Dalton Transactions Dynamic Article Links Cite this: DOI: 10.1039/c2dt30537b www.rsc.org/dalton PAPER ε-Keggin-based coordination networks: Synthesis, structure and application toward green synthesis of polyoxometalate@graphene hybrids† L. Marleny Rodriguez-Albelo, a Guillaume Rousseau, b Pierre Mialane, b Jérôme Marrot, b Caroline Mellot-Draznieks, c A. Rabdel Ruiz-Salvador, a Shiwen Li, d Rongji Liu, e Guangjin Zhang, e Bineta Keita* d and Anne Dolbecq* b Received 6th March 2012, Accepted 22nd June 2012 DOI: 10.1039/c2dt30537b Four coordination networks based on the {ε-PMo V 8 Mo VI 4 O 40 (OH) 4 Zn 4 } Keggin unit (εZn) have been synthesized under hydrothermal conditions. (TBA) 3 {PMo V 8 Mo VI 4 O 36 (OH) 4 Zn 4 }[C 6 H 4 (COO) 2 ] 2 (ε(isop) 2 ) is a 2D material with monomeric εZn units connected via 1,3 benzenedicarboxylate (isop) linkers and tetrabutylammonium (TBA) counter-cations lying between the planes. In (TPA) 3 {PMo V 8 Mo VI 4 O 37 (OH) 3 Zn 4 }[C 6 H 3 (COO) 3 ](TPA[ε(trim)] ∞ ), 1D inorganic chains formed by the connection of εZn POMs, via Zn–O bonds, are linked via 1,3,5 benzenetricarboxylate (trim) ligands into a 2D compound with tetrapropylammonium (TPA) cations as counter-cations. (TBA) {PMo V 8 Mo VI 4 O 40 Zn 4 }(C 7 H 4 N 2 ) 2 (C 7 H 5 N 2 ) 2 ·12H 2 O(ε(bim) 4 ) is a molecular material with monomeric εZn POMs bound to terminal benzimidazole (bim) ligands. Finally, (TBA)(C 10 H 10 N 4 ) 2 (HPO 3 ) {PMo V 8 Mo VI 4 O 40 Zn 4 } 2 (C 10 H 9 N 4 ) 3 (C 10 H 8 N 4 )(ε 2 ( pazo) 4 ) is a 1D compound with dimeric (εZn) 2 POMs connected by HPO 3 2− ions and terminal para-azobipyridine ( pazo) ligands. In this compound an unusual bond cleavage of the central NvN bond of the pazo ligand is observed. We report also a green chemistry- type one-step synthesis method carried out in water at room temperature using ε 2 ( pazo) 4 and ε(isop) 2 as reducing agent of graphite oxide (GO) to obtain graphene (G). The POM@G hybrids were characterized by X-ray photoelectron spectroscopy, Raman spectroscopy, powder X-ray diffraction, energy dispersive X-ray analysis, infrared spectroscopy, scanning electron microscopy, transmission electron microscopy and cyclic voltammetry. Introduction Metal organic frameworks (MOFs) 1 have attracted intensive interest because of their structural diversity as well as their potential applications, for example in gas storage, separation, catalysis, drug delivery and imaging. 2 Their properties depend both on the nature of their constituent inorganic building units and their spatial arrangement. In this respect, polyoxometalates (POMs), a large family of soluble anionic metal oxide clusters of d-block transition metals in high oxidation states (W VI , Mo V,VI , V IV,V ), with a wide range of magnetic, 3 redox, 4 and catalytic properties, 5 constitute ideal building blocks for multifunctional materials, combining the properties of POMs and those of MOFs. POMs can be easily functionalized by organic molecules either by direct linking to the oxygen atoms of the POM or via transition metal or rare earth ions grafted at the surface of the POM. 6 Connections of the POMs can then be achieved via the use of multidentate ligands, leading to the formation of oligo- mers 7 or polymers, depending on the number of coordination sites and their geometric orientation. The polymers can be described as POM-based MOFs, so-called POMOFs. 8 Note that this name has also been used for fully inorganic POM-based frameworks as an abbreviation of polyoxometalate open frame- work. 9 An increasing amount of POM-based coordination polymers have been reported over the last decade, with a diver- sity of structures ranging from 1D to 3D frameworks. 10 Some of them exhibit catalytic properties 11 but their electrocatalytic prop- erties have rarely been exploited. 12 Another promising approach consists of building MOF materials around inorganic POM † Experimental conditions for the synthesis and characterizations of POM@G hybrids, bond distances and BVS calculations, figures of the crystallographic structures and of the hypothetical structure of ε(bim) 4 , PXRD patterns, XPS, Raman and IR spectra, TEM and SEM images, CVs. CCDC 870381–870384. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2dt30537b a Group of Materials Developed by Design, Division of Chemistry and Technologyof Materials, Institute of Materials Research and Engineering (IMRE), University of Havana, Havana, 10400, Cuba b Institut Lavoisierde Versailles, UMR 8180, Université de Versailles Saint-Quentin en Yvelines, 45 Avenue des Etats-Unis, 78035 Versailles cedex, France. E-mail: [email protected] c Laboratoire de Chimie des Processus Biologiques, FRE 3488, Collège de France, 11 Place Marcellin Berthelot, Paris 75005, France d Laboratoire de Chimie Physique, Groupe d’Electrochimie et de Photoélectrochimie, UMR 8000, CNRS, Université Paris-Sud, Bâtiment 350, 91405 Orsay cedex, France. E-mail: [email protected] e Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, 100190 Beijing, China This journal is © The Royal Society of Chemistry 2012 Dalton Trans. View Online / Journal Homepage

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
DaltonTransactions
Dynamic Article Links
Cite this: DOI: 10.1039/c2dt30537b
www.rsc.org/dalton PAPER
ε-Keggin-based coordination networks: Synthesis, structure and applicationtoward green synthesis of polyoxometalate@graphene hybrids†
L. Marleny Rodriguez-Albelo,a Guillaume Rousseau,b Pierre Mialane,b Jérôme Marrot,b
Caroline Mellot-Draznieks,c A. Rabdel Ruiz-Salvador,a Shiwen Li,d Rongji Liu,e Guangjin Zhang,e
Bineta Keita*d and Anne Dolbecq*b
Received 6th March 2012, Accepted 22nd June 2012DOI: 10.1039/c2dt30537b
Four coordination networks based on the {ε-PMoV8MoVI4O40(OH)4Zn4} Keggin unit (εZn) have beensynthesized under hydrothermal conditions. (TBA)3{PMoV8MoVI4O36(OH)4Zn4}[C6H4(COO)2]2(ε(isop)2) is a 2D material with monomeric εZn units connected via 1,3 benzenedicarboxylate (isop)linkers and tetrabutylammonium (TBA) counter-cations lying between the planes. In(TPA)3{PMoV8MoVI4O37(OH)3Zn4}[C6H3(COO)3] (TPA[ε(trim)]∞), 1D inorganic chains formed by theconnection of εZn POMs, via Zn–O bonds, are linked via 1,3,5 benzenetricarboxylate (trim) ligands intoa 2D compound with tetrapropylammonium (TPA) cations as counter-cations. (TBA){PMoV8MoVI4O40Zn4}(C7H4N2)2(C7H5N2)2·12H2O (ε(bim)4) is a molecular material with monomericεZn POMs bound to terminal benzimidazole (bim) ligands. Finally, (TBA)(C10H10N4)2(HPO3){PMoV8MoVI4O40Zn4}2(C10H9N4)3(C10H8N4) (ε2(pazo)4) is a 1D compound with dimeric (εZn)2 POMsconnected by HPO3
2− ions and terminal para-azobipyridine (pazo) ligands. In this compound an unusualbond cleavage of the central NvN bond of the pazo ligand is observed. We report also a green chemistry-type one-step synthesis method carried out in water at room temperature using ε2(pazo)4 and ε(isop)2 asreducing agent of graphite oxide (GO) to obtain graphene (G). The POM@G hybrids were characterizedby X-ray photoelectron spectroscopy, Raman spectroscopy, powder X-ray diffraction, energy dispersiveX-ray analysis, infrared spectroscopy, scanning electron microscopy, transmission electron microscopyand cyclic voltammetry.
Introduction
Metal organic frameworks (MOFs)1 have attracted intensiveinterest because of their structural diversity as well as theirpotential applications, for example in gas storage, separation,catalysis, drug delivery and imaging.2 Their properties depend
both on the nature of their constituent inorganic building unitsand their spatial arrangement. In this respect, polyoxometalates(POMs), a large family of soluble anionic metal oxide clusters ofd-block transition metals in high oxidation states (WVI, MoV,VI,VIV,V), with a wide range of magnetic,3 redox,4 and catalyticproperties,5 constitute ideal building blocks for multifunctionalmaterials, combining the properties of POMs and those ofMOFs. POMs can be easily functionalized by organic moleculeseither by direct linking to the oxygen atoms of the POM or viatransition metal or rare earth ions grafted at the surface of thePOM.6 Connections of the POMs can then be achieved via theuse of multidentate ligands, leading to the formation of oligo-mers7 or polymers, depending on the number of coordinationsites and their geometric orientation. The polymers can bedescribed as POM-based MOFs, so-called POMOFs.8 Note thatthis name has also been used for fully inorganic POM-basedframeworks as an abbreviation of polyoxometalate open frame-work.9 An increasing amount of POM-based coordinationpolymers have been reported over the last decade, with a diver-sity of structures ranging from 1D to 3D frameworks.10 Some ofthem exhibit catalytic properties11 but their electrocatalytic prop-erties have rarely been exploited.12 Another promising approachconsists of building MOF materials around inorganic POM
†Experimental conditions for the synthesis and characterizations ofPOM@G hybrids, bond distances and BVS calculations, figures of thecrystallographic structures and of the hypothetical structure of ε(bim)4,PXRD patterns, XPS, Raman and IR spectra, TEM and SEM images,CVs. CCDC 870381–870384. For ESI and crystallographic data in CIFor other electronic format see DOI: 10.1039/c2dt30537b
aGroup of Materials Developed by Design, Division of Chemistry andTechnology of Materials, Institute of Materials Research andEngineering (IMRE), University of Havana, Havana, 10400, CubabInstitut Lavoisier de Versailles, UMR 8180, Université de VersaillesSaint-Quentin en Yvelines, 45 Avenue des Etats-Unis, 78035 Versaillescedex, France. E-mail: [email protected] de Chimie des Processus Biologiques, FRE 3488, Collègede France, 11 Place Marcellin Berthelot, Paris 75005, FrancedLaboratoire de Chimie Physique, Groupe d’Electrochimie et dePhotoélectrochimie, UMR 8000, CNRS, Université Paris-Sud, Bâtiment350, 91405 Orsay cedex, France. E-mail: [email protected] Laboratory of Green Process and Engineering, Institute of ProcessEngineering, Chinese Academy of Sciences, 100190 Beijing, China
This journal is © The Royal Society of Chemistry 2012 Dalton Trans.
View Online / Journal Homepage
templates via the so-called “ship in a bottle” approach,13 essen-tially for catalytic applications but also to improve the stabilityof the MOF framework.14
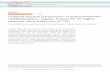
We have developed for a few years a new family of POMOFsusing ε-Keggin POMs as building blocks (Fig. 1). This POMhas the general formula {ε-PMoV8MoVI4O40−x(OH)xM4} (M =ZnII, LaIII; x = 0–5) and contains an ε-Keggin core capped byfour M metallic ions. The ε-isomer has been shown to be themost stable isomer of highly reduced Keggin POMs15 and thecations grafted on its surface compensate its high negativecharge. The overall charge of the ε-Keggin depends on thenumber of protonated oxo bridging ligands which may varyfrom 0 to 5. These Keggin entities are therefore versatile build-ing blocks that may be used either as anions (M = Zn, x = 0) orcations (M = Zn, La, x = 3–5). The ε-Keggin La-derivative wassynthesized at room temperature16 and reacted under mild con-ditions with a variety of carboxylates leading to a new family of1D to 3D materials.17 Although the Zn-based ε-Keggin POM(noted εZn) could not be isolated under similar conditions, itwas shown to form in situ under hydrothermal conditions.17b,18
A family of POMOF structures built from εZn Keggin buildingblocks was thus successfully isolated, with bipyridine,17b,18
benzene-1,4-dicarboxylic acid (bdc),19 imidazole (im),20 andmore recently benzene-1,3,5-tricarboxylic acid (trimesic acid,noted trim).21 The 3D materials with bdc linkers show a highelectrocatalytic activity for the reduction of bromates19 whilethose with trim linkers exhibit a remarkable activity as hetero-geneous electrocatalyst for hydrogen-evolution reaction.21 Thesevery promising results have motivated further studies on thePOMOF materials with εZn units. The influence of the nature ofthe counter-ions and of the linker on the structure and theelectrochemical properties has thus been investigated and wedescribe herein the synthesis and characterization of a series ofnovel POMOFs containing monomeric, dimeric or chain-likeεZn Keggin building blocks connected by trim, isophthalic(isop), benzimidazole (bim) or para-azobipyridine (pazo)linkers. The first three ligands are rigid ligands with two or threecoordination sites while the fourth one is more flexible. Besidesthe structural data, the use of two representative POMOFs of this
family as reductants for the synthesis of graphene from graphiteoxide is also presented.
Experimental
Synthesis
Hydrothermal syntheses were carried out in 23 mL poly-tetrafluoroethylene lined stainless steel containers under auto-genous pressure. Commercially available reagents were used asreceived, without further purification. All reactants were stirredbriefly before heating. The pH mixture was measured before(pHi) and after the reaction (pHf). The products were isolated byfiltration and washed with ethanol.
Preparation of (TBA)3{PMoV8MoVI4O36(OH)4Zn4}[C6H4(COO)2]2(ε(isop)2). A mixture of (NH4)6Mo7O24·4H2O (0.618 g,0.50 mmol), molybdenum powder 99.99% (0.060 g,0.62 mmol), H3PO3 (0.020 g, 0.25 mmol), zinc chloride(0.136 g, 1 mmol), benzene-1,3-dicarboxylic acid (0.166 g,1 mmol), tetrabutylammonium (TBA) hydroxide 40 wt% solu-tion in water (200 μL, 0.30 mmol) and H2O (10 mL) was stirredand the pH was adjusted to 5 (pHi) with 2 M NaOH. Themixture was heated to 200 °C over a period of 1 h, kept at200 °C for 70 h and cooled down to room temperature over aperiod of 80 h (pHf = 4.8). Black crystals suitable for X-ray dif-fraction study were collected after filtration (0.220 g, 28% basedon P). Anal. calc. (found) for C64H120Mo12N3O48PZn4 (3143.5)C 24.45 (24.36), H 3.85 (3.79), Mo 36.62 (37.20), N 1.34(1.30), P 0.99 (0.98), Zn 8.32 (8.30). IR (KBr pellets, ν/cm−1):1604 (m), 1564 (m), 1481 (sh), 1470 (m), 1352 (s), 1274 (w),1204 (w), 1159 (w), 1069 (w), 1008 (sh), 983 (m), 967 (m),940 (s), 812 (s), 781(s), 747 (m), 704 (m), 592 (m), 564 (w),549 (w), 488 (m), 473 (w).
Preparation of (TPA)3{PMoV8MoVI4O37(OH)3Zn4}[C6H3(COO)3](TPA[ε(trim)]∞). A mixture of (NH4)6Mo7O24·4H2O (0.309 g,0.25 mmol), molybdenum powder 99.99% (0.030 g,0.31 mmol), H3PO3 (0.010 g, 0.12 mmol), zinc chloride(0.068 g, 0.5 mmol), 1,3,5 benzenetricarboxylic acid (0.105 g,0.5 mmol), tetrapropylammonium (TPA) hydroxide 40 wt%solution in water (125 μL, 0.3 mmol) and H2O (10 mL) wasstirred and the pH was adjusted to 5 (pHi) with 2 M HCl. Themixture was heated to 180 °C over a period of 1 h, kept at180 °C for 70 h and cooled down to room temperature over aperiod of 80 h (pHf = 4.3). Red platelets suitable for X-ray dif-fraction study were collected after filtration (0.090 g, 26% basedon P). Anal. calc. (found) for C45H90Mo12N3O46PZn4 (2853): C18.94 (19.05), H 3.18 (3.26), Mo 40.35 (40.80), N 1.47 (1.50),P 1.08 (1.04), Zn 9.17 (8.89). IR (KBr pellets, ν/cm−1): 1713(m), 1617 (m), 1574 (m), 1481 (m), 1359 (m), 1239 (w), 1176(w), 1062 (w), 979 (m), 952 (s), 812 (m), 783 (s), 763 (s), 711(m), 673 (sh), 619 (w), 589 (w), 535 (w), 478 (w), 402 (w), 343(w), 316 (w).
Preparation of (TBA){PMoV8MoVI4O40Zn4}(C7H4N2)2(C7H5N2)2·12H2O (ε(bim)4). A mixture of (NH4)6Mo7O24·4H2O (0.618 g,0.50 mmol), molybdenum powder 99.99% (0.060 g, 0.62 mmol),H3PO3 (0.020 g, 0.25 mmol), zinc acetate (0.219 g, 1 mmol),benzimidazole (0.118 g, 1 mmol), tetrabutylammonium hydroxide
Fig. 1 Ball and stick representation of the {ε-PMoV8MoVI4O36-(OH)4Zn4} Keggin core.
Dalton Trans. This journal is © The Royal Society of Chemistry 2012
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
40 wt% solution in water (200 μL, 0.3 mmol) and H2O (10 mL)was stirred and the pH was adjusted to 5 (pHi) with 2 M HOAc.The mixture was heated to 200 °C over a period of 1 h, kept at200 °C for 70 h and cooled down to room temperature over aperiod of 80 h (pHf = 5.6). Red platelets suitable for X-raydiffraction study were collected after filtration (0.210 g, 28%based on P). Anal. calc. (found) for C44H78Mo12N9O52PZn4(3009): C 17.56 (16.99), H 2.62 (2.09), Mo 38.26 (38.92),N 4.19 (4.22), P 1.03 (0.87), Zn 8.69 (8.84). IR (KBr pellets,ν/cm−1):, 1621 (w), 1596 (sh), 1510 (w), 1482 (m), 1464 (w),1413 (m), 1375 (w), 1303 (w), 1274 (sh), 1254 (w), 1150 (w),1109 (w), 1056 (w), 964 (sh), 940 (s), 898 (sh), 815 (s), 781 (s),709 (m), 587 (m), 543 (w), 479 (m), 434 (w).
Preparation of (TBA)(C10H10N4)2(HPO3){PMoV8MoVI4O40Zn4}2-(C10H9N4)3(C10H8N4) (ε2(pazo)4). (NH4)6Mo7O24·4H2O (0.618 g,0.50 mmol), molybdenum powder 99.99% (0.060 g,0.62 mmol), H3PO3 (0.020 g, 0.25 mmol), zinc acetate (0.219 g,1 mmol), azopyridine (0.184 g, 1 mmol), tetrabutylammoniumhydroxide 40 wt% solution in water (200 μL, 0.3 mmol) andH2O (10 mL) was stirred and the pH was adjusted to 5 (pHi)with 2 M NaOH. The mixture was heated to 200 °C over aperiod of 1 h, kept at 200 °C for 80 h and cooled down to roomtemperature over a period of 90 h (pHf = 5.8). Red platelets suit-able for X-ray diffraction study were collected after filtration(0.240 g, 33% based on P). Anal. calc. (found) for C76H93Mo24-N25O83P3Zn8 (5603.3): (found): C 16.29 (15.97), H 1.66 (1.47),Mo 41.09 (43.50), N 6.24 (5.24), P 1.66 (1.39), Zn 9.33 (9.04).IR (KBr pellets, ν/cm−1): 1635 (m), 1616 (sh), 1519 (m), 1496 (w),1464 (w), 1202 (m), 1059 (w), 936 (s), 814 (s), 780 (s), 714 (w),592 (m), 547 (w), 522 (w), 482 (w).
Characterizations
Infrared spectra were recorded on an FTIR Magna 550 Nicoletspectrophotometer as pressed KBr pellets.
Single crystal X-ray diffraction studies were carried out usinga Siemens SMART three-circle diffractometer for all the struc-tures except for TPA[ε(trim)]∞ for which data were collectedusing a Bruker Nonius X8 APEX 2 diffractometer. Both wereequipped with a CCD bidimensional detector using the mono-chromatized wavelength λ(Mo-Kα) = 0.71073 Å. Absorptioncorrection was based on multiple and symmetry-equivalentreflections in the data set using the SADABS program22 basedon the method of Blessing.23 The structure was solved by directmethods and refined by full-matrix least-squares using theSHELX-TL package.24 For all the structures it was not possibleto locate all the counter-cations (tetrabutyl or tetrapropylammo-nium ions) and hydration water molecules due to severe disorderand the data set was corrected with the program SQUEEZE,25 apart of the PLATON package of crystallographic software usedto calculate the solvent or counter-ions disorder area and toremove its contribution to the overall intensity data. Crystallo-graphic data are given in Table 1.
Powder diffraction data was obtained on a Bruker D5000diffractometer using Cu radiation (1.54059 Å).
The experimental conditions for the POM@G hybridssynthesis and characterizations are reported in the ESI† section.
Results and discussion
The formula and abbreviation of the ligands, of the coordinationnetworks, the dimensionality of the inorganic building unit aswell as of the materials, for the four new members of thisgrowing family of POMOFs based on the εZn Keggin units aswell as for the five ones previously reported are gathered inTable 2.
Structures
The four coordination networks are based on the well-known{ε-PMoV8MoVI4O40Zn4} POM (Fig. 1), a reduced ε-Kegginisomer with four ZnII ions in tetrahedral coordination grafted atits surface. The d electrons are localized in MoV–MoV bonds.Accordingly the MoV⋯MoV distances are equal to ∼2.6 Å whileMoVI⋯MoVI distances are quite longer (∼3.2 Å). The exami-nation of the Mo⋯Mo distances have proved to be the best cri-terion to assess the oxidation degree of the Mo ions, valencebond calculations being sometimes less conclusive (Fig. SI1–SI4in ESI†). The presence of 8 MoV and 4 MoVI ions have thusbeen evidenced in ε(isop)2, TPA[ε(trim)]∞ and ε2(pazo)4. Thishas not been possible in ε(bim)4 because of its location on asymmetry element inducing disorder. Valence bond calculations(Fig. SI1–SI4 in ESI†) have allowed identifying protonated brid-ging oxygen atoms. The remaining charges are compensated bycounter-cations. The diversity of crystal structures observedrelies mainly on the orientation of the four ligands around eachsingle POM unit, which determines the symmetry and the con-nectivity of the {ligand-POM} building block.
The detailed formulae of ε(isop)2 and the previously reportedZ-POMOF119 are identical (Table 2), (TBA)3{PMo12O36-(OH)4Zn4}L2, with L being the isop or the bdc linker but theirstructures are strikingly different. For both compounds, the inor-ganic building unit is the monomeric {ε-PMoV8MoVI4O40Zn4}POM. The four ZnII ions in tetrahedral coordination are bound toan oxygen atom of the carboxylate group of the isop linker. Asalready observed in Z-POMOF119 the dicarboxylate ligandadopts a monodentate coordination mode (Fig. 2a). However, itsconnection to two Zn ions of two different POMs allows the for-mation of a 2D grid (Fig. 2b) while in Z-POMOF1 a 3Darrangement was generated. This may be due to the Zn–Ci–Znangle (Ci being the center of the phenyl ring) which is equal to140° in ε(isop)2 and 180° in Z-POMOF1 (Fig. 2a and SI5 inESI†). The 2D hybrid planes stack on top of each other alongthe b axis but are shifted so that a POM of one plane faces thehole of the grid below and above it in a pseudo CsCl arrange-ment (Fig. SI6 in ESI†). The voids left by this arrangement areoccupied by TBA counter-ions. This structure is strikinglyreminiscent of the ε(im)2 layered crystal structure obtained withimidazolate ligands20 however obtained with dimerized Zn-cappedPOMs. While the individual POM units in ε(isop)2 still exhibittheir original tetrahedral symmetry, it is visible that the orientationof the isop linkers around a single POM are responsible for theloss of the regular tetrahedral symmetry as they generate a rathersquare-plane building block (Fig. 3), therefore propagating of agrid-like structure in a similar fashion than in ε2(im)4.
TPA[ε(trim)]∞ was synthesized following a synthetic proto-col identical to that of ε(trim)4/3 (Table 2) replacing TBAOH by
This journal is © The Royal Society of Chemistry 2012 Dalton Trans.
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
TPAOH, with the idea that less bulky counter-cations wouldleave some void space free. However the structures of these twomaterials are very different. In TPA[ε(trim)]∞ the inorganicconnectivity1b is 1 instead of 0 as found in ε(trim)4/3. The εZnPOMs are connected to each other via Zn–O bonds into 1D inor-ganic chains (Fig. 4a), as also observed in {ε-GeMo12} molybdo-germanate ions capped with NiII ions.26 These chains are linked
via trim linkers acting only as bidentate ligands and not triden-tate as expected, forming mixed organic–inorganic layers(Fig. 3b). The structure of TPA[ε(trim)]∞ is thus very close tothat observed for [ε(trim)]∞
21 (Table 2), showing that the threedifferent arrangements combining εZn POMs and trim mole-cules, observed in ε(trim)4/3, [ε(trim)]∞ and ε2(trim)2
21
(Table 2), are very close in energy and that a slight change in the
Table 1 Crystallographic data
ε(isop)2 TPA[ε(trim)]∞ ε(bim)4 ε2(pazo)4
Formula C64H120Mo12N3O48PZn4 C45H90Mo12N3O46PZn4 C44H78Mo12N9O52PZn4 C76H93Mo24N25O83P3Zn8Mr (g mol−1) 3143.5 2853.0 3009.0 5603.3T (K) 293 293 293 293Space group Pna21 Pcab I4̄ P21/nCrystal system Orthorhombic Orthorhombic Tetragonal Monoclinica (Å) 21.899(5) 17.563(1) 15.270(3) 13.475(7)b (Å) 20.306(5) 30.229(2) 15.270(3) 17.479(9)c (Å) 22.900(5) 31.886(3) 18.496(4) 28.988(15)β (°) 90 90 90 96.52(1)V (Å3) 10 183 (4) 16 929 (2) 4313(2) 6791(6)Z 4 8 2 2Dc (g cm−3) 2.055 2.203 2.317 2.740μ (mm−1) 2.447 2.928 2.888 3.659Data/parameters 26 789/964 24 676/650 5881/187 11 956/802Rint 0.0654 0.0993 0.0538 0.0734GOF 0.904 0.961 0.957 1.119R (>2σ(I)) R1
a = 0.0578 R1a = 0.0414 R1
a = 0.0615 R1a = 0.0586
wR2b = 0.1771 wR2
b = 0.1303 wR2b = 0.1474 wR2
b = 0.1543
a R1 ¼P
Foj j� Fcj jPFcj j
bwR2 ¼ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiP
wðF2o�F2
c Þ2PwðF2
o Þ2
r
Table 2 Synthetic conditions and structural features of the POMOFs structures with {ε-PMoV8MoVI4O36(OH)4Zn4} Keggin units
Organic linker Name ReactantsT(°C)
Inorganicbuildingunit Formula Dimensionality Ref.
bdc (NH4)6Mo7O24,ZnCl2, TBAOH
180 εZn (TBA)3{PMo12O36(OH)4Zn4}[bdc]2(Z-POMOF1)
3D 19
isop (NH4)6Mo7O24,ZnCl2, TBAOH
200 εZn (TBA)3{PMo12O36(OH)4Zn4}[isop]2(ε(isop)2)
2D Thiswork
trim (NH4)6Mo7O24,ZnCl2, TBAOH
180 εZn (TBA)3{PMo12O36(OH)4Zn4}[trim]4/3(ε(trim)4/3)
3D 21
Na2MoO4, ZnCl2,TBAOH
180 (εZn)2 (TBA)6{PMo12O37(OH)3Zn4}2[trim]2(ε2(trim)2)
3D 21
Na2MoO4, ZnCl2,TBAOH
180 (εZn)∞ (TBA)3{PMo12O37(OH)3Zn4}[trim]([ε(trim)]∞)
2D 21
(NH4)6Mo7O24,ZnCl2, TPAOH
180 (εZn)∞ (TPA)3{PMo12O37(OH)3Zn4}[trim](TPA[ε(trim)]∞)
2D Thiswork
im (NH4)6Mo7O24,ZnCl2, TBAOH
180 (εZn)2 (TBA){PMo12O37(OH)3Zn4}[(im)(Him)] (ε2(im)4)
2D 20
bim (NH4)6Mo7O24, Zn(OAc)2, TBAOH
200 εZn (TBA){PMo12O37(OH)3Zn4}(bim)2(bimH)2 (ε(bim)4)
0D Thiswork
pazo (NH4)6Mo7O24, Zn(OAc)2, TBAOH
200 (εZn)2 (TBA){PMo12O37(OH)3Zn4}2(pazo)4(ε2(pazo)4)
1D Thiswork
Dalton Trans. This journal is © The Royal Society of Chemistry 2012
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
nature of the counter-ions can orient the formation of one phaseover another. This observation is also in agreement with the evi-dencing of the key structure directing role of the TBA templatesfor the formation of the 3D phase ε(trim)4/3.
21 Also, while theTPA could not be located in the crystal structure, it can be notedthat the distance between the planes (Fig. 4a) is slightly shorterin TPA[ε(trim)]∞ (15.11 Å), compared to [ε(trim)]∞(16.20 Å), a consequence of the replacement of TBA by TPA.Also, it is interesting to note that this TPA[ε(trim)]∞ structurehas some common features in terms of connectivity with ε(im)2or ε(isop)2: having one uncomplexed carboxylic function, thetrim ligand plays the same role as the imidazole or isophthalicligand. All three ligands exhibit a similar range of Zn–Ci–Znangle (145° for imidazole and 140° for trim and isop).
ε(bim)4 is a molecular material with isolated εZn POMs. Thisligand was used with the idea of increasing inter-POM distancesby introducing steric encumbrance, so that the formation oflarger rings (and pores) than those observed in ε2(im)4 would beinduced. We were also aware that this ligand was extensivelyused for the synthesis of zinc imidazolate frameworks (ZIFs) andhas been recognized as beneficial in orientating the synthesistowards large-pore frameworks, notably SOD and RHO-type
ZIFs.27 Despite its molecular structure, ε(bim)4 probably exhi-bits the most interesting feature among the four crystal structurespresented in this work. Four benzimidazole ligands are con-nected to the four Zn ions of the POM (Fig. 5a). Also, the fourbim ligands around a single POM unit are oriented in such a waythat the regular tetrahedral symmetry of the {ligand-POM} build-ing block is maintained (Fig. 3b), allowing in principle thepropagation of a 4-connected net like in dense silicates and zeo-lites. The absence here of a 3D architecture is to be related toone protonated N atom of the imidazole ligand, a probable con-sequence of the low synthetic pH. These POMs are located at thevertices and at the center of the orthorhombic unit-cell (Fig. SI7in ESI†). Intriguingly, a careful analysis of ε(bim)4 shows thatthe POMs are ideally placed (modulo a slight rotation of thecentral POM) to potentially generate a 3D architecture of thecristobalite-type. Fig. SI8† shows this “potential” structure,which is identical to Z-POMOF1,19 however with a doublyinterpenetrated network instead of a triply interpenetrated one inthe case of Z-POMOF1, suggesting a target formula ε(bim)2and a candidate porous crystal structure. Whilst we did notsucceed in producing a 3D material, this structure demonstratesthat the use of bim leads to the ideal tetrahedral targeted buildingblock. The question is still open regarding the negative impact ofthe benzene ring in preventing the inter-POM connection. Againin this structure, disordered TBA cations occupy the spacebetween the POMs.
The inorganic building unit in ε2(pazo)4 is the dimeric (εZn)2POM (Fig. SI9 in ESI†) resulting from the connection of twoPOMs via Zn–O bonds, as already observed in ε2(im)4
20 and
Fig. 2 (a) View of the connecting mode of the isop linker with theindication of the Zn–Ci–Zn angle. (b) View along the b axis of the bi-dimensional structure of ε(isop)2. Blue spheres: Mo, yellow spheres: Zn,green spheres: P, red spheres: O, black spheres: C, small black spheres:H, blue polyhedra: MoO6.
Fig. 3 Building block in (a) ε(isop)2 showing a flattened tetrahedralshape in pink and (b) in ε(bim)4 showing a regular tetrahedral shape.
This journal is © The Royal Society of Chemistry 2012 Dalton Trans.
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
ε2(trim)221 (Table 2). There are three kinds of pazo molecules in
the structure (Fig. 5b). Four of the six remaining accessible ZnII
ions on the dimeric POM are connected to A or B type pazomolecules while C type molecules are free. The A type pazoligands are connected via one N atom of the pyridine moiety andthe B type pazo via one N atom of the central NvN bond. Thislatter coordination mode is unusual. Indeed, in the CambridgeStructural Data Base, in only two structures, among the 160structures with the pazo molecule, the nitrogen atoms of thecentral bond are connected to metal ions.28 Generally, trans-azobipyridines are planar and connected via the nitrogen atomsof the pyridine moieties. This unusual coordination mode hasbeen explained by a two-electron reduction process, leading tothe formation of a quinoid-like bonding with the possibility of afree rotation around the N–N bond (Scheme 1).28 Such unusualbond cleavage of the central NvN bond has also been observedin azobenzene molecules.29 The lengthening of the N–N centralbond in ε2(pazo)4 (A: 1.435(11), B: 1.425(16) Å) compared tofree pazo ligand (∼1.23 Å) is in agreement with this mechanism.The quinoid form A (Scheme 1) is probably observed in the Atype pazo molecules, which are bound via a central N atom(Fig. 5b) while the tautomeric form B (Scheme 1) is most prob-ably encountered in the B type pazo molecule connected to theZnII ions via the N atom of a pyridine ring (Fig. 5b). Note alsothat the free pazo molecules C have also been reduced (dN–N =1.434(16) Å). Also unexpectedly, the two remaining ZnII ions onthe (εZn)2 POMs are bound to one oxygen atom of a free HPO3
2−
ion, coming from the H3PO3 precursor. This allows the
connection of the dimers into a 1D chain (Fig. 5c). Besides proto-nated free pazo molecules, disordered TBA ions play the role ofcounter-cations. The pyridine rings of the connected and the freepazo molecules interact via π–π interactions (Fig. SI10 in ESI†).
Fig. 4 (a) View along the c axis of the 1D chains in TPA[ε(trim)]∞.(b) View along the b axis of the hybrid organic–inorganic planes.Yellow spheres: Zn, red spheres: O, black spheres: C, small blackspheres: H, blue polyhedra: MoO6, green tetrahedra: PO4.
Fig. 5 (a) The molecular structure of the POM unit in ε(bim)4showing the connection of the four terminal benzimidazole ligands.(b) The three types of pazo molecules in ε2(pazo)4. (c) View of thehybrid chain in ε2(pazo)4. Yellow spheres: Zn, green spheres: P, redspheres: O, blue spheres: N, black spheres: C, small black spheres: H,blue polyhedra: MoO6, green tetrahedra: PO4.
Dalton Trans. This journal is © The Royal Society of Chemistry 2012
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
Overall, the four crystal structures presented here possessvarious degrees of POM-ligand-POM connectivities, rangingfrom molecular structure (ε(bim)4) to chain (ε2(pazo)4) or sheetsystems (ε(isop)2, TPA[ε(trim)]∞). Among all, the ε(isop)2structure exhibits expected features: the isop ligand allows amaximum POM-ligand-POM connectivity, with the isop ligandhaving all carboxylic functions complexed, suggesting that athermodynamic control is at play. Still, the resulting structurepossesses a layered structure due to the absence of control overthe orientation of the isop linker to generate a regular tetra-hedron. Instead a largely flattened tetrahedral building block isobserved, explaining the 2D assembly.
In ε(bim)4 the four Zn ions are connected to an organicligand assorted with a remarkably regular tetrahedral molecularbuilding block. It is apparent that the kinetics of complexation ofligand-POM pairs must be extremely rapid, driving the formationof isolated molecular entities that further assemble through non-bonded interactions to form a molecular crystal structure. Still,this structure yields some proof of concept that imidazole ligandmight be well placed to provide 3D POMOFs architectures pro-vided that control of the POM-ligand complexation is achieved(already done with Z-POMOF1 as the cristobalite-type). The tri-mesate TPA[ε(trim)]∞ crystal structure suggests that full com-plexation is not achieved therefore limiting the dimensionality ofthe resulting structure.
Synthesis and characterizations
The synthetic protocol was similar to the one used for the syn-thesis of the previous POMOFs.18–20 The synthesis of the fourcoordination networks was performed by the reaction of a MoVI
precursor, Mo as reducing agent, H3PO3, ZnII ions and anorganic linker in water. These conditions are known to generatein situ the {ε-PMoV8MoVI4O36(OH)4Zn4} core. The syntheticconditions are very close for the four phases (Table 2), the differ-ences arising from the nature of the MoVI precursor (sodiumversus ammonium salt), of the ZnII salt (chloride versus acetatesalt), of the counter-ions (TBA versus TPA) and the temperature(180 °C versus 200 °C). As previously observed, the value ofthe initial pH (around 5.0) is critical. The four compounds areisolated as dark red almost black crystals in moderate yield (ca.30%). ε(isop)2, TPA[ε(trim)]∞ and ε2(pazo)4 are insoluble inall common solvents while ε(bim)4 is moderately soluble inDMF and DMSO. The purity of the phases has been checked bycomparison of the experimental X-ray powder pattern with thepowder pattern calculated from the structure solved from single-crystal X-ray diffraction data (Fig. SI11 in ESI†).
In the infrared spectra of the four phases several regions canbe distinguished: in ε(isop)2 and TPA[ε(trim)]∞, the νC–Ovibrations of the carboxylate linker are identified around 1610and 1570 (νasym) and 1380 and 1350 cm−1 (νsym). In ε2(pazo)4
and ε(bim)4, the vibrations of the organic linker is found around1600 (νC–N and νN–H) and 1250 cm−1 (νC–N), the N–N stretchingvibration of the pazo ligand is found at 1464 cm−1. In all fourcompounds, the signature of the TBA or TPA counter-ions isidentified around 1480 cm−1 while the P–O and MovO vibrationsof the inorganic skeleton of the POM are encountered around1060 cm−1 (weak absorption) and 940 cm−1 (strong absorption)respectively. The Mo–O–Mo vibrations are found below 940 cm−1.
Synthesis and characterizations of polyoxometalate@graphenehybrids
A variety of strategies have been employed to develop POM-based hybrids because of their usefulness in, e.g., environmentaland energy applications.6,21,30 Given the recognized advantagesof graphene31 and the well-known strong chemisorption betweenPOMs and carbon materials, we set out to elaborate poly-oxometalate@graphene hybrids (POM@G) via a reductionprocess of graphite oxide (GO) in which a POMOF was used asreducing agent. Specifically, we are interested in finding a facileway to immobilize insoluble POMs on large surfaces whileenhancing their beneficial properties. Recent reports describedthe synthesis of graphene-based materials via UV photoreduc-tion32 or hydrazine assisted reduction33 of Keggin-type POMs inpresence of GO. Herein, we report for the first time, a greenchemistry-type one-step synthesis method carried out in purewater at room temperature. GO was synthesized by oxidation ofgraphite using Hummer’s method (see the ESI† for details). Thetwo selected POMOFs were ε(isop)2 and ε2(pazo)4. In a typicalexperiment, the desired amount of POMOF was added to anaqueous exfoliated GO dispersion (0.1 mg mL−1) then, theresulting mixture was continuously stirred for at least 6 h.During the reduction process, the brown-colored suspensionturns black progressively. This observation is attributed to thewell-known black color of the reduced GO (Fig. 6). The suspen-sions were then centrifuged at 16 000 rpm for 10 min, redis-persed and washed four times with pure water. At last, the blackprecipitates were redispersed in pure water or dried and charac-terized by X-ray photoelectron spectroscopy (XPS), Ramanspectroscopy, powder X-ray diffraction (XRD), energy dispersiveX-ray analysis (EDX), infrared spectroscopy (IR), scanning elec-tron microscopy (SEM), transmission electron microscopy (TEM)and cyclic voltammetry (CV). Based on the known propensity ofPOMs to adsorb strongly on various materials including gra-phene,33,34 it is anticipated that these thoroughly washed samplesare POM@G hybrids.
The degree of GO reduction and the chemical composition ofPOM@G were monitored by XPS. Fig. 7a and 7b feature theXPS C 1s spectra of ε(isop)2@G and GO. The C 1s deconvolu-tion spectrum of GO presents the well-known four types ofcarbon which appear at 284.8 eV (graphite-like C, C–C/CvC),286.8 eV (C–O), 287.8 eV (CvO) and 289 eV (O–CvO)
Scheme 1
This journal is © The Royal Society of Chemistry 2012 Dalton Trans.
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
respectively. After reduction, all the peaks corresponding to theoxygen containing groups decreased remarkably indicating thatmost of these groups are removed after reduction. The consider-able increase of the graphitic-like C content (from 39.1% to87.5%) is attributed to the expected restoration of sp2 carbonnetwork. Table 3 gathers the contents of the different types ofcarbon obtained with GO, ε(isop)2@G, ε2(pazo)4@G and thecorresponding literature values obtained after UV photoreductionof GO in presence of PW12O40
3− (PW12).32b The efficiency of
POM for the restoration of sp2 carbon decreases in the followingsequence order ε(isop)2 (87.5%) > ε2(pazo)4 (68.5%) ≈ PW12
(65.6%). It is worth noting the high capabilities of ε(isop)2 andε2(pazo)4 in eliminating the CvO and O–CvO groups.
Fig. 7c and SI12† show that for both ε(isop)2@G andε2(pazo)4@G, the presence of molybdenum in valence statesV and VI was detected. The presence of phosphorous and zincwas also detected in the hybrids. These observations confirm theformation of POM@G hybrids during the reduction of GO byε(isop)2 and ε2(pazo)4, in agreement with the mechanismreported for the one-step synthesis of POM-functionalized metalnanostructutres.30d
Raman spectroscopy is widely used for probing defects andstructural properties of carbon materials.35 Fig. 8 exhibits theG (1564.3 cm−1) and D (1340.4 cm−1) bands of the Ramanspectra of ε(isop)2@G, GO and G. The G band is attributed tothe first-order scattering of the E2g vibration mode observed forthe sp2 domains while the D band is a breathing mode of k-pointphonons of A1g symmetry.35 Specifically, the intensity ratio ofD and G bands (ID/IG) provide disorder degree and average sizeof the sp2 domains. The ID/IG intensity ratio of GO (0.949) is, asexpected, much higher than that of G (0.129) indicating the pres-ence of defects and the diminution in size of the sp2 domainsdue to the considerable oxidation. For ε(isop)2@G, the ID/IGintensity ratio decreases to 0.74 after reductions and this is dueto restoration of aromatic structure, i.e. increase of the averagesize of the crystalline graphene domains. To the best of ourknowledge, such a result was not reported for POM@G systems.The ID/IG intensity ratios of these hybrids are higher than that ofGO.35 Fig. SI13† shows that the ratio (ID/IG = 1.15) correspondingto ε2(pazo)4@G is significantly higher. The increase of the ID/IGratio compared to that in GO is usually attributed to the presenceof a larger number of small sp2 domains.32a,36 Thus, in agreementwith the XPS results, ε2(pazo)4 is less efficient than ε(isop)2.
XRD measurements have been performed on GO,ε(isop)2@G and ε2(pazo)4@G. As expected37 the XRD powderpattern of GO exhibits an intense peak located at 2θ = 11.7°(Fig. SI14†), matching with a layered system with an interlayerdistance of ca. 7.56 Å. In contrast, the XRD powder patterns ofε(isop)2@G and ε2(pazo)4@G (Fig. SI15†) do not present apeak characteristic of GO, while a broad band centered at ca.2θ = 26° is observed. This corresponds to an interlamellard-spacing of 3.42 Å, confirming that the starting GO has beenhighly reduced in the presence of POM.38 Moreover, a compari-son of the XRD powder patterns of the POMs@G and of thecorresponding starting POM materials shows that crystallites ofPOMs have been immobilized on the reduced GO. Moreover, acomparison of the IR spectra of the POMs@G systems and ofthe related POM precursor confirms that the integrities of thepolyanionic compounds have been retained throughout the syn-thetic process (Fig. SI16 and SI17†).
The TEM image (Fig. SI18a†) reveals that the GO consists ofrandomly aggregated, crumpled, thin nanosheets closely asso-ciated with each other and forming a disordered solid. After theywere reduced by ε(isop)2 and ε2(pazo)4, respectively, the aggre-gated nanosheets were separated, and the typical TEM images ofthe transparent thin graphene sheets looking like crumpled silk
Fig. 6 Pictures of GO, ε(isop)2/GO and ε(isop)2@G.
Fig. 7 C 1s XPS spectra of (a) GO before reduction and (b) as-pre-pared ε(isop)2@G; (c) XPS spectra of Mo3d in the ε(isop)2 preparedhybrids.
Dalton Trans. This journal is © The Royal Society of Chemistry 2012
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
veil waves were observed (Fig. SI18b and SI19b† forε(isop)2@G and ε2(pazo)4@G, respectively), which is a featurestructure of graphene nanosheets. From the SEM images(Fig. SI18c and SI19a† for ε(isop)2@G and ε2(pazo)4@G,respectively) of the product, we can also clearly observe thecurled and overlapped nanosheets. In situ EDX analysis of theas-prepared ε(isop)2@G hybrid (Fig. SI18d†) showed that strongMo peaks exist as well as the Zn peaks, confirming the existenceof the POMOF (ε(isop)2) in the hybrids. A quantitative analysisgive a Mo–Zn ratio of 4.04 : 0.96 for ε2(pazo)4@G and of4.02 : 0.98 for ε(isop)2@G.
Finally, cyclic voltammetry (CV) was used to test the chemi-cal stability of the ε(isop)2@G hybrids in a pH 1 medium. TheCV measurements were performed in solid-state by immobiliz-ing the desired amount of hybrids in a perfluorinated polymer(Nafion). Fig. 9 displays well-defined and chemically reversiblereduction waves associated to the MoVI centers in ε(isop)2@Gsystem. They feature the known Mo-reduction bielectronic wavesfor polymolybdates with Keggin or Dawson structures. In linewith previous CV data for other POMOFs,19,21 the third wavelocated at −0.105 V vs. SCE is broader than that of the εPMo12building units. This observation is attributed to an overlap of twoclosely spaced monoelectronic waves. The splitting of theMo-reduction third wave into two waves was even observedfor Z-POMOF1.19 Thus the six electron reduced form ofε(isop)2@G is less basic than the corresponding form of
εPMo12 in agreement with previous results for other POMOFs.In other words, an electrochemical–chemical–electrochemical(ECE) or electrochemical–electrochemical–chemical (EEC)-typeprocess governing waves merging are probably less favorablewith ε(isop)2@G. This observation is similar to the one forε(isop)2@Vulcan XC72 (ε(isop)2@V) composites. Fig. 9 pre-sents the evolution of the CVof ε(isop)2@G as a function of thepotential scan rate, and it can be seen that all the CVs displaythe classical symmetrical shape featuring the reversible reductionand oxidation of surface confined reactants.39 The dependence ofcathodic and anodic peak currents of the second wave as a func-tion of the scan rate is also shown in Fig. 9 (inset). The good lin-earity (R = 0.998) of these curves confirms the surface-confinedcharacter of the observed waves. The MoV centers are associatedwith a broad and large irreversible oxidation waves in accord-ance with the CV characteristics of many MoV polymoly-bdates.19,21 The electrochemical results altogether underscore thesuccessful entrapment of ε(isop)2 on the graphene surface. Thus,the POM@G hybrids are promising candidates for several appli-cations, especially for photo-electro-catalysis. For this purpose,one of the important requirements is the high stability of thehybrids under various conditions. Perfect reproducibility of theCV over thousands of cycles in different conditions shows thatthe POM@G based electrodes are very stable in solution phase.
Table 3 Fitting of the C 1s peak binding energy (eV) (relative atomic percentage %)
Samples Graphite-like C–O CvO O–CvO
GO 284.8 (39.1%) 286.8 (51.2%) 287.8 (8.5%) 289.0 (1.2%)ε(isop)2@G 284.8 (87.5%) 286.8 (12.5%) 287.8 (0) 288.5 (0)ε(pazo)4@G 284.8 (68.5%) 286.8 (31.4%) 287.8 (0.1%) 289.0 (0)PW12@Ga 284.5 (65.6%) 286.6 (20.9%) 287.8 (9.7) 289.0 (3.8%)
aObtained under irradiation (ref. 32b).
Fig. 8 Raman spectra of natural G, GO before reaction and as-preparedε(isop)2@G.
Fig. 9 Cyclic voltammograms (CVs) and peak current intensity vari-ations for ε(isop)2@G in a [1 M LiCl +HCl (pH 1)] medium. The refer-ence electrode was a saturated calomel electrode (SCE). CVs as afunction of scan rate (from inner to outer curve: 10, 20, 30, 40, 50, 60,70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190 and200 mV s−1, respectively). (inset) the dependence of cathodic andanodic peak currents of the second reduction and oxidation waves as afunction of the scan rate. R = 0.998.
This journal is © The Royal Society of Chemistry 2012 Dalton Trans.
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
For example, after 5000 continuous cycles, no significant changeof the CV characteristics of the ε(isop)2@G-modified electrodewas observed (Fig. SI20†). The electrodes are also very durablein the solid state. After several months of storage in the air,their electrochemical properties are similar to the ones forfreshly prepared electrodes. Furthermore, a comparison betweenthe CV characteristics of ε(isop)2@G and ε(isop)2@V modifiedelectrodes, under the same experimental conditions, discloses theexpected salient advantages of graphene over Vulcan XC72 assupports (Fig. 10): (i) the peak current responses for ε(isop)2@Gare ca. 6.5 times higher and occur at lower over potentials (by35–75 mV depending on the wave). As expected, theε(isop)2@G-modified electrode exhibits also larger capacitivebackground charging current which is due to the inherentimpressive surface area of graphene. However the remarkablecurrent response enhancement is probably not simply due to anarea effect but also to the strength of the interaction betweenPOMs and graphene30 and the uptake capacity of graphene forPOMs. (ii) faster electron transfer kinetics is observed forε(isop)2@G through the comparison of the anodic-to-cathodicpeak potential differences (ΔEp) on the waves of the two systems(for example the ΔEp values for a second redox couple at100 mV s−1 are 10 mV and 30 mV respectively for ε(isop)2@Gand ε(isop)2@V). It is worth noting that the ΔEp forε(isop)2@G is close to the theoretical value (ΔEp = 0) predictedfor surface-attached electroactive species.39 It is worth notingthat such electrode performances could be obtained with a tinyamount of ε(isop)2@G (0.4 mg cm−2). Future work to finelytune the POM@G hybrids properties will include the influenceof the strength of the interaction between POM and G and conse-quently the POM structure and composition on their electricalconductivity and photo (electro) chemical behaviors.
Conclusions
In conclusion, four new POM-based hybrid materials based onεZn Keggin anions and organic linkers have been isolated. The
structural diversities of these four materials confirm that thenature of the organic linker and of the counter-ions plays acrucial role in the assembly of POM-based metal organic frame-works. Several conclusions can be drawn: (i) the replacement ofthe TBA counter-ions with TPA in the 3D ε(trim)4/3 POMOFmaterial leads to a structural change and thus does not releaseany free space, as could have been expected, (ii) N-donorligands such as imidazole or pyridine derivatives only lead tomaterials with low dimensionalities probably due to the proto-nation of the N atoms, (iii) di or tricarboxylate are thus the bestcandidates for the formation of 3D structures. We have success-fully demonstrated a green and simple method for efficientelaboration of POM@G hybrids. The POM@G hybrids with thehigh quality of the graphene constituent, enhanced electrochemi-cal properties, large surface area and remarkable stability undervarious conditions, offer great promise for producing a new classof nanomaterials for a wide range of applications in, e.g., thephoto (electro) catalytic and electroanalytical domains.
References
1 (a) Chem. Soc. Rev., 2009, 38, 1201, special issue on Metal OrganicFrameworks; (b) C. N. R. Rao, A. K. Cheetham and A. Thirumurugan,J. Phys.: Condens. Matter, 2008, 20, 083202; (c) G. Ferey, Chem. Soc.Rev., 2008, 37191.
2 (a) W. Xuan, C. Zhu, Y. Liu and Y. Cui, Chem. Soc. Rev., 2012, 41,1677; (b) D. Farruseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed.,2009, 48, 7502; (c) S. R. Miller, D. Heurtaux, T. Baati, P. Horcajada,J.-M. Grenèche and C. Serre, Chem. Commun., 2010, 46, 4526;(d) P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas,M. Vallet-Regi, M. Sebban, F. Taulelle and G. Ferey, J. Am. Chem. Soc.,2008, 130, 6774.
3 (a) J. M. Clemente-Juan and E. Coronado, Coord. Chem. Rev., 1999,193–195, 361; (b) U. Kortz, A. Müller, J. van Slageren, J. Schnacke,N. S. Dalal and M. Dressel, Coord. Chem. Rev., 2009, 253, 2315;(c) P. Mialane, A. Dolbecq and F. Sécheresse, Chem. Commun., 2006,3477; (d) P. Kögerler, B. Tsukerblat and A. Müller, Dalton Trans., 2010,39, 21.
4 B. Keita and L. Nadjo, Electrochemistry of Polyoxometalates, Encyclo-pedia of Electrochemistry, ed. A. J. Bard and M. Stratmann, Wiley-VCH,2006, vol. 7, 607.
5 C. L. Hill (Guest Editor), J. Mol. Catal. A: Chem., 2007, 262, 1–242,special issue on polyoxometalates.
6 A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010,110, 6009.
7 M.-P. Santoni, A. K. Pal, G. S. Hanan, M.-C. Tnag, K. Venne, A. Furtos,P. M. Tremblay, C. Malveau and B. Hasenknopf, Chem. Commun., 2012,48, 200.
8 (a) S. T. Zheng, J. Zhang and G. Y. Yang, Angew. Chem., Int. Ed., 2008,47, 3909; (b) S.-T. Zheng, J. Zhang, X.-X. Li, W.-H. Fang andG.-Y. Yang, J. Am. Chem. Soc., 2010, 132, 15102.
9 (a) C. Streb, C. Ritchie, D.-L. Long, P. Kögerler and L. Cronin, Angew.Chem., Int. Ed., 2007, 46, 7579; (b) S. G. Mitchell, T. Boyd,H. N. Miras, D.-L. Long and L. Cronin, Inorg. Chem., 2011, 50, 136;(c) T. McGlone, C. Streb, M. Busquets-Fité, J. Yan, D. Gabb, D.-L. Longand L. Cronin, Cryst. Growth Des., 2011, 11, 2471.
10 (a) J. Niu, S. Zhang, H. Chen, J. Zhao, P. Ma and J. Wang, Cryst. GrowthDes., 2011, 11, 3769; (b) H.-Y. Liu, H. Wu, J. Yang, Y.-Y. Liu, B. Liu,Y.-Y. Liu and J.-F. Ma, Cryst. Growth Des., 2011, 11, 2920;(c) X.-L. Wang, Y.-G. Li, Y. Lu, H. Fu, Z.-M. Su and E.-B. Wang, Cryst.Growth Des., 2010, 10, 4227; (d) H.-Y. An, E.-B. Wang, D.-R. Xiao,Y.-G. Li, Z.-M. Su and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 904;(e) E. V. Chubarova, C. Klöck, M. H. Dickman and U. Kortz, J. ClusterSci., 2007, 18, 697; (f ) H. Y. Liu, H. Wu, J. Yang, Y.-Y. Liu, J.-F. Ma andH.-Y. Bai, Cryst. Growth Des., 2011, 11, 1786.
11 (a) Y. Ren, C. Du, S. Feng, C. Wang, Z. Kong, B. Yue and H. He, Crys-tEngComm, 2011, 13, 7143; (b) J. W. Han and C. L. Hill, J. Am. Chem.Soc., 2007, 129, 15094; (c) D. Dang, Y. Bai, C. He, J. Wang, C. Duanand J. Niu, Inorg. Chem., 2010, 49, 1280.
Fig. 10 Superposition of the CVs of ε(isop)2@G and ε(isop)2@V in apH 1 medium (1 M LiCl/HCl). The scan rate was 100 mV s−1. Thereference electrode was a saturated calomel electrode (SCE).
Dalton Trans. This journal is © The Royal Society of Chemistry 2012
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
12 (a) H. Fu, Y.-G. Li, Y.-H. Wang, X.-L. Wang, X.-J. Feng and E.-B. Wang,Inorg. Chim. Acta, 2009, 362, 3231; (b) X.-F. Sun, W.-S. You,X.-M. Han, L. Ren, C.-Y. Huang and M.-Y. Liu, Z. Anorg. Allg. Chem.,2011, 637, 1341.
13 R. Yu, X.-F. Kuang, X.-Y. Wu, C.-Z. Lu and J. P. Donahue, Coord.Chem. Rev., 2009, 253, 2872.
14 (a) C.-Y. Sun, S.-X. Liu, D.-D. Liang, K.-Z. Shao, Y.-H. Ren andZ.-M. Su, J. Am. Chem. Soc., 2009, 131, 1883; (b) J. Juan-Alcañiz,E. V. Ramos-Frenandez, U. Lafont, J. Gascon and F. Kapteijn, J. Catal.,2010, 269, 229; (c) R. Canioni, C. Roch-Marchal, F. Sécheresse,P. Horcajada, C. Serre, M. Hardi-Dan, G. Ferey, J.-M. Greneche,F. Lefebvre, J.-S. Chang, Y.-K. Hwang, O. Lebedev, S. Turner andG. Van Tendeloo, J. Mater. Chem., 2011, 21, 1226; (d) F.-J. Ma,S.-X. Liu, C.-Y. Sun, D.-D. Liang, G.-J. Ren, F. Wie, Y.-G. Chen andZ.-M. Su, J. Am. Chem. Soc., 2011, 133, 4178; (e) S. R. Bajpe,C. E. A. Kirschhock, A. Aerts, E. Breynaert, G. Absilis, T. N. Parac-Vogt,L. Giebeler and J. A. Martens, Chem.–Eur. J., 2010, 16, 3926;(f ) J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi,K. I. Hardcastle and C. L. Hill, J. Am. Chem. Soc., 2011, 133, 16839;(g) N. V. Maksimchuk, K. A. Kovalenko, S. S. Arzumanov,Y. A. Chesalov, M. S. Melgunov, A. Stepanov, V. P. Fedin andO. A. Kholdeeva, Inorg. Chem., 2010, 49, 2920.
15 H. El Moll, B. Nohra, P. Mialane, J. Marrot, N. Dupré, B. Riflade,M. Malacria, S. Thorimbert, B. Hasenknopf, E. Lacôte, P. A. Aparicio,X. López, J. M. Poblet and A. Dolbecq, Chem.–Eur. J., 2011, 17, 14129.
16 P. Mialane, A. Dolbecq, L. Lisnard, A. Mallard, J. Marrot andF. Sécheresse, Angew. Chem., Int. Ed., 2002, 41, 2398.
17 (a) A. Dolbecq, P. Mialane, L. Lisnard, J. Marrot and F. Sécheresse,Chem.–Eur. J., 2003, 9, 2914; (b) A. Dolbecq, C. Mellot-Draznieks,P. Mialane, J. Marrot, G. Férey and F. Sécheresse, Eur. J. Inorg. Chem.,2005, 3009.
18 C. Lei, J.-G. Mao, Y.-Q. Sun and J.-L. Song, Inorg. Chem., 2004, 43,1964.
19 L. M. Rodriguez-Albelo, A. R. Ruiz-Salvador, A. Sampieri, D. W. Lewis,A. Gómez, B. Nohra, P. Mialane, J. Marrot, F. Sécheresse, C. Mellot-Draznieks, R. Ngo Biboum, B. Keita, L. Nadjo and A. Dolbecq, J. Am.Chem. Soc., 2009, 131, 16078.
20 L. M. Rodriguez-Albelo, A. R. Ruiz-Salvador, D. W. Lewis, A. Gómez,P. Mialane, J. Marrot, A. Dolbecq, A. Sampieri and C. Mellot-Draznieks,Phys. Chem. Chem. Phys., 2010, 12, 8632.
21 B. Nohra, H. El Moll, L. M. Rodriguez Albelo, P. Mialane, J. Marrot,C. Mellot-Draznieks, M. O’Keeffe, R. Ngo Biboum, J. Lemaire,B. Keita, L. Nadjo and A. Dolbecq, J. Am. Chem. Soc., 2011, 133,13363.
22 G. M. Sheldrick, SADABS; Program for Scaling and Correction of AreaDetector Data, University of Göttingen, Germany, 1997.
23 R. Blessing, Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, 51, 33.24 G. M. Sheldrick, SHELX-TL version 5.03, Software Package for the
Crystal Structure Determination, Siemens Analytical X-ray InstrumentDivision, Madison, WI, USA, 1994.
25 P. van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crys-tallogr., 1990, 46, 194.
26 W. Wang, L. Xu, G. Gao, L. Liu and X. Liu, CrystEngComm, 2009, 11,2488.
27 A. N. H. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler,M. O’Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58.
28 O. Theilmann, W. Saak, D. Haase and R. Beckhaus, Organometallics,2009, 28, 2799.
29 J. M. Smith, R. J. Lachicotte and P. L. Holland, J. Am. Chem. Soc., 2003,125, 15752.
30 (a) A. Hiskia, A. Mylonas and E. Papaconstantinou, Chem. Soc. Rev.,2001, 30, 62; (b) Y. Guo and C. Hu, J. Mol. Catal. A: Chem., 2007, 262,136; (c) B. Keita and L. Nadjo, J. Mol. Catal. A: Chem., 2007, 262, 190;(d) B. Keita, T. Liu and L. Nadjo, J. Mater. Chem., 2009, 19, 19;(e) U. Kortz (Guest Editor), Eur. J. Inorg. Chem., 2009, 34, 5055–5276(Issue dedicated to Polyoxometalates) (f ) B. S. Bassil and U. Kortz,Dalton Trans., 2011, 40, 9649; (g) D.-L. Long, R. Tsunashima andL. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736; (h) S. Berardi,M. Carraro, A. Sartorel, G. Modugno and M. Bonchio, Isr. J. Chem.,2011, 51, 259.
31 (a) C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj,Angew. Chem., Int. Ed., 2009, 48, 7752; (b) M. J. Allen, V. C. Tung andR. B. Kaner, Chem. Rev., 2010, 110, 132; (c) M. Pumera, Chem. Soc.Rev., 2010, 39, 4146; (d) Y. Xu and G. Shi, J. Mater. Chem., 2011, 21,3311; (e) K. R. Ratinac, W. Yang, J. J. Gooding, P. Thordarson andF. Braet, Electroanalysis, 2011, 23, 803; (f ) X. Huang, X. Qi, F. Boeyand H. Zhang, Chem. Soc. Rev., 2012, 41, 666; (g) Q. Xiang, J. Yu andM. Jaroniec, Chem. Soc. Rev., 2012, 41, 782.
32 (a) H. Li, S. Pang, X. Feng, K. Müllen and C. Bubeck, Chem. Commun.,2010, 46, 6243; (b) H. Li, S. Pang, S. Wu, X. Feng, K. Müllen andC. Bubeck, J. Am. Chem. Soc., 2011, 133, 9423; (c) L. Cao, H. Sun, J. Liand L. Lu, Anal. Methods, 2011, 3, 1587.
33 (a) D. Zhou and B.-H. Han, Adv. Funct. Mater., 2010, 20, 2717;(b) Y. Sun, X. Hu, W. Luo and Y. Huang, ACS Nano, 2011, 5, 7100.
34 (a) J.-P. Launay, Doctoral thesis, Université Paris VI, Pars, 1974;(b) Y. Izumi, R. Hasebe and K. Urabe, J. Catal., 1983, 84, 402;(c) M. A. Schwegler, P. Vinke, M. Van der Ejik and H. Van Bekkum, Appl.Catal., A, 1992, 80, 41; (d) B. Keita and L. Nadjo, J. Electroanal. Chem.,1993, 354, 295; (e) A. Kuhn and F. C. Anson, Langmuir, 1996, 12, 5481.
35 (a) A. C. Ferrari, J. C. Meyer, V. C. Scardaci, C. M. Lazzeri, F. Mauri,S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys.Rev. Lett., 2006, 97, 187401; (b) A. C. Ferrari, Solid State Commun.,2007, 143, 47; (c) K. N. Kudin, B. Ozbas, H. C. Schniepp,R. K. Prud’homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36.
36 S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas,A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon,2007, 45, 1558.
37 D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner,G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff,Nature, 2007, 448, 457.
38 D. R. Dreyer, S. Murali, Y. Zhu, R. S. Ruoff and C. W. Bielawski,J. Mater. Chem., 2011, 21, 3443.
39 E. Laviron, in Electroanalytical Chemistry, ed. A. J. Bard, MarcelDekker, New York, 1983, 12, 53.
This journal is © The Royal Society of Chemistry 2012 Dalton Trans.
Dow
nloa
ded
by M
cGill
Uni
vers
ity o
n 25
Jul
y 20
12Pu
blis
hed
on 1
8 Ju
ly 2
012
on h
ttp://
pubs
.rsc
.org
| do
i:10.
1039
/C2D
T30
537B
View Online
Related Documents