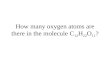How many Atoms are in the K 3 PO 4 molecule? A.8 B.3 C.2 D.7 :30

How many Atoms are in the K 3 PO 4 molecule?
Dec 31, 2015
How many Atoms are in the K 3 PO 4 molecule?. 8 3 2 7. :30. How many different Atoms are in the KCl 3 Na 2 molecule?. 4 2 3. 1. :30. 21.3 g of sugar are mixed with water in a beaker. The resulting solution of sugar water has a mass of 81.6g. How much water was in the beaker?. - PowerPoint PPT Presentation
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

How many Atoms are in the K3PO4 molecule?
A. 8B. 3C. 2D. 7
:30

How many different Atoms are in the KCl3Na2 molecule?
A. 4B. 2C. 3.D. 1
:30

21.3 g of sugar are mixed with water in a beaker. The resulting solution of sugar water has a mass of 81.6g.
How much water was in the beaker?
A. 81.6gB. Not enough infoC. 102.9gD. 60.3g
:30

Choose the correct balanced equation Na2S + HCl NaCl +H2S
A. 2Na2S + 2HCl2Nacl + H2S
B. Na2S + 2HCl 2NaCl + H2S
C. Na2S + 2HCl 2NaCl +H2S
D. It’s already balanced.
:30

KI + Cl2 KCl + I2 This equation can be classified as a
A. Combination reaction
B. Single replacement reaction
C. Double replacement reaction
D. Decomposition reaction
:30

This equation can be classified as a H2O + SO3 H2SO4 .
A. Combination reaction
B. Single replacement reaction
C. Double replacement reaction
D. Decomposition reaction
:30

This equation can be classified as a Ag2O Ag + O2
A. Combination reaction
B. Single replacement reaction
C. Double replacement reaction
D. Decomposition reaction
:30

This equation can be classified as a AgNO3 + KCl AgCl + KNO3
A. Combination reaction
B. Single replacement reaction
C. Double replacement reaction
D. Decomposition reaction
:30

How does a balanced equation demonstrate the conservation of mass?
A. It has the same mass before and after the reaction
B. It has the same number of atoms before and after the reaction
C. It has the same type of atoms before and after the reaction
D. All of the above
:30

What do all floating objects have in common?
A. They displace an equal mass of liquid as their own mass
B. They displace an equal volume of the liquid as their own volume
C. They are less dense than the liquid they are floating in
D. They displace more liquid mass than their own mass :30

What do all sinking objects have in common?
A. They are more dense than the liquid they are floating in
B. They displace less volume of the liquid as their own volume
C. They displace an equal mass of liquid as their own mass
D. The mass of displaced liquid is less than their mass.
:30

Which displaces more water?
A. shipB. icebergC. NeitherD. Unable to determine
:30

Which is more dense?
A. shipB. icebergC. NeitherD. Unable to determine

Based on this diagram, what is the most likely density of the iceberg? (Assume a density of 1.03 g/mL for
seawater.) A. 0.91 g/ccB. 0.28 g/ccC. 1.08 g/ccD. 0.56 g/cc
:30

My gold ring had a mass of 102g. It displace 10.4 mL of H2O. What was its density?
A. 9.81 g/ccB. 9.80 g/ccC. 0.12 g/ccD. 1061 g/cc
:30

My boat has a mass of 534 kg, a length of 7M, width of 3M and a height of 2M, what mass of water will it displace when floating in the lake?
A. 42 cubic metersB. 42,000 KgC. 534 KgD. 22,428 Kg
:30

A toy boat sits in a bucket of water. A rock is then placed in the boat. Does the water level in
the bucket
A. Get lowerB. Stay the sameC. Get higherD. Not change
:30

An object sits at the bottom of a beaker as shown. Assuming that the graduated cylinder was empty when the object was placed in the beaker and that the beaker was full to the level
of the spout, what must be true?
A. The mass of the water in the graduated cylinder is greater than the mass of the object.
B. The mass of the water in the graduated cylinder is equal to the mass of the object...
C. The volume of the water in the graduated cylinder is greater than the volume of the object.
D. The volume of the water in the graduated cylinder is equal to the volume of the object.
:30

When the ball was put into the beaker, it displaced an amount of water into the graduated cylinder at the left. Given that the liquid in the beaker is water (density = 1 g/mL), what is the mass of the object?
A. 10 g.B. 5 gC. 10 mLD. 1 g/mL
:30

Assuming that the graduated cylinder was empty when the object was placed in the beaker and that the beaker was full to the level of the spout, what
must be true?
A. The density of the object is greater than the density of water
B. The mass of the water in the graduated cylinder is equal to the mass of the object.
C. The volume of the water in the graduated cylinder is greater than the volume of the object
D. The volume of the water in the graduated cylinder is equal to the volume of the object
:30

Which characteristic is not true of Acids?
A. Acids are sourB. Acids burn skin tissueC. Acids are slipperyD. Acids have more H+ ions
than OH- ions
:30

Which characteristic is not true of bases?
A. Bases are bitterB. Bases burn skin
tissueC. Bases are slipperyD. Bases have more H+
ions than OH- ions
:30

The pH scale runs from 0 to 14 with 0 representing the highest concentration of OH- ions
A. TrueB. False
:30

Alkaline substances have a pH of greater than 7
A. TrueB. False
:30

Drano is a strong base that dissolves organic matter to unclog drains. What is the most likely pH of Drano?
A. 7B. 9C. 13D. 2
:30

Suppose you have already compared the pH values of two substances using 0-14 paper. When would a
follow-up test using 4.5-7.5 paper be most helpful?
A. When both substances have a pH of about 3
B. When both substances have a pH of about 8.
C. When both substances have a pH of about 5
D. When one substance has a pH of 3 and the other a pH of 8
:30

What is the most likely pH of apple juice?
A. 3.8
B. 7.2C. 6.5D. 1.2
:30

Chemicals in the air can combine with rain to produce acid rain, which is harmful to the environment. Which
of these is most likely to be the pH of acid rain?
A. 1.5B. 3.8C. 5.6D. 6.9
:30

Place these unknown pH test papers in order from
most acidic to most alkaline
A. C,D,A,BB. D,C,A,BC. D,C,B,AD. B,A,C,D
:30

Enter question text...
A. Enter answer text...B. Enter answer text...C. Enter answer text...D. Enter answer text...
:30
Related Documents