by nucleic acid staining with DAPI (blue). White arrows indicatecell–cell contacts and cell–membrane adhesions. Scale bar 10 �m. (For interpretation of the references to colour in this figure legend, the reader is referred to theweb version of the article.)
may decrease cell adhesion by inhibiting focal adhesion assem-bly and preventing actin polymerization. On the other hand, theoverexpression of vinculin may restore adhesion and spread-ing by promoting the recruitment of cytoskeletal proteins to thefocal adhesion complex at the site of integrin binding [38]. Vin-culin’s ability to interact with integrins to the cytoskeleton at thefocal adhesion appears to be critical for control of cytoskeletalmechanics and cell spreading. Thus, vinculin appears to playa key role in shape control based on its ability to modulatethe focal adhesion structure and function. In human hepato-cytes cultured in the galactosylated membrane bioreactor thelocalization of vinculin demonstrated the formation of cell–cellcontacts that should provide better conditions for the mainte-nance of liver-specific functions as confirmed also by metabolicdata.
The high metabolic competence of hepatocytes inside thebioreactor is also an indication of the high energy status of cellsand of high efficiency of the bioreactor. The development ofa system that improves the process oxygenation to help in end-product removal and optimise the distribution of fluid moleculesinside the cell environment is likely to be of considerable valuein improving cell maintenance.
5. Conclusions
This study has demonstrated that the galactosylated PESmembrane bioreactor is able to promote attachment, aggregateformation and the long-term maintenance of differentiated func-tions of cells outside of the body providing a microenvironmentable to elicit specific cellular responses of tissue analogues. Thishepatocyte specific bioreactor should find applications in drugtesting, toxicological studies and in tissue engineering to helpsolve problems related to human diseases.
Acknowledgements
The authors acknowledge the financial support of the ItalianMinistry of University and Research, MIUR, for the grant to theFIRB research project RBNE012B2K.
References
[1] M.N. Berry, A.M. Edwards, The Hepatocyte Review, Kluwer AcademicPublishers, Dordrecht, 2000, pp. 365–585.
Author's personal copy
S. Morelli et al. / Journal of Membrane Science 302 (2007) 27–35 35
[2] A. Bader, L. De Bartolo, A. Haverich, High level benzodiazepine andammonia clearance by flat membrane bioreactors with porcine liver cells,J. Biotechnol. 81 (2000) 95.
[3] A. Guillozo, J.M. Begue, J.P. Campion, M.N. Gascoin, C. Guguen-Guillozo, Human hepatocyte culture: a model of pharmaco-toxicologicalstudies, Xenobiotica 15 (1985) 635.
[4] M.T. Donato, J.V. Castell, M.J. Gomez-Lechon, Co-cultures of hepato-cytes with epithelial-like cell lines: expression of drug biotransformationactivities by hepatocytes, Cell Biol. Toxicol. 7 (1991) 1.
[5] J.C. Dunn, R.G. Tompkins, M.L. Yarmush, Long-term in vitro function ofadult hepatocytes in a collagen sandwich configuration, Biotechnol. Prog.7 (1991) 237.
[6] A. Sanchez, A.M. Alvarez, R. Pagan, C. Roncero, S. Vilaro, M. Benito,I. Fabregat, Fibronectin regulates morphology, cell organization and geneexpression of rat fetal hepatocytes in primary culture, J. Hepatol. 32 (2000)242.
[7] L. De Bartolo, S. Morelli, L. Lopez, L. Giorno, C. Campana, S. Salerno,M. Rende, P. Favia, L. Detomaso, R. Gristina, R. d’Agostino, E. Drioli,Biotransformation and liver specific functions of human hepatocytes inculture on RGD-immobilised plasma-processed membranes, Biomaterials26 (2005) 4432.
[8] Y. Watanabe, X. Liu, I. Shibuya, T. Akaike, Functional evaluation of poly-(N-p-vinylbenzyl-O-b-d-galactopyranosyl-[1-4]-d-gluconamide) (PVLA)as a liver specific carrier, J. Biomater. Sci. Polym. Ed. 11 (2000) 564.
[9] M.L. Yarmush, M. Toner, J.C. Dunn, A. Rotem, A. Hubel, R.G. Tomp-kins, Hepatic tissue engineering. Development of critical technologies,Ann. N.Y. Acad. Sci. 665 (1992) 238.
[10] J.W. Allen, T. Hassanein, S.N. Bathia, Advance in bioartificial liver devices,Hepatology 34 (2001) 447.
[11] A.J. Strain, J.M. Neuberger, A bioartificial liver—state of the art, Science295 (2002) 1005.
[12] L.M. Flendrig, J.W. La Soe, G.G.A. Joerning, A. Steenbeck, O.T. Karlsen,W.M.M. Bovee, N.C.J.J. Ladiges, A.A. te Velde, A.F.M. Chamuleau, Invitro evaluation of a novel bioreactor based on an integral oxygenator anda spirally wound nonwoven polyester matrix for hepatocyte culture as smallaggregates, J. Hepatol. 26 (1997) 1379.
[13] J. Gerlach, N. Schnoy, M.D. Smith, P. Neuhaus, Hepatocyte culture betweenwoven capillary networks—a microscopy study, Artif. Organs 18 (1994)226.
[14] H.O. Jauregui, C.J.P. Mullon, D. Trenkler, S. Naik, H. Santangini, P. Press,T.E. Muller, B.A. Solomon, In vivo evaluation of a hollow fiber liver assistdevice, Hepatology 21 (1995) 460.
[15] S.L. Nyberg, R.A. Shatford, M.W. Peshwa, J.G. White, F.B. Cerra, W.S. Hu,Evaluation of a hepatocyte-entrapment hollow fiber bioreactor: a potentialbioartificial liver, Biotechnol. Bioeng. 41 (1992) 194.
[16] J.F. Patzer, Advances in bioartificial liver assist devices, Ann. N.Y. Acad.Sci. 944 (2001) 320.
[17] L. De Bartolo, G. Jarosch-Von Schweder, A. Haverich, A. Bader, A novelfull-scale flat membrane bioreactor utilizing porcine hepatocytes: cell via-bility and tissue-specific functions, Biotechnol. Prog. 16 (2000) 102.
[18] K.E. Dionne, B.M. Cain, R.H. Li, W.J. Bell, E.J. Doherty, D.H. Rein,M.J. Lysaght, F.T. Gentile, Transport characterization of membranes forimmunoisolation, Biomaterials 17 (1996) 257.
[19] E. Curcio, L. De Bartolo, G. Barbieri, M. Rende, L. Giorno, S. Morelli, E.Drioli, Diffusive and convective transport through hollow fiber membranesfor liver cell culture, J. Biotechnol. 117 (2005) 309.
[20] Y. Martin, P. Vermette, Bioreactor for tissue mass culture: design, charac-terization and recent advances, Biomaterials 26 (2005) 7481.
[21] A. Bader, E. Knop, A. Kern, K. Boker, N. Fruhauf, O. Crome, H. Esselmann,C. Pape, G. Kempka, K.-Fr. Sewing, 3-D coculture of hepatic sinusoidalcells with primary hepatocytes design of an organotypical model, Exp. CellRes. 226 (1996) 223.
[22] J. Gerlach, N. Schnoy, J. Vienken, M.D. Smith, P. Neuhaus, Comparisonof hollow fibre membranes for hepatocyte immobilisation in bioreactors,Int. J. Artif. Organs 19 (1996) 610.
[23] F.J. Wu, J.R. Friend, C.C. Hsiao, M.J. Zilliox, W.J. Ko, F.B. Cerra, W.S.Hu, Efficient assembly of rat hepatocytes spheroids for tissue engineeringapplications, Biotechnol. Bioeng. 50 (1996) 404.
[24] J. Rozga, F. Williams, M.S. Ro, D.F. Neuzil, T.D. Giorgio, G. Backfisch,A.D. Moscioni, R. Hakim, A.A. Demetriou, Development of a bioartificialliver: properties and function of a hollow-fiber module inoculated with livercells, Hepatology 17 (1993) 258.
[25] M.J. Powers, D.M. Janigian, K.E. Wack, C.S. Baker, D. Beer Stolz,L.G. Griffith, Functional behaviour of primary rat liver cells in athree-dimensional perfused microarray bioreactor, Tissue Eng. 8 (2002)499.
[26] T.G. Park, Perfusion culture of hepatocytes within galactose-derivatizedbiodegradable poly(lactide-co-glycolide) scaffolds prepared by gas foam-ing of effervescent salts, J. Biomed. Mater. Res. 59 (2002) 127.
[27] L. De Bartolo, S. Morelli, M. Rende, S. Salerno, L. Giorno, L.C. Lopez,P. Favia, R. d’Agostino, E. Drioli, Galactose derivative immobilized glowdischarge processed PES membranes maintain the metabolic activity ofhuman and pig liver cells, J. Nanosci. Nanotechnol. 6 (2006) 2344.
[28] L. Detomaso, R. Gristina, G.S. Senesi, R. d’Agostino, P. Favia, Stableplasma-deposited acrylic acid surfaces for cell culture applications, Bio-materials 26 (2005) 3831.
[29] S. Moore, K. Link, Carbohydrate characterization, J. Biol. Chem. 133(1940) 293.
[30] Yin, L. Ying, P.C. Zhang, R.X. Zhuo, E.T. Kang, K.W. Leong, H.Q. Mao,High density of immobilized galactose ligand enhances hepatocyte attach-ment and function, J. Biomed. Mater. Res. 67 (2003) 1093.
[31] L. De Bartolo, S. Salerno, L. Giorno, S. Morelli, G. Barbieri, E. Curcio, M.Rende, E. Drioli, Membrane bioreactor using pig hepatocytes for in vitroevaluation of anti-inflammatory drugs, Catal. Today 118 (2006) 172.
[32] N. Krasteva, T.H. Groth, F. Fey-Lamprecht, G. Altankov, The role of surfacewettability on hepatocytes adhesive interactions and function, J. Biomater.Sci. Polym. Ed. 12 (2001) 613.
[33] W. Xu, H. Baribault, E.D. Adamson, Vinculin knockout results in heartand brain defects during embryonic development, Development 125 (1998)327.
[34] S.T. Lopina, G. Wu, E.W. Merrill, C.L. Griffith, Hepatocyte culture oncarbohydrate-modified star polyethylene oxide hydrogels, Biomaterials 17(1996) 559.
[35] H.F. Lu, W.S. Lim, Z.Q. Tang, P.C. Zhang, K.W. Leong, S.M. Chia, H. Yu,H.Q. Mao, Galactosylated PVDF membrane promotes hepatocyte attach-ment and functional maintenance, Biomaterials 24 (2003) 893.
[36] L. Ying, C. Yin, R.X. Zhuo, K.W. Leong, H.Q. Mao, E.T. Kang, K.G. Neoh,Immobilization of galactose ligands on acrylic acid graft-copolymerizedpoly(ethylene terephthalate) film and its application to hepatocyte culture,Biomacromolecules 4 (2003) 157.
[37] C.P. Swaminatahn, A. Gupta, N. Surolia, A. Surolia, Plasticity in the pri-mary binding site of galactose/N-acetylgalactosamine-specific lectins, J.Biol. Chem. 275 (2000) 28483.
[38] R.M. Ezzell, W.H. Goldmann, N. Wang, N. Parasharama, D.E. Ingber,Vinculin promotes cell spreading by mechanically coupling integrins tothe cytoskeleton, Exp. Cell Res. 231 (1997) 14.
Related Documents