Enzymes Organic Catalysts

Enzymes Organic Catalysts. Enzymes are Proteins! Proteins contain the elements: C, H, O, N The monomers of proteins are Amino acids.
Dec 18, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Enzymes are Proteins!• Proteins contain the elements: C, H, O, N• The monomers of proteins are Amino acids
• Facilitate or catalyze chemical reactions in cells– Speed them up– By lowering the activation energy (the energy needed to start the reaction)
– W/O enzymes, chemical reactions would occur too slowly at the temperatures found in organisms to sustain life processes
Characteristics of Enzymes
A Catalyst Analogy*
• You are at the American Museum of Natural History (because you are a science nerd and like to look at Dinosaur Bones
• You need to get to Babo (because you are a big fan of Iron Chef)
• What are your transportation options?
*Analogy provided thanks to Ms.Walker
• You can walk…• If you walk like a New Yorker – about 1 block a minute – you would walk over 80 blocks – so an hour and 20 minutes.
• Longer if you walk like a Livingston High School student on their way to class
• You can take the C from 81st to West 4th St
• Once the train arrives – about 15-17 minutes.
• Less time if you can transfer to the A
A Catalyst Analogy*
*Analogy provided thanks to Ms.Walker
• 3D shape (tertiary or quaternary) confers specificity to the substrate upon which it binds to facilitate the reaction
• Many enzymes are named after the substrate on which they act and end in
–ase • Sucrase (enzyme) acts upon sucrose (substrate)
Characteristics of Enzymes
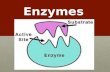
• Substrate = Reactant Molecule
• Active site= specific binding site on the enzyme for the substrate to bind to
Lock and Key Model
An exact fit between enzyme and substrate; The enzyme works with one specific
substrate
Induced Fit ModelEnzyme can react with similar substrate molecules to produce the same product; The active site of enzyme undergoes shape change
How Enzymes WorkEnzymes bind to the substrate and weaken the chemical bonds, so less energy is needed for
the reaction to proceed
Factors that affect Enzymes
• Enzymes are affected by their environment• There are optimal conditions for every enzyme
• Optimal conditions = optimal performance• Deviations= Denaturization = Loss of Function– Substrate & Enzyme Concentration– Higher temperatures– Changes in salt concentrations & pH
Substrate Concentration
• Enzymes must “bump into” substrate molecules
• More substrate =more collisions =more enzymes being used=more activity
• At some point activity levels off because all possible enzymes are already in use
Enzyme Concentration
• Again, enzymes must “bump into” substrate molecules
• More enzymes= more collisions = more activity
• At some point all substrates are being broken down and so increasing enzymes no longer increases activity
pH• Every enzyme has an “optimal” pH– a pH at which the enzyme functions most effectively
• Most enzymes in the human body work best between pH 6-8 (near neutral)
Temperature• Every enzyme has an
optimal temperature• Most enzymes in the
human body work best between 35-40˚C (near normal body temps)
• Increase in Temp = increase in molecular collisions = increase in enzyme activity
• However, this only hold true to a point!– Too much heat can denature
the protein and slow activity down or cause loss of function
What is Energy?• Energy– The ability to do work
• Potential– Stored Energy– Chemical Energy = potential energy of molecules
• Kinetic– Energy of doing work (motion)– Heat
• Energy associated with the movement of molecules
Life Depends on Energy Conversion
Thermodynamics =The study of energy conversions• Two laws govern energy conversion:– First Law of Thermodynamics• The law of energy conservation• Energy can be transferred and transformed, but not created or destroyed
– Second Law of Thermodynamics• Energy conversions reduce order (increase disorder)• Amount of disorder = Entropy
Chemical Reactions• Chemical reactions are involved when energy conversions occur– Atoms are rearranged– Law of Conservation of Matter– Matter is neither created nor destroyed
• The sum of all chemical reactions in an organism– Metabolism
• Chemical reactions either store or release energy
Endothermic and Exothermic ReactionsChemical reactions either store or release energy
• Endergonic Reactions = Endothermic Reactions– Store energy– Yield products high in potential energy– Photosynthesis
• Exergonic Reactions = Exothermic Reactions– Release energy– Potential energy is high in the reactants– Cellular Respiration
Comparing Endothermic and Exothermic
ReactionsExothermic Reaction Endothermic Reactions
Net release of energy as products are formed (often in the form of light or heat)
Net input of energy needed as products are formed
Reactants have more PE than products Reactants have less PE than products
Reactants are less stable than the products
Reactants are more stable than the products
Less energy is needed to start reaction than is released as products are formed
More activations energy is needed than energy released as products form
Includes Cell Respiration and Hydrolysis Includes Photosynthesis and Synthesis
Coupled Reactions
• Many reactions use the energy released from exothermic reactions to drive or fuel endothermic reactions
• These are called “Coupled Reactions”– ATP Regeneration is the main example in cells• The usable energy released by most exothermic reactions is stored in ATP• The energy used in most endothermic reactions comes from ATP
Related Documents