%E JOURNAL OF ~IOWGIGAL CHEMISTRY 0 1994 by The American Society for Biochemistry and Molecular Biology, Inc. Val. 269, No. 33, Issue of August 19, pp. 20995-21002, 1994 Printed in U.S.A. Chimeric Enzymes STRUCTURE-FUNCTION ANALYSIS OF SEGMENTS OF sn-1,Z-DIACYLGLYCEROL CHOLINE- AND ETHANOLAMINEPHOSPHOTRANSFERASES* (Received forpublication, May 5, 1994, and in revised form, June 14, 1994) Russell H. yjelmstad, Sherry C. MorashS, Christopher R. McMasterS, and Robert M. Bell§ From the Departments of Molecular Cancer Biology and Biochemistry, Duke University Medical Center, Durham, Nokh Carolina 27710 The Saccharomyces cereuisiae CPTl and EPTl genes represent structural genes that encode distinct choline- and choline/ethanolaminephosphotransferases, respec- tively. To explore the function of linear segments of these enzymes, a series of 14 EPTl-CPT1 chimeric gene constructs and the parental wild-type genes were ex- pressed in a cptl eptl double null mutant background completely devoid of phosphoamino alcohol transferase activity. Eleven of the chimeric genes expressed func- tional enzymes. The CDP-amino alcohol and sn-l,%di- acylglycerol (DAG) substrate specificities and essential phospholipid cofactor requirements of the parental and chimeric enzymes wereinvestigated using a mixed mi- cellar assay system. Chimeric enzymes exhibited a pat- tern of CDP-amino alcohol affinities that defined a structural domain sufficientto confer CDP-amino alco- hol specificity. When wild-type enzymes were investi- gated using a chemically defined series of DAGS, each possessed a distinct characteristic pattern of utilization. Chimeric enzymes exhibited DAG acyl chain specificity profiles that either conformed to parental wild-type pat- terns or represented novel substrate specificities. Cor- relation of these outcomes with their underlying struc- tural modifications permitted the assignment of an internal, linear region of 218 amino acids sufficient to confer DAG acyl chain specificity; this region contained three predicted transmembrane segments. Neither wild- type enzyme showed significant acyl chain selectivity with respect to phospholipid activation when a homol- ogous series of chemically defined phosphatidylcholines were employed, suggesting that enzyme recognition of the fatty acyl moieties of the DAG substrate and phos- pholipid activator is fundamentally different. Analysis of chimeric enzymes dependenceon phospholipid acti- vators suggested the involvement of discontinuous pro- tein segments participating in the interaction with phospholipid cofactors. Phosphatidylcholine (PC)’ is the major phospholipid compo- nent of most eukaryotic cells (Zinser et al., 1991; Henry, 1982; Grant GM20015 (to R. M. B.). The costs of publication of this article * This work was supported in part by National Institutes of Health were defrayed in part by the payment of page charges. This article must therefore be hereby marked “aduertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. ?j Recipient of Medical Research Council of Canada fellowship awards. $ To whom correspondence should be addressed: Dept. of Molecular Cancer Biology, Box 3711, Duke University Medical Center, Durham, ‘The abbreviations used are: PC, phosphatidylcholine; PE, phos- phatidylethanolamine; DAG, diacylglycerol; CFT, cholinephosphotrans- ferase; EPT, ethanolaminephosphotransferase; DiC18:1, l,%-dioleoyl- glycerol; DiC16:1, 1,2-dipalmitoleoylglycerol. NC 27710. Tel.: 919-684-5316; Fax: 919-684-6134. White, 1973) and is a reservoir for second messenger signaling molecules (Exton, 1990; Pelech and Vance, 1989; Billah and Anthes, 1990). Previous work from this laboratory resulted in the isolation of genomic clones for distinct structural genes en- coding a cholinephosphotransferase (CPTl ) (Hjelmstad and Bell, 1987, 1990; Hosaka and Yamashita, 1987) and a choline/ ethanolaminephosphotransferase (EPTl ) (Hjelmstad and Bell, 1988, 1991a) in Saccharomyces cereuisiae. These genes encode distinct enzymes that catalyze the synthesis of PC and PCiPE in vitro from DAG using CDP-choline or CDP-cholineXDP-eth- anolamine, as phosphoalcohol donors (Hjelmstad and Bell, 1991b). Chromosomalsingle anddouble disruption mutants in these loci were generated anddefinitively established that the CPTl and EPTl genes represent the complete set of genes en- coding choline- and ethanolaminephosphotransferases in yeast (Hjelmstad and Bell, 1987, 1988, 1991~). The complete nucle- otide sequences of both genes were determined and predicted highly similar gene products with 53% amino acid identity. Expression of the wild-type EPTl and CPTl genes in strains bearing a null mutation in the cognate locus permitted detailed enzymological analysis of the individual gene products without the need for enzyme purification. Using a mixed micellar assay system, characteristic biochemical properties of the CPTl and EPTl gene products with respect to substrate specificities, es- sentialactivation by divalent metal ions and phospholipid, and inhibition by the end-product CMP were determined (Hjelmstad and Bell, 1991b). In this work, the genetic and biochemical system developed in our laboratory for the study of yeast choline- and ethanol- aminephosphotransferases was further exploited for the con- struction, expression, and characterization of EPT1:CPTl gene product chimeras. Using this approach, internal linear subseg- ments of these enzymes were identified that confer substrate specificity and structural determinants of lipid activation. Cor- relation of these findings to structural models for these pro- teins provided penetrating insight into their structure and function. EXPERIMENTAL PROCEDURES Materials-Defined acyl species of PCs were products of Avanti Polar Lipids, and homologous DAGs were prepared from them by digestion with phospholipase C. Concentrations of the resulting DAGs were de- termined by measurement of total acyl ester (Stern and Shapiro, 1953). CMP was purchased from Sigma. Growth media and reagents for mo- lecular biological manipulations were obtained as described previously (Hjelmstad and Bell, 1987, 1991a, 1991b). Seaplaque low gelling agarose was a product of FMC Bioproducts. [-p32P]ATP was obtained from Du Pont NEN and radiolabeled substrates [32P]CDP-choline and [32P1CDP-ethanolamine were prepared as described previously (Hjelmstad and Bell, 1991). Strains, Media, Growth Conditions, and PLasmidsS. cereuisiae strain DBY746 (a leu2-3 leu2-112 his3-1 ura3-52 trpl-289) was ob- tained from the Yeast Genetics Stock Center. Strain HJO91, used for expression of chimeric enzymes, was constructed by integrative trans- 20995

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
%E JOURNAL OF ~ I O W G I G A L CHEMISTRY 0 1994 by The American Society for Biochemistry and Molecular Biology, Inc.
Val. 269, No. 33, Issue of August 19, pp. 20995-21002, 1994 Printed in U.S.A.
Chimeric Enzymes STRUCTURE-FUNCTION ANALYSIS OF SEGMENTS OF sn-1,Z-DIACYLGLYCEROL CHOLINE- AND ETHANOLAMINEPHOSPHOTRANSFERASES*
(Received for publication, May 5, 1994, and in revised form, June 14, 1994)
Russell H. yjelmstad, Sherry C. MorashS, Christopher R. McMasterS, and Robert M. Bell§ From the Departments of Molecular Cancer Biology and Biochemistry, Duke University Medical Center, Durham, Nokh Carolina 27710
The Saccharomyces cereuisiae CPTl and EPTl genes represent structural genes that encode distinct choline- and choline/ethanolaminephosphotransferases, respec- tively. To explore the function of linear segments of these enzymes, a series of 14 EPTl-CPT1 chimeric gene constructs and the parental wild-type genes were ex- pressed in a cptl eptl double null mutant background completely devoid of phosphoamino alcohol transferase activity. Eleven of the chimeric genes expressed func- tional enzymes. The CDP-amino alcohol and sn-l,%di- acylglycerol (DAG) substrate specificities and essential phospholipid cofactor requirements of the parental and chimeric enzymes were investigated using a mixed mi- cellar assay system. Chimeric enzymes exhibited a pat- tern of CDP-amino alcohol affinities that defined a structural domain sufficient to confer CDP-amino alco- hol specificity. When wild-type enzymes were investi- gated using a chemically defined series of DAGS, each possessed a distinct characteristic pattern of utilization. Chimeric enzymes exhibited DAG acyl chain specificity profiles that either conformed to parental wild-type pat- terns or represented novel substrate specificities. Cor- relation of these outcomes with their underlying struc- tural modifications permitted the assignment of an internal, linear region of 218 amino acids sufficient to confer DAG acyl chain specificity; this region contained three predicted transmembrane segments. Neither wild- type enzyme showed significant acyl chain selectivity with respect to phospholipid activation when a homol- ogous series of chemically defined phosphatidylcholines were employed, suggesting that enzyme recognition of the fatty acyl moieties of the DAG substrate and phos- pholipid activator is fundamentally different. Analysis of chimeric enzymes dependence on phospholipid acti- vators suggested the involvement of discontinuous pro- tein segments participating in the interaction with phospholipid cofactors.
Phosphatidylcholine (PC)’ is the major phospholipid compo- nent of most eukaryotic cells (Zinser et al., 1991; Henry, 1982;
Grant GM20015 (to R. M. B.). The costs of publication of this article * This work was supported in part by National Institutes of Health
were defrayed in part by the payment of page charges. This article must therefore be hereby marked “aduertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
?j Recipient of Medical Research Council of Canada fellowship awards.
$ To whom correspondence should be addressed: Dept. of Molecular Cancer Biology, Box 3711, Duke University Medical Center, Durham,
‘The abbreviations used are: PC, phosphatidylcholine; PE, phos- phatidylethanolamine; DAG, diacylglycerol; CFT, cholinephosphotrans- ferase; EPT, ethanolaminephosphotransferase; DiC18:1, l,%-dioleoyl- glycerol; DiC16:1, 1,2-dipalmitoleoylglycerol.
NC 27710. Tel.: 919-684-5316; Fax: 919-684-6134.
White, 1973) and is a reservoir for second messenger signaling molecules (Exton, 1990; Pelech and Vance, 1989; Billah and Anthes, 1990). Previous work from this laboratory resulted in the isolation of genomic clones for distinct structural genes en- coding a cholinephosphotransferase (CPTl ) (Hjelmstad and Bell, 1987, 1990; Hosaka and Yamashita, 1987) and a choline/ ethanolaminephosphotransferase (EPTl ) (Hjelmstad and Bell, 1988, 1991a) in Saccharomyces cereuisiae. These genes encode distinct enzymes that catalyze the synthesis of PC and PCiPE in vitro from DAG using CDP-choline or CDP-cholineXDP-eth- anolamine, as phosphoalcohol donors (Hjelmstad and Bell, 1991b). Chromosomal single and double disruption mutants in these loci were generated and definitively established that the CPTl and EPTl genes represent the complete set of genes en- coding choline- and ethanolaminephosphotransferases in yeast (Hjelmstad and Bell, 1987, 1988, 1991~). The complete nucle- otide sequences of both genes were determined and predicted highly similar gene products with 53% amino acid identity.
Expression of the wild-type EPTl and CPTl genes in strains bearing a null mutation in the cognate locus permitted detailed enzymological analysis of the individual gene products without the need for enzyme purification. Using a mixed micellar assay system, characteristic biochemical properties of the CPTl and EPTl gene products with respect to substrate specificities, es- sential activation by divalent metal ions and phospholipid, and inhibition by the end-product CMP were determined (Hjelmstad and Bell, 1991b).
In this work, the genetic and biochemical system developed in our laboratory for the study of yeast choline- and ethanol- aminephosphotransferases was further exploited for the con- struction, expression, and characterization of EPT1:CPTl gene product chimeras. Using this approach, internal linear subseg- ments of these enzymes were identified that confer substrate specificity and structural determinants of lipid activation. Cor- relation of these findings to structural models for these pro- teins provided penetrating insight into their structure and function.
EXPERIMENTAL PROCEDURES Materials-Defined acyl species of PCs were products of Avanti Polar
Lipids, and homologous DAGs were prepared from them by digestion with phospholipase C. Concentrations of the resulting DAGs were de- termined by measurement of total acyl ester (Stern and Shapiro, 1953). CMP was purchased from Sigma. Growth media and reagents for mo- lecular biological manipulations were obtained as described previously (Hjelmstad and Bell, 1987, 1991a, 1991b). Seaplaque low gelling agarose was a product of FMC Bioproducts. [-p32P]ATP was obtained from Du Pont NEN and radiolabeled substrates [32P]CDP-choline and [32P1CDP-ethanolamine were prepared as described previously (Hjelmstad and Bell, 1991).
Strains, Media, Growth Conditions, and PLasmidsS. cereuisiae strain DBY746 (a leu2-3 leu2-112 his3-1 ura3-52 trpl-289) was ob- tained from the Yeast Genetics Stock Center. Strain HJO91, used for expression of chimeric enzymes, was constructed by integrative trans-
20995
20996 Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases
i m E
CPTl 1 1 1
EPTl
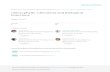
ture of CPT1-EPT1 chimeric en- FIG. 1. Construction and nomencla-
zymes. Chimeric enzymes were synthe- Y sized from appropriate restriction sites within the parent enzymes as described under "Experimental Procedures."
z - B
- 0
z 5 I Coding Frame I
formation of the cptl::LEU2 null mutation carried on the previously described plasmid pRH105 (Hjelmstad and Bell, 1987) into the eptl point mutant HJ729 (eptl-1) (Hjelmstad and Bell, 1988). The resulting cptl::LEU2 eptl-l strain was designated HJO91, and its genetic char- acteristics were contirmed by cholinephosphotransferase colony auto- radiography, mitotic stability of the cpt1::LEUZ integrant on nonselec- tive media, and cosegregation of the cpt- and leu+ phenotypes in tetrad analysis as reported previously for the construction of HJOOO (Hjelms- tad and Bell, 1991a). Yeast were cultured in synthetic minimal media containing the appropriate supplements at 20 pg/ml as required.
Recombinant DNA Techniques-The coding portions of the CPTl and EPTl genes were analyzed for restriction enzyme sites, pre-existing or inducible by single or double mutations that would preserve amino acid primary structure, which would facilitate precise joining of segments of the two genes within the context of their homology alignment. As shown in Fig. 1, six sites were chosen that satisfied these criteria and required the introduction of nine new mutations. The latter were produced in M13 phage subclones available from CPTl and EPTl DNA sequencing projects (Hjelmstad and Bell, 1990, 1991a) using custom synthesized mutant oligonucleotides and a commercially available oligonucleotide- directed in vitro mutagenesis kit from Amersham Corp. For the CPTl gene, the precise locations of the restriction enzyme cleavage sites, the required nucleotide mutations (sense strand), and the locations of the splice junctions within the amino acid reading frames were as follows: NarI, nucleotides 705-710, nucleotide 708 A + G, Ala6'-Profi2; ApaI, nucleotides 894-899, none, within Pro'26; EcoRI, nucleotides 1081- 1086, none, within Phels7; HincII, nucleotides 1210-1215, nucleotide 1215 A + C: within GluZ3'; BamII, nucleotides 1366-1371, nucleotide 1368 T + G and nucleotide 1371 T + C, within Gly2"; and ScaI, nucleotides 1563-1568, nucleotide 1563 G + A and nucleotide 1567 T +
C, within For the EPTl gene, the analogous locations and mu- tations were as follows: NarI, none, Ala47-Pro48; ApaI, nucleotides 978- 983, nucleotide 980 A- G, within Pro"'; EcoRI, nucleotides 1164-1169, nucleotide 1166 G --* A and nucleotide 1169 T + C, within Phe'73; HincII, nucleotides 1293-1298, nucleotide 1295 A + C, Va1215-Asp216; BamII, nucleotides 1443-1448, nucleotide 1445 T - G and nucleotide 1448 T + C; and ScaI, nucleotides 1640-1645, nucleotide 1644 T + C, within Val331.
Following oligonucleotide-directed in vitro mutagenesis, the desired nucleotide changes were directly confirmed by dideoxynucleotide se- quence analysis of the M13 phage inserts using universal primers or primers previously synthesized for original gene sequencing.
Using standard plasmid construction techniques, the mutation-bear- ing M13 inserts were reconstituted into the appropriate full-length genes. Together with previously reported CPT1- and EPTI-bearing plasmids, these were then utilized in the construction of the chimeric genes shown in Fig. 1. All of the EPTl-CPTl chimeric genes were inserted into the multiple cloning site of the standard yeast plasmid
The A + C mutation at CPTl nucleotide 1215 mutates GluZ3' to Asp23o, but since the EPTl amino acid at this position is Asp, this effectively shifts the splice junction to Va1229-G1u230 without a mutation.
10-60
m.. 10-50
Lpu\" - 10-40
10-30
" 7 30-60
30-50
40-60
40-50
YEp352 (Hill et al., 1986). In each case, the 5' sequences proximal to the coding region were derived from EPTl and extended to the EcoRI site depicted in Fig. 1. In chimeric constructs bearing CPTl-derived car- boxyl-terminal sequences, the 3' sequences distal to the coding frame were derived from CPTl and extended to near the EcoRV site depicted in Fig. 1. In chimeric constructs bearing EPT1-derived carboxyl-termi- nal sequences, the 3' sequences distal to the coding frame were derived from and extended to near the XhoI site depicted in Fig. 1.
Isolation of Membranes and Enzyme Assays-Yeast cultures were grown and membranes prepared from them as described previously (Hjelmstad and Bell, 1987). All membranes were prepared from cul- tures grown on appropriately supplemented minimal media to mid- logarithmic phase.
Enzyme Assays-Cholinephosphotransferase and ethanolamine- phosphotransferase colony autoradiography assays and mixed micellar assays were performed as described previously (Hjelmstad and Bell, 1992). The radiochemical specific activity of [y-32PlCDP-choline and CDP-ethanolamine were varied as necessary to efficiently detect the varying enzymatic activities of the chimeric enzymes.
RESULTS AND DISCUSSION
Rationale-The biochemical analysis of novel chimeric gene products has been widely used to study the structure and func- tion of closely related proteins, such as receptors that exhibit high affinity interaction with small molecules (Czech et al., 1993; McAIlister et al., 1993; Gether et al., 1993; Schaffer et al., 1993; Schumacher et al., 1993; Heguy et al., 1993). More lim- ited and less systematic studies have been performed on en- zymes (Blotstein et al., 1993). In this approach, linear and segmental protein determinants of a biochemical property can be deduced on the basis of the biochemical effect of substituting a segment derived from one protein into the homologous region of a related protein when the two proteins are distinguishable with respect to the analytic property. This approach can pro- vide powerful insight into the function of primary structural regions.
The yeast system developed for the study of choline- and ethanolaminephosphotransferases manifested three prerequi- site features, which rendered it suitable for structure-function studies using a chimeric enzyme approach. First, the CPTl and EPTl loci are paired structural genes that bear sufficient DNA sequence homology to engineer splice junctions while avoiding point mutations. Furthermore, the predicted gene products are highly similar with uninterrupted homology alignments and similar predicted secondary structures. Thus, EPTl-CPTl chi- meric genes could be constructed and reasonably anticipated to express functional products. Second, multiple discriminating
Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases 20997
TABLE I In vitro activities of parent and chimeric choline- and
ethanolaminephosphotransferases Enzyme activities were determined in the membrane fraction of
strain HJO91 harboring the indicated chimeric genes on the high copy plasmid YEp352 as described under “Experimental Procedures.” DiC18:l was the diacylglycerol source except where indicated.
Cholinephosphotransferase Ethanolamine- Enzyme activity
transgrase phos ho
DiC,,., DiC,,:, activity
1.4
2.0
n.dl
0.01
0.1 0
0.49
0.78
10.4
nmol/min/rng
7.4
8.9
n.d
0.01
0.1 2
0.22
0.31
4.3
0.00
0.04
n.d
0.02
0.74
1.8
1.4
21.9
’ n.d., not detectable.
TABLE I1 In vitro activities of chimeric choline- and
ethanolaminephosphotransferases Enzyme activities were determined in the membrane fraction of
strain HJO91 harboring the indicated chimeric genes on the high copy plasmid YEp352 as described under “Experimental Procedures.” DiC18:l was the diacylglycerol source except where indicated.
Cholinephosphotransferase Ethanolarnine- Enzyme activity phos ho
transiraie activity DiC,,, DiC,,.,
10-60 Va
10-50 W‘x” - 10-40
10-30
30-60 -ssl
30-50 - 40-60 -* - 40-50
2.5
0.67
n.dl
0.42
n.d
0.01
0.05
0.1 5
nmol/mm/rng
13.4
5.5
n.d
0.55
n.d
0.02
0.06
0.09
0.03
0.01
n.d
0.01
n.d
0.04
0.27
0.60
n.d., not detectable.
biochemical properties exist that distinguish the enzymatic ac- tivities of the parental wild-type gene products; these proper- ties constitute the necessary tools to analyze the function of novel EPTl-CPTl gene product chimeras. Finally, genetic tools permitting the construction of a suitable expression system were available, facilitating analysis of the properties of EPT1- CPTl gene product chimeras in membrane preparations devoid of measurable wild-type activity.
While the properties of chimeric proteins can provide defin- itive and powerful conclusions about structure-function rela- tionships, the unknown effects of segmental substitutions on adjacent and distant structural features of the protein, as well as the technical limitations to the number and variety of chi- meric constructs that can be evaluated necessarily, constrain the information that can be derived by such an approach. In the case of chimeric proteins evaluated on the basis of enzymo- logical properties as in the present work, three potential ex- perimental outcomes can be anticipated: 1) the chimeric pro- tein exhibits enzymatic activity that conforms to either wild- type parental activity with respect to a given biochemical
TABLE I11 CDP-amino alcohol specificities of parent and chimeric enzymes
strain HJO91 harboring the indicated chimeric genes on the high copy Enzyme activities were determined in the membrane fraction of
plasmid YEp352 as described under ”Experimental Procedures.” DiC18:I was the diacylglycerol source except where indicated.
Enzyme Kr“ ChoF’TIEthFT specific activity
CDP-choline CDP-ethanolamine ratio
” CPT 1 I 66 473 97
10 “‘1 2 22 21 8 45
20 ”- 1 n.dl n.d
30 1 683 78 0.39
40 1 172 18 0.28
50 -- I 74 25 0.59
60 75 15 0.56
EPT 75 20 0.56
’ n.d., not detectable.
TABLE IV CDP-amino alcohol specificities of chimeric enzymes
strain HJ091 harboring the indicated chimeric genes on the high copy Enzyme activities were determined in the membrane fraction of
plasmid YEp352 as described under “Experimental Procedures.” DiC18:l was the diacylglycerol source except where indicated.
Enzyme Kr” ChoF‘TlEthqT
CDP-choline CDP-ethanolamine specific activlty
ratio
10-60 m‘‘” - 335 n.d.’ 196
10-50 sD\\.. -,- 1 75 n.d. 159
10-30 E%,- - 393 n.d. 402
30-50 n.d. 55 0.67
404jO n.d. 45 0.45
40.50 n.d. 16 0.64
’ n.d., not determined.
property, 2) the chimeric protein exhibits enzymatic activity with novel biochemical properties, or 3) the chimeric protein lacks enzymatic activity. As will be shown, both the first and second outcomes provide useful information about the role of the underlying structural changes in determining the analytic property.
Construction and Nomenclature of EPTl-CPTl Chimeric Genes--The yeast CPTl and EPTl genes contain open reading frames encoding 407 and 391 amino acids, respectively, which are disrupted near their 5’ ends by small introns (Hjelmstad and Bell, 1990, 1991a). The predicted gene products bear 54% amino acid identity and are essentially collinear in their opti- mal alignment except for a 14-amino acid NH,-terminal seg- ment in the CPTl gene product, which is not predicted in the EPTl gene product (see Fig. 1) (Hjelmstad and Bell, 1991b). To facilitate the construction of EPTl-CPTl chimeric genes, the nucleotide sequences of EPTl and CPTl cDNAs were evalu- ated for the presence of existing restriction enzyme cleavage sites or sites that could be created by in vitro mutagenesis, which could serve as splice junctions between the EPTl and CPTl coding frames. The potential splice junctions were fur- ther required to result in precise union of parental sequences within the homology alignment and prohibited from inducing amino acid mutations. From multiple identified sites meeting these criteria, six were chosen and divided the coding frames into seven approximately equal contiguous segments of 50-60
20998 Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases
I I CPTl
” ha- 10-50
10-30
1 30
30-50
I 40
40-50
EPTl
0 20 40 60 80 100 % Remaining Activity
FIG. 2. CMP inhibition of chimeric enzymes. Choline- and eth- anolaminephosphotransferase activities were determined in the mixed micelle assay in the presence of 1 mM CMP. Inhibition for chimeras that could utilize both CDP-choline and CDP-ethanolamine was independ- ent of the CDP-amino alcohol supplied. The results are the mean of at least three separate determinations.
amino acids (Fig. 1). Restriction sites were introduced as re- quired by in vitro mutagenesis in M13 phage subclones as described under “Experimental Procedures.” Each mutation was confirmed by direct DNA sequencing and then reconsti- tuted into full length wild-type genes for subsequent use in the construction of chimeric genes.
As depicted in Fig. 1, two sets of chimeric genes were con- structed utilizing the available splice sites. In the first genera- tion of chimeric constructs (Fig. l, lower left), the contribution of the parental sequences was smoothly varied to generate a set of genes predicted to encode proteins containing progressively less COOH-terminal CPTl-derived sequence. Based on data derived from the first generation of chimeras, a second genera- tion of chimeric genes (Fig. 1, lower right) was constructed which consisted of a set of internal substitutions of CPT1-de- rived segments. It is evident that all of the chimeric genes contain EPT1-derived NH,-terminal regions. Two factors guided this choice. First, the EPTl promoter was known to support higher levels of overexpression (5-15-fold) in high copy number yeast vectors (Hjelmstad and Bell, 1988) compared to the CPTl promoter (3-5-fold) (Hjelmstad and Bell, 1987). This offered a significant advantage in terms of the sensitivity of enzymatic assays performed on membranes prepared from chi- mera-expressing strains. Second, alternate transcripts from the CPTl locus have been observed under certain growth con- d i t i o n ~ . ~ The precise nature of the protein products derived from alternate transcripts is currently not completely under- stood, and the possibility of expressing chimeric proteins of uncertain primary structure was deemed inadvisable. As pre- sented in Fig. 1 and in subsequent text and figures, a simplified graphical depiction and textual nomenclature was adopted to refer to the chimeric genes and their gene products. Precise amino acid sequences for each chimeric construct can be con- strued from previous reports defining the nucleotide and in- ferred amino acid sequences (Hjelmstad and Bell, 1990,1991b). The contribution of CPTl (open) and EPTl (cross-hatched) se- quences to a chimera was standardized in graphical represen- tations. The chimeric genes and gene products were named according to their splice junctions with the 5’ (NH,-terminal) end understood to be derived from the EPTl locus. The restric- tion enzyme splice junctions were arbitrarily numbered in mul- tiples of 10 from the 5’ (NH,-terminal) end (10 = NarI, 20 = ApaI, etc.).
Expression of EPTl-CPTl Chimeric Gene Products-To as- sess the ability of the EPT1-CPT1 chimeric genes to direct the synthesis of functional hybrid gene products, a strain bearing
S. C. Morash, R. H. Hjelmstad, C. R. McMaster, and R. M. Bell, unpublished observations.
CPTl
EPTl
18:0/20:4 16:0/20:4
18:1/18:1 16:1/16:1 18:0/18:1 18:1/16:0 16:0/18:1 18:0/16:0 16:0/18:0 18:0/18:0 16:0/1 6:O 14:0/14:0 12:0/12:0 I I I , I I ,
18:0/20:4 16:0/20:4 18:1/18:1 16:1/16:1 18:0/18:1 18:1/16:0
18:0/16:0 16:0/18:0 18:0/18:0 16:0/16:0 14:0/14:0 12:0/12:0
16:0/18:1
0 1 2 3 4 5 6 7 8 nrnol/mg prolmin
1 0 5 10 15 20 25
nmoVrng prolmin
FIG. 3. Diacylglycerol acyl chain specificity of CPTl and EPTl gene products. Choline- and ethanolaminephosphotransferase activi- ties were determined in a mixed micelle system in the presence of 5 mol % different diacylglycerol donors and CDP-choline andor CDP-ethanol- amine. The results are similar regardless of the CDP-amino alcohol substrate employed and are the mean of at least three separate deter- minations.
mutations in EPTl and CPTl, as well as a marker for the selection of transformants, was required. Each of the EPTl- CPTl chimeric plasmids, as well as the parental wild-type genes, was introduced into HJ091, a strain fulfilling this re- quirement, and membranes prepared from the resulting trans- formants were evaluated for choline- and ethanolaminephos- photransferase activities using dioleoylglycerol as lipid substrate. The activities of the individual CPTl and EPTl gene products were characterized previously, exploiting the avail- ability of overexpressing clones and null mutations (Hjelmstad and Bell, 1991b). A mixed micellar assay was devised to facili- tate the delivery of the dioleoylglycerol lipid substrate and remove endogenous lipids present in crude membranes by sur- face dilution (Hjelmstad and Bell, 1987). As shown in Tables I and 11, all but three chimeric genes supported the expression of activity. The magnitude of the activities was quite variable; constructs that failed to express detectable enzyme activities (chimeric enzymes 20, 10-40, and 30-60) or expressed seri- ously defective levels of activity (chimeric enzymes 30 and 30- 50) could contain primary sequence mismatches in the corre- sponding gene products that were catastrophic to protein function. However, an examination of the structures of these defective chimeric enzymes failed to reveal a simple sequence mismatch common to them, suggesting that a complex set of segmental mismatches may manifest such defects. The success- ful expression of a set of active EPTl-CPTl chimeric enzymes permitted the application of enzymological analysis in evalu-
Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases 20999
18:1/18:1
16:1/16:1
18:1116:0
16:0/18:1
16:0/16:0
14:0/1 4:O
nrnollminlmg protein
1 8 : 1 / 1 8 : 1 7
16:1/16:'
18:1/16:(
16:0/18:1
16:0/16:0
14:0/14:0p , , , 1 0 2 4 6 8 1 0
nmoVrnin/mg protein
CPTl I 1
10 ms==- I
18:1/18:1 7 1 16:1/16:1 I 18:1116:0
16:0118:1
10-50
16:0/16:0
14:0/14:0
0 1 2 3 4 5 6 nrnoVrninlmg protein
ficities similar to the CPTl gene product. Cholinephosphotrans- FIG. 4. Chimeric enzymes with diacylglycerol acyl chain speci-
ferase activities were determined in a mixed micelle system in the presence of 5 mol % different diacylglycerol donors and CDP-choline. The results are the mean of at least three separate determinations.
ating structure-function relationships of the choline- and eth- anolaminephosphotransferases.
CDP-amino Alcohol Specificities ofparental and EPTl -CPTl Chimeric Enzymes-The parental and chimeric enzymes exhib- ited characteristic CDP-amino alcohol specificities (Tables I11 and IV). Under standard conditions, the cholinephosphotrans- ferase activity of the CPTl gene product was nearly 200-fold greater than the enzyme's ethanolaminephosphotransferase activity, as previously shown (Hjelmstad and Bell, 1991b). In contrast, the EPTl gene product exhibited approximately 2-fold greater ethanolaminephosphotransferase activity than cholinephosphotransferase activity. Indeed, the simple ratio of cholinephosphotransferase to ethanolaminephosphotransfer- ase activity proved to be a useful expression of substrate selec- tivity and an index to which CDP-amino alcohol specificities of chimeric enzymes could be compared to the parental property. Chimeric enzymes 10, 10-60, 10-50, and 10-30 displayed a CDP-amino alcohol substrate specificity very similar to the parental CPTl gene product, whereas chimeric enzymes 30,40, 50,60,30-50,40-50, and 40-60 exhibited activity ratios highly similar to that of the EPTl gene product (Tables I11 and IV). In the chimeric construct series depicted in Table 111, it can be seen that substitution of NH,-splice junction 10 EPT1-derived sequences did not alter the CDP-amino alcohol specificity of the largely CPTI-derived chimeric enzyme 10. Additional substitu- tion of EPT1-derived 10-30 region sequences converted chi- meric enzyme 30 to an EPTl-like activity. Other changes in this series did not significantly affect this property. In this series, shown in Table IV, internal substitution of CPTl-derived se- quences, which included the 10-30 region (chimeric enzymes
18:1/18:1
16:1/16:1
18:1/16:0
16:0118:1
16:0/16:0
14:0/1 4:O 0 5 10 15 20 25
nrnoVrninlmg protein
18:1/18:1
16:1/16:1
18:1/16:0
16:0/18:1
16:0/1 6:O
14:0/1 4:O 0 0.1 0.2 0.3 0.4 0.5
nrnoVrninlrng protein
18:1/18:1
16:1/16:1
18:1/16:0
16:0/1 8:l
EPTl
50
40-50
16:0/1 6:O
14:0/14:0
0 50 100 150 pmol/min/rng protein
FIG. 5. Chimeric enzymes with diacylglycerol acyl chain speci- ficities similar to the EPTl gene product. Choline- and ethanol- aminephosphotransferase activities were determined in a mixed micelle system in the presence of 5 mol % different diacylglycerol donors and CDP-choline or CDP-ethanolamine, The results are similar regardless of the CDP-amino alcohol substrate employed and are the mean of at least three separate determinations.
10-60, 10-50, and 10-301, was sufficient to confer CPTl-like CDP-amino alcohol selectivity, while substitutions not involv- ing this region exhibited EPT1-like behavior.
This analysis definitively identified the region encoded within splice junctions 10-30 sufficient to confer CDP-amino alcohol substrate specificity. In this regard, homologies within this region to other enzymes are of interest (Hjelmstad and Bell, 1991c; Paltauf et al. 1992). Yeast phosphatidylinositol syn- thase, phosphatidylserine synthase, and Escherichia coli phos- phatidylglycerophosphate synthase together with the yeast CPTl and EPTl gene products, exhibit a conserved sequence (Nikawa et al., 1987a, 1987b;) within the core of this linear segment. All these enzymes catalyze the displacement of CMP from a CDP-alcohol by a second alcohol with the formation of a phosphodiester bond and the breaking of a phosphoric anhy- dride bond. Additionally, the region extending from splice junc- tion 20-30 is homologous to glyceraldehyde-3-phosphate dehy- drogenase and phosphoglycerate kinase (Holland and Holland, 1980; Michelson et al., 19831, both of whose crystal structures have been solved (Buehner et al., 1974; Blake and Evans, 1974; Banks et al., 1979) and wherein these regions encode for nucle- otide binding domains. Furthermore, these proteins, along with the CPTl and EPTl gene products, form phosphorylated glyc- erol intermediates during catalysis. Thus, the structure-func- tion relationship determining CDP-amino alcohol specificity revealed in this analysis of chimeric enzymes substantiates relationships suggested by sequence homology analysis.
CMP as an Inhibitor of Parental and EPTl-CPTl Chimeric Enzymes-Previous work revealed that CMP, an end product of the phosphotransferase reaction, strongly inhibited EPTl gene
21000 Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases
18:1/18:1 16:1116:1 18:1116:0 16:0/18:1 16:0/16:0 14:0/14:0
0 2 4 6 8 nrnoUrniWmg protein
18:1/18:1 16:1/16:1 18:1/16:0 16:0/16:1 16:0/16:0 14:0/14:0
o i i i i pmoUrniWmg protein
18:1118:1 16:1/16:1 18:1116:0 16:0/18 16:0/16:0 14:0/14:0c . , . I
0 0:5 1 i.5 2 nrnol/rniWmg protein
CPTl I I
EPTl
30 1
40 1
10-30 L
18:1118:1 16:lll 6:l 18:1/16:0 16:0/1 S:1 16:0/16:0 I I 14:0/14:01 , , , , I
0 5 10 15 20 25 nmoUrniWrng protein
18:1118:1 16:1116:1 16:1116:0 16:0/18:1 16:0/16:0 14:0/14:0
0 0.03 0.06 0.09 0.12 nmoVrniWmg protein
18:1/18:1 16:1/16:1 18:1116:0 16:0/18:1 16:0/16:0 14:0/14:0
0 5 10 15 pmoVmin/rng protein
were determined in a mixed micelle system in the presence of 5 mol % different diacylglycerol donors and CDP-choline or CDP-ethanolamine. The FIG. 6. Chimeric enzymes with novel diacylglycerol acyl chain specificities. Choline- and ethanolaminephosphotransferase activities
results are the mean of at least three separate determinations.
product activity while only weakly inhibiting the CPTl gene product activity, regardless of the CDP-amino alcohol employed (Hjelmstad and Bell, 1991b). This property was exploited to further evaluate functional domains using the chimeric en- zymes (Fig. 2). Chimeras 30, 30-50,40, and 40-50 were inhib- ited by CMP to an extent similar to that of parental EPTl, while 10-50 and 10-30 were not inhibited by CMP. Both 10-50 and 10-30 preferred CDP-choline as a substrate based on CPTI EPT activity ratios (Table IV), whereas the other chimeras depicted were classed as preferring CDP-ethanolamine similar to the EPTl gene product. In general, the inhibition by CMP was consistent with the assignment of the chimeras as either preferentially utilizing CDP-ethanolamine or CDP-choline. That is, chimeras that preferentially used CDP-ethanolamine were inhibited by CMP, as is the EPTl gene product, while chimeras that preferred CDP-choline as the amino alcohol do- nor were not inhibited by CMP similar to CPT1-derived activ- ity. As a product of the reaction, CMP would be released from the active site of the enzyme; hence, it appears that this inhi- bition is consistent with and exclusive to the EPTl CDP-amino alcohol substrate specificity domain and reaction mechanism.
Diacylglycerol Acyl Chain Specificity of Wild-type CPTl and EPTl Gene Products-In preliminary studies of the individual CPTl and EPTl gene product activities, sn-1,2-dioleoylglycerol was utilized as the lipid substrate and differences in the ap- parent K,,, value for this molecular species of DAG were re- ported for each enzyme (Hjelmstad and Bell, 1991b). Attempts to exploit the characteristic apparent K,,, values of the EPTl and CPTl gene products as an analytic property to facilitate the assignment of DAG recognition structure-function relation- ships using the EPT1-CPTl chimeric enzymes proved inad- equate. Thus, additional discriminating properties pertaining to the DAG substrate specificities were sought. A series of chemically defined DAGS were prepared from their correspond- ing phosphatidylcholines and evaluated as substrates at con- stant concentrations of 5 mol %. As shown in Fig. 3, both the CPTl and EPTl gene products exhibited broad specificities for
their diacylglycerol substrates. The preferred DAG substrate for the CPTl gene product was 16:1/16:1; 18:1/18:1 actually proved to be a relatively poor substrate for this enzyme. The overexpressed EPTl gene product showed maximal activity using the 18:1/18:1 species but was also active using the 16:1/ 16:l species. The 18:1/16:0 supported greater CPTl gene prod- uct activity, while 16:0/18:1 supported higher EPTl gene prod- uct activity. Most notably, the CPTl gene product employed the shorter chain saturated species 16:0/16:0 and 14:0/14:0 to an appreciable extent, while EPTl gene product activity using these DAG substrates was nearly undetectable. These DAG substrate specificity profiles provided the required distinguish- ing biochemical properties for which studies exploring struc- tural regions involved in DAG acyl chain recognition could be based.
Diacylglycerol Acyl Chain Specificity of Chimeric EPTl- CPTl Gene Products-The DAG acyl chain specificity of each of the chimeric enzymes was determined using six molecular spe- cies chosen from the set used to characterize the parental en- zymes. The observed patterns of activity could be easily com- pared visually to the wild-type profiles and fell into three groups: 1) similar to the CPTl gene product (chimeric enzymes 10 and 10-50, Fig. 4); 2) similar to the EPTl gene product (chimeric enzymes 30, 40, 10-30, and 40-50, Fig. 5); and 3) novel (Fig. 6). Sequences derived from the 10-50 region were sufficient to determine the DAG acyl chain specificities, as best exemplified by the CPT1-like profile of the internally substi- tuted chimeric enzyme 10-50 (Fig. 4). Chimeric enzymes con- taining mismatches in the 10-50 region exhibited novel DAG acyl chain specification (Fig. 6), except for chimeric enzyme 40-50 which expressed a pattern in accordance with its 10-40 region (Figs. 4 and 5). Unfortunately, chimeric enzyme 10-40 was inactive (Table 111, precluding rigorous testing of the pos- sibility that the 10-40 region was sufficient to determine this property. The acyl chains of DAG exist in the hydrocarbon core of the membrane bilayer. Interaction of DAG with the phospho- transferase likely involves transmembrane protein segments.
Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases 21001
Svbstrate
CPTl r t COP-choline COP-choline EPTl
EPTl COP-ethanolamine 40 ""' I COP-ethanolamine 50 ""\"
10-60 I COP-ethanolamine
-a COP-ethanolamtne 3 0 - 5 0 -- --- COP-ethanolamine 40-50 - COP-ethanolamine 40-60 -'" .=@x COP-ethanolamlne o" ~.
Actlv!U-wlth_!?E x .:# , I EO 100
Actwlty wlth PC
FIG. 7. Activation of enzyme activities by phosphatidyletha- nolamine. Phosphatidylethanolamine (5 mol %) was the phospholipid activator in the mixed micellar assay of choline- or ethanolaminephos- photransferase activity. The results are the mean of at least three separate experiments.
18:0/20:4 16:0/20:4 18:1/18:1 16:1/16:1 18:0/18:1 18:1/16:0 16:0/18:1
CPT1 18:0/16:0 16:0/18:0
16:0/16:0 14:0/14:0
0 1 2 3 4 5 6 7
nmol mg prolmin
18:0/20:4 16:0/20:4 18:1/18:1 16:1/16:1 18:0/18:1
EPTl 16:0/18:1 18:0/16:0 16:0/18:0 18:0/18:0
14:0/14:0 12:0/12:0
nmol/mg prolmin
FIG. 8. Acyl chain dependence of phosphatidylcholine activa- tion. Phosphatidylcholines of different acyl chain species were included in the choline- and ethanolaminephosphotransferase reaction mixture at a concentration of 5 mol %. The results are the mean of at least three separate experiments.
The 10-50 region contain three predicted membrane spanning domains (Hjelmstad and Bell, 1990, 1991a). Interestingly, the E. coli DAG kinase also contains three predicted transmem- brane domains (Loomis et al., 1985).
Phospholipid Cofactor Requirement of Parental and EPTl- CPTl Chimeric Gene Products-In the course of previous en- zymological studies, an absolute requirement of both CPTl and EPTl gene products for a phospholipid activator was discov- ered (Hjelmstad and Bell, 1991b). Both enzymes were activated to the greatest degree by PC, while PE could support CPTl derived enzyme activity to 65% of that of PC. The EPTl gene product was unique in its response to PE activation. When CDP-choline was utilized, the degree of activation by PE was similar to that observed for the CPTl gene product (65%); how- ever, when CDP-ethanolamine was the substrate, PE-sup-
LUMINAL SURFACE
YTOPCASMIC SURFACE
e CDP-AMINOALCOHOL SPECIFICITY
DIACYLGLVCEROL ACYL SPECIFICITY
FIG. 9. Structural domains within the predicted CPTl and/or EPTl gene product structures. CpTlp is depicted. Large bold num- bers indicate chimeric enzyme splice junctions. Smaller numbers iden- tify amino acid residues.
ported activity was only 13% that of PC. Hence, phospholipid activation has a profound effect on substrate utilization by the EPTl gene product. This phenomenon was used to search for phospholipid activation domains within CPTl and EPT1. In- formative chimeras were those that could utilize CDP-ethanol- amine as a substrate. Chimeras 40, 50, 10-60, and 30-50 all had low ethanolaminephosphotransferase activity using PE as the activator as compared to PC, similar to that of parental EPTl (Fig. 7 ) . Since chimeras 40 and 50 respond to phospho- lipid activation similar to the EPTl gene product, one would predict the phospholipid activation domain to reside NH,-ter- minal of splice junction 40 and COOH-terminal of junction 60. Additionally, since chimeras 10-60 and 30-50 also responded to lipid activation in the same mode as the EPTl parental en- zyme, this further narrowed the predicted phospholipid activa- tion domain to reside between junctions 10 through 30. How- ever, chimeras 40-50 and 40-60 also contain region 10-30 of the EPTl coding region, but the degree of activation by PE is well beyond the levels observed for the EPTl parental enzyme when CDP-ethanolamine is the substrate (Fig. 7 ) . Hence, al- though the phospholipid activation of the EPTl and CPTl gene products most likely involves region 10-30, other discontinuous regions within the linear segments of these enzymes may also contribute to the phospholipid activation domain.
In order to further discern regions that may be required for phospholipid activation of the enzymes, phosphatidylcholines of different acyl chain specificities were utilized to test for their abilities to support both CPTl and EPTl derived activities (Fig. 8). Both enzymes exhibited a broad specificity with regard to both acyl chain length and the degree of saturation within the fatty acid chains. A specific acyl specificity was not unique to either of the enzymes and hence could not be used as a criteria for the further dissection of phospholipid activation domains.
Concluding Discussion: Structure /Function Relationships in Yeast Choline- and Ethanolaminephosphotransferases-The successful expression of chimeric CPTl IEPTl proteins re- sulted in enzymes displaying either parental or novel biochemi- cal characteristics facilitating the assignment of functional do- mains within the parental enzymes (Fig. 9). The active site can be inferred to lie within a continuous region within splice junc- tions 10 through 40. The DAG binding site can be predicted to contain membrane-spanning regions of this site with all three spanning regions being involved in DAG bindinglcatalysis. The CDP-amino alcohol specificity domain is within regions 10-30. A high degree of homology to other phospholipid synthesizing enzymes that catalyze-the displacement of CMP from a CDP-
21002 Chimeric Enzymes: Choline- and Ethanolaminephosphotransferases
alcohol with the formation of a new phosphodiester bond are consistent with this assignment. Indeed, this region is most likely a common domain required for the efficient catalysis of this reaction mechanism. CMP inhibition of the EPTl gene product also maps to this region. Furthermore, the binding of the two substrates to the enzyme appear to be independent events, since the diacylglycerol specificity profile of the EPT- like chimeras is independent of the CDP-amino alcohol em- ployed. Although it is apparent that phospholipids regulate both CPT and EPT activities, an obvious phospholipid activa- tion region(s) was not apparent from these studies. "he lipid activation domain appears to consist of many binding sites or a discontinuous region of peptides, which are required for phos- pholipid association. This work has led to a significant ad- vancement in the structure/function relationship within the CPTl and EPTl gene products, some of which were extended to include other enzymes catalyzing similar reactions. These chi- meras should prove useful in vivo in the study of the regulation of PC and PE synthesis.
REFERENCES
Banks, R. D., Blake, C. C. F., Evans, P. R., Haser, R., Rice, D. W., Hardy, G., Merrett, M., and Phillips, A. W. (1979) Nature 279,773-777
Billah, M. M., and Anthes, J. C. (1990) Biochem. J. 269,281-291 Blake, C. C. F., and Evans, P. R. (1974) J. Mol. Biol. 84, 585-601 Blotstein, R., Zhang, R., Gottardi, C. J., and Caplan, M. J. (1993) J. Biol. Chem.
Buehner, M., Ford, G. C., Moras, D., and Olsen, K W. (1974) J. Mol. Bid. 90,2549 Czech, M. P., Chawla, A., Woon, C-W., Buxton, J., A r m o N , M., Tang, W., Joly, M.,
Exton, J. H. (1990) J. Biol. Chem. 266, 1-4 Gether, U., Johansen, T. E., and Schwartz, T. W. (1993) J. B i d . Chem. 268,7893-
268,10654-10658
and Corvera, S. (1993) J. Cell B i d . 123, 127-135
7898
Heguy, A., Baldari, C. T., Censini, S., Ghiara, P., and Telford, J. L. (1993) J. Biol. Chem. 268, 10490-10494
Henry, S. A. (1982) in The Molecular Biology of the East Saccharomyces: Metab- olism and Gene Expression (Strathem, J. N., Jones, E. W., and Broach, J. R.,
Hill, J. E., Myers, A. M., Koemer, T. J., and Tzagalott, A. (1986) East 2, 163-167 eds) pp. 101-158, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Hjelmstad, R. H., and Bell, R. M. (1987) J. Biol. Chem. 262,3909-3917 Hjelmstad, R. H., and Bell, R. M. (1988) J. Bid. Chem. 263, 19748-19757 Hjelmstad, R. H., and Bell, R. M. (1990) J. Bid. Chem. 266, 1755-1764 Hjelmstad, R. H., and Bell, R. M. (1991a) J. Biol. Chem. 266, 5094-5103 Hjelmstad, R. H., and Bell, R. M. (1991b) J. B i d . Chem. 266, 4357-4365
Hjelmstad, R. H., and Bell, R. M. (1992) Methods Enzymol. 209, 272-278 Hjelmstad, R. H., and Bell, R. M. (1991~) Biochemistry 30,1731-1740
Holland, J. P., and Holland, M. J. (1980) J. Biol. Chem. 266, 2596-2605 Hosaka, K, and Yamashita, S. (1987) Eul: J . Biochem. 162.7-13 Loomis, C. R., Walsh, J. P., and Bell, R. M. (1985) J. B i d . Chem. 260, 4091-4097 MeAllister, G., Knowlee, M. R., Patel, S., Marwood, R., Emms, F., Seabrook, G. R.,
Graziano, M., Borkowski, D., Hey, P. J., and Freedman, S. B. (1993) FEES Lett.
Michelson, A. M., Markham, A. F., and Orkin, S. (1983) Proc. Natl. Acad. Sci. 324,81-86
Nikawa, J., Kodaki, T., and Yamashita, S. (1987a) J, Biol. Chem. 262, 4876-4881 U. S. A. 80,472-476
Nikawa, J., Tsukagoshi, Y., Kodaki, T., and Yamashita, S. (1987b) Eur. J . Biochem. 167, 7-12
Paltauf, F., Kohlwein, S. D., and Henry, S. A. (1992) in The Molecular and Cellular Biology of the East Saccharomyces (Jones, E. W., Pringle, J. R., and Broach, J. R., eds) pp. 415-500, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Pelech, S. L., and Vance, D. E. (1989) %rids Biochem. Sci. 14,2E-30 Peterson, G. L. (1977) Anal. Biochem. 83,346-356 Schaf€er, L., Kjeldsen, T., Anderson, A. S., Wiberg, F. C., Larsen, U. D., Cara, J. F.,
Mirmira, R. G., Nakagawa, S. H., and Tager, H. S. (1993) J. Biol. Chem. 268, 3044-3047
Schumacher, R., Soos, M. A,, Schlessinger, J., Brandenburg, D., Siddle, K., and Ullrich, A. (1993) J. Biol. Chem. 268, 1087-1094
Stem, I., and Shapiro, B. (1953) J. Clin. Pathol. 6, 158-160 White, D. E. (1973) in Form and Function of Phospholipids (Ansell, G. B., Haw-
Zinser, E., Sperka-Gottlieb, C. D. M., Fasch, E. V., Kohlwein, s. D., Paltauf, F., and thome, J. N., and Dawson, R. M. C., eds) pp. 441-482, Elsevier, Amsterdam
Daum, G. (1991) J. Bacteriol. 173,202G2034
Related Documents