Journal of Chromatography A, 1109 (2006) 111–119 Double gradient ion chromatography using short monolithic columns modified with a long chained zwitterionic carboxybetaine surfactant Colm´ an ´ O R´ ıord´ ain a , Leon Barron a , Ekaterina Nesterenko a , Pavel N. Nesterenko b , Brett Paull a,∗ a National Centre for Sensor Research, School of Chemical Sciences, Dublin City University, Glasnevin, Dublin 9, Ireland b Department of Analytical Chemistry, Lomonosov Moscow State University, Moscow 119899, Russian Federation Available online 19 January 2006 Abstract The rapid separation of inorganic anions on short monolithic columns permanently coated with a long chained zwitterionic carboxybetaine-type surfactant is shown. The surfactant, N-dodecyl-N,N-(dimethylammonio)undecanoate (DDMAU), was used to coat 2.5, 5.0 and 10 cm long reversed- phase silica monoliths, resulting in a permanent zwitterionic exchange surface when used with aqueous based eluents. The unique structure of the surfactant results in a charge double layer structure on the surface of the stationary phase, with strong internal anionic and weak external cationic exchange groups. The dissociation of the weak external carboxylic acid group acts to shield the inner anionic exchange site, resulting in substantial effective capacity changes with eluent pH. Utilising this effect with the application of an eluent pH gradient, simultaneously combined with eluent flow-rate gradients, very rapid simultaneous separations of both weakly retained anions and strongly retained polarisable anions was possible, with up to 10-fold decreases in overall run times. Coating stability and retention times under isocratic and isofluentic eluent conditions were shown to be reproducible over >450 repeat injections, with peak efficiency values averaging 29,000 N/m for the 2.5 cm column and 42,000 N/m for the 10 cm monolithic column, again under isocratic elution conditions. © 2006 Elsevier B.V. All rights reserved. Keywords: Ion chromatography; Inorganic anions; Monolithic column; N-dodecyl-N,N-(dimethylammonio)undecanoate 1. Introduction Since the early application of zwitterionic stationary phases to the separation of small ions in 1991 [1], interest in the use of novel zwitterionic stationary phases for ion chromatography (IC) has received much attention [2–20]. The principle reason for such interest is the unusual selectivity often exhibited by zwitterionic phases compared to simple ion exchangers, and the added possibilities to manipulate such selectivity. A number of groups have proposed general mechanisms for understanding the unusual selectivities exhibited for small anions and cations by zwitterionic phases, although the large variations in size, shape and charge distribution of the zwitterionic groups used to-date mean consensus on a ‘fit-all’ model may not be possible [6,15–17]. ∗ Corresponding author. Tel.: +353 1 7005060; fax: +353 1 7005503. E-mail address: [email protected] (B. Paull). Much of the work on zwitterionic stationary phases for ion analysis has utilised zwitterionic surfactants to modify (coat) reversed-phase substrates. Within this class of zwitterionic modifiers, there are four structural parameters that will influence the final selectivity exhibited towards ionic solutes when coated onto the stationary phase support. The parameters involved are: (i) the length and number of hydrophobic tail regions (which will impact upon coating stability and column capacity); (ii) the spacing between the cationic and anionic groups within the immobilised zwitterions; (iii) the order of the two ionic groups within the molecule (which governs which ionic groups are ‘shielded’ from solute ions by the oppositely charged sites; and (iv) the relative strength of the two ionic groups (e.g. strong acid and strong base, weak acid and weak base, strong acid and weak base or weak acid and strong base). To-date the majority of studies have utilised strong–strong type surfactants, with hydrophobic tail region lengths ranging from C 8 to C 14 , and with relatively short spacing between ionic sites, typically C 2 to C 4 [3,4,6–8,15]. However, recently a number of studies into alterna- 0021-9673/$ – see front matter © 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.chroma.2006.01.002

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Journal of Chromatography A, 1109 (2006) 111–119
Double gradient ion chromatography using short monolithiccolumns modified with a long chained zwitterionic
carboxybetaine surfactant
Colman O Rıordain a, Leon Barron a, Ekaterina Nesterenko a,Pavel N. Nesterenko b, Brett Paull a,∗
a National Centre for Sensor Research, School of Chemical Sciences, Dublin City University, Glasnevin, Dublin 9, Irelandb Department of Analytical Chemistry, Lomonosov Moscow State University, Moscow 119899, Russian Federation
Available online 19 January 2006
Abstract
The rapid separation of inorganic anions on short monolithic columns permanently coated with a long chained zwitterionic carboxybetaine-typesurfactant is shown. The surfactant, N-dodecyl-N,N-(dimethylammonio)undecanoate (DDMAU), was used to coat 2.5, 5.0 and 10 cm long reversed-phase silica monoliths, resulting in a permanent zwitterionic exchange surface when used with aqueous based eluents. The unique structure of theseeflut1©
K
1
to(fzagtbst[
0d
urfactant results in a charge double layer structure on the surface of the stationary phase, with strong internal anionic and weak external cationicxchange groups. The dissociation of the weak external carboxylic acid group acts to shield the inner anionic exchange site, resulting in substantialffective capacity changes with eluent pH. Utilising this effect with the application of an eluent pH gradient, simultaneously combined with eluentow-rate gradients, very rapid simultaneous separations of both weakly retained anions and strongly retained polarisable anions was possible, withp to 10-fold decreases in overall run times. Coating stability and retention times under isocratic and isofluentic eluent conditions were showno be reproducible over >450 repeat injections, with peak efficiency values averaging 29,000 N/m for the 2.5 cm column and 42,000 N/m for the0 cm monolithic column, again under isocratic elution conditions.
2006 Elsevier B.V. All rights reserved.
eywords: Ion chromatography; Inorganic anions; Monolithic column; N-dodecyl-N,N-(dimethylammonio)undecanoate
. Introduction
Since the early application of zwitterionic stationary phaseso the separation of small ions in 1991 [1], interest in the usef novel zwitterionic stationary phases for ion chromatographyIC) has received much attention [2–20]. The principle reasonor such interest is the unusual selectivity often exhibited bywitterionic phases compared to simple ion exchangers, and thedded possibilities to manipulate such selectivity. A number ofroups have proposed general mechanisms for understandinghe unusual selectivities exhibited for small anions and cationsy zwitterionic phases, although the large variations in size,hape and charge distribution of the zwitterionic groups usedo-date mean consensus on a ‘fit-all’ model may not be possible6,15–17].
∗ Corresponding author. Tel.: +353 1 7005060; fax: +353 1 7005503.E-mail address: [email protected] (B. Paull).
Much of the work on zwitterionic stationary phases for ionanalysis has utilised zwitterionic surfactants to modify (coat)reversed-phase substrates. Within this class of zwitterionicmodifiers, there are four structural parameters that will influencethe final selectivity exhibited towards ionic solutes when coatedonto the stationary phase support. The parameters involved are:(i) the length and number of hydrophobic tail regions (whichwill impact upon coating stability and column capacity); (ii)the spacing between the cationic and anionic groups within theimmobilised zwitterions; (iii) the order of the two ionic groupswithin the molecule (which governs which ionic groups are‘shielded’ from solute ions by the oppositely charged sites; and(iv) the relative strength of the two ionic groups (e.g. strongacid and strong base, weak acid and weak base, strong acid andweak base or weak acid and strong base). To-date the majorityof studies have utilised strong–strong type surfactants, withhydrophobic tail region lengths ranging from C8 to C14, and withrelatively short spacing between ionic sites, typically C2 to C4[3,4,6–8,15]. However, recently a number of studies into alterna-
021-9673/$ – see front matter © 2006 Elsevier B.V. All rights reserved.
oi:10.1016/j.chroma.2006.01.002112 C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119
tive strong–weak carboxybetaine-type surfactants as stationaryphase modifiers have been reported [18–20]. Hu et al. investi-gated a 3-(heptadecafluorooctylsulfonylamino)-N,N-dimethyl-propanammonioethyl-carboxylate (C8F17SO2NHC3H6N+
(CH3)2-C2H4-COO−) coated reversed-phase column andapplied the resultant phase to anion separations [18]. The rathercomplex reagent contained a strong quarternary ammoniumgroup and a weak carboxylic acid terminal group, which whenused at high pH (NaHCO3 eluents from 1.0 to 30 mM at pH∼8.5) showed no retention of inorganic anions, presumed to bedue to strong repulsion effects of the terminal carboxylic acidgroup at this pH. When used with dilute acid eluents, such as1 mM H2SO4, the column exhibited very strong retention ofthe same inorganic anions with a selectivity and retention ordersimilar to that of standard anion exchange separations. Morerecently, O Rıordain et al. used dodecyldimethylaminoaceticacid to dynamically coat (through inclusion within the operatingeluent) reversed-phase monolithic columns of 10.0 cm [19]and 1.0 cm length [20]. The reagent used consists of a C12hydrophobic tail and a single methylene group between thestrong inner anion exchange site and weak terminal carboxylicacid group. As with the earlier work of Hu et al. [18], the car-boxybetaine coated columns exhibited pH dependent selectivitydue to the shielding effect of the terminal carboxylic acid groupand so retention of strongly retained anions could be shortenedconsiderably through an increase in eluent pH. As the columnsucn[
bierwawNChas
itmttshtDacfs
2. Experimental
2.1. Apparatus
The chromatographic system used was a Waters Model 600EMulti-solvent Delivery System (Waters, Milford, MA, USA),fitted with a 20 �L injection loop, and coupled to a ShimadzuSPD-6AV UV–vis Spectrophotometric Detector (Kyoto, Japan),monitoring at 214 nm. For column stability studies, a DionexICS-3000 ion chromatograph with conductivity detection wasused (Dionex, Sunnyvale CA, USA). The pH of prepared mobilephases was measured using an Orion Model 420 pH meter(Thermo Orion, Beverly, MA, USA) with a glass electrode,whilst the column outlet pH was monitored using a flow throughpH electrode assembly. Processing of chromatograms was car-ried out using a PeakNet 6.30 chromatography workstation(Dionex, Sunnyvale, CA, USA).
2.2. Reagents
The zwitterionic surfactant used to dynamically coat thestationary phase was N-dodecyl-N,N-(dimethylammonio)un-decanoate (DDMAU) (Calbiochem, La Jolla, CA, USA). Allother chemicals used were of analytical reagent grade, andwere supplied by Sigma–Aldrich (Tallaght, Dublin, Ireland). Alleluents and standard solutions were prepared using deionisedwfdu
2
w(2GsDac4cMpbc
3
3
rHe
sed were monolithic, this applied pH gradient was used inonjunction with applied eluent flow-rate gradients, and theew technique termed ‘double gradient ion chromatography’20].
However, a drawback of the above work was the poor sta-ility of the carboxybetaine coating, which necessitated thenclusion of small amounts of the surfactant within the elu-nt to stabilise column capacity. The following paper describesesults obtained using a new carboxybetaine-type surfactant,hich exhibits considerably higher coating stability than the
bove materials, thus removing the requirement for inclusionithin the eluent. The surfactant chosen, namely N-dodecyl-,N-(dimethylammonio)undecanoate (DDMAU), has a similar12 hydrophobic tail and strong internal anion exchange site, butas an additional C10 chain linking the anion exchange site withterminal carboxylic acid, which acts to improve its coating
tability on a reversed-phase column.It has been shown [21] that increasing the number of
nter-charge methylene groups in carboxybetaine-type surfac-ants increases the total hydrophobicity of the zwitterionic
olecule, which tends to lower the critical micelle concen-ration (cmc), as well as causing the charge separation inhe zwitterionic head group to grow, thereby increasing thetrength of the repulsive dipole–dipole interaction betweenead groups at the interface. Most of the references madeo DDMAU in the literature have involved the utilisation ofDMAU in the extraction of mycoplasma membrane protein
ntigens [22]. However, here it is shown that the above reagentan provide very versatile and unusual selectivity when used toorm a zwitterionic stationary phase for ion chromatographiceparations.
ater from a Millipore Milli-Q water purification system (Bed-ord, MA, USA), and were filtered through a 0.45 �m filter andegassed by sonication. Dilute solutions of NaOH and HCl weresed to adjust the pH of the mobile phases.
.3. Column preparation
The silica based monolithic separation columns usedere Chromolith monolithic reversed-phase C18 columns
100 mm × 4.6 mm I.D., 50 mm × 4.6 mm I.D. and5 mm × 4.6 mm I.D.) obtained from Merck (Darmstadt,ermany). The columns were coated with the zwitterionic
urfactant, by passing 100 mL of a 20 mM aqueous solution ofDMAU through the column at a flow-rate of 0.7 mL/min forperiod of greater than 60 min. Following initial coating, the
olumns were then rinsed with Milli-Q water for approximately5 min at a flow-rate of 1.0 mL/min. The columns were thenonditioned with the eluent until a steady baseline was achieved.onitoring the column eluate absorbance during the coating
rocedure allowed a calculation of the column capacity, using thereakthrough method. It was found that for the 2.5 cm column, aoating of 157 �mol DDMAU was obtained using this method.
. Results and discussion
.1. DDMAU
Prior to commencing this work, a number of possible zwitte-ionic surfactants were considered as stationary phase modifiers.owever, DDMAU was selected, firstly based upon its consid-
rable hydrophobic character, which would improve coating
C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119 113
Table 1Retention factors (k) of ionic and zwitterionic surfactants on C18 modified columns
Surfactant k
Column
Chromolith PerformanceRP-18-e 100 cm × 0.46 cm
YMC Pack ODS-2 150 mm× 4.6 mm
Gemini 5 � C18 50 mm× 2.0 mm
% MeCN % MeCN % MeCN
90% 60% 90% 60% 90% 60%
Dodecyliminodiacetic acid (log P = 2.34) 3.9 26.4 10.1 23.6 10.3 16.3Dodecylamine (log P = 4.76) 7.9 48.6 19.0 43.6 15.5 65.7DDAB (log P = 6.62) 0.9 4.5 3.3 6.1 2.3 4.0DDMAU (log P = 6.89) 19.0 >140 44.0 ∼128 – ∼122Dodecyldimethylamino acetic acid (log P = 4.44) 2.5 15.8 4.9 16.6 8.3 55.7Didodecylamine (log P = 10.63) 29.0 >140 36.9 ∼106 76.7 99.0
stability, and secondly due to the extended number of inter-charge methylene groups within the molecule, which wouldgive a distinct terminal weak acid layer once adsorbed ontothe stationary phase surface. To evaluate the degree of sur-factant adsorption on C18 reversed-phase substrates, the reten-tion of DDMAU was determined on a range of C18 modi-fied stationary phases and compared to the retention of sev-eral other common ionic and zwitterionic surfactants, includingthose previously used in IC to coat reversed-phase monolithiccolumns, such as dodecyldimethyl-aminoacetic acid [19,20]and didodecyldimethylammonium bromide (DDAB) [23,24].Table 1 shows the retention factors for three C18 reversed-phasecolumns, including a 10 cm Chromolith Performance RP 18-ecolumn, for five different surfactants, using mobile phases con-sisting of 66 and 90% MeCN with 5 mM phosphate buffer atpH 2.6. The significant retention of DDMAU, even at such highMeCN concentrations, illustrates the strong interaction with theC18 phase, such that at MeCN concentrations of 66% and below,no peak was observed for DDMAU on the monolithic column.As expected, the retention of DDMAU was considerably higherthan that seen with both dodecyldimethylaminoacetic acid andDDAB. The relative retention of surfactants observed was inreasonable agreement with their calculated hydrophobicity val-ues (log P [25]) (Table 1), which can be used for predicting thestability of coatings on different reversed-phase materials.
The exact nature of the adsorption of DDMAU onto thesdtdcbo[csrmrma
bic surfaces, there is less information, even though the use ofzwitterionic surfactants in micellar liquid chromatography usingreversed-phase columns is well established [31–33]. However,on charged surfaces, there are some limited studies, for examplework by Baryla et al. [30] investigated the adsorption of a sin-gle chained zwitterionic surfactant on bare silica surfaces andagain showed micellar structures at the surface at concentrationsabove the CMC.
From analysis of the above body of work, it is apparent thatprediction of the exact structure of the adsorbed surfactant coat-ing is non-trivial, and that there are a number of parametersthat would affect this structure when coating C18 modified silicabased surfaces with surfactants containing one or more weakionic groups. For example, these include the degree of residualsilanols on the silica surface, the carbon loading of the silica andthe pH and ionic strength of the mobile phase or eluent. The lat-ter two parameters may of course vary during use of the coatedphase and so continual rearrangement of the surface structuremay also be a possibility. It is also clear that any surface struc-tures formed during coating at high DDMAU concentrationswould almost certainly differ to those remaining in place withinsolutions that no longer contain the surfactant, such as the elu-ents used within this study.
The pH effect shown previously with dodecyldimethy-laminoacetic acid [19,20] was assumed to be due to the repul-sive action of the terminal carboxylic acid group shielding theittesDtue
3D
p
urface of the reversed-phase monolithic column is difficult toetermine precisely. It is known that the critical micelle concen-ration for DDMAU is very low, being only 0.13 mM. Therefore,uring the column coating phase, the coating solution wouldontain the surfactant in micellar form. There have been a num-er of studies into the elucidation of the alignment and structuref surface absorbed surfactants using atomic force microscopy26–30]; however, most of these studies have focused uponharged surfaces and cationic or anionic surfactants. The analy-is of hydrophobic surfaces modified with ionic surfactants haseceived less attention, although some work on graphitic carbonodified with cetyltrimethylammonium bromide (CTAB) has
evealed what appears to be the formation of cylindrical hemi-icelle structures upon the surface at surfactant concentrations
bove the CMC [26]. For zwitterionic surfactants on hydropho-
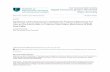
nternal anion exchange site. However, this shielding action oferminal weak acid site does not help elucidate the surface struc-ure of the coating, as the observed effect would be apparent withither a bi-layered or hemi-micellar structure, as suggested in thechematic representations of how such alignments may occur forDMAU shown in Fig. 1. As can be seen, with both structures,
he external weak acid layer should have a pH dependent effectpon the interaction of anionic species with the internal anionxchange site.
.2. Selectivity for common inorganic anions usingDMAU coated monoliths
Initial experiments used a 10 cm coated column with a sim-le eluent comprised of 10 mM KCl and no additional buffer or
114 C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119
Fig. 1. Schematic representation of the DDMAU coating formed upon thereversed-phase surface showing (a) possible bi-layer formation, (b) the forma-tion of hemi-micelles upon the surface.
DDMAU. Retention times of the UV absorbing anions iodate,bromate, nitrite, bromide, nitrate, iodide and thiocyanate in indi-vidual standard solutions were recorded. However, it was notedthat when carrying out consecutive injections using the newlycoated column, retention times tended to actually increase overtime. This was the opposite effect to that usually seen with coatedcolumns, which are often subject to initial column bleed. Whena 1.0 mM mixture of all seven anions was repeatedly injectedonto the newly coated 10 cm monolith, retention times for allof the analytes increased steadily, being particularly noticeablewith those anions that were retained the longest, i.e. iodide andthiocyanate. For example, in the case of thiocyanate, retentionincreased from ∼24 to ∼34 min for identical sample solutionsinjected for the first and fourth time. Initially this was thoughtto be due to a gradual change in the structural arrangement ofthe adsorbed surfactant, but measurement of the eluent pH pre-and post-column showed the above effect to be predominantlydue to significant column buffering. The pH of the solutionpre-column (pH 5.55) and post-column (pH 6.58) differed by1 pH unit. As a means of addressing this problem, buffered elu-
ents were subsequently used, such as 10 mM sodium phosphatebuffer (at pH 6.01). Using this new eluent, a 1.0 mM mixture ofiodate, bromate, nitrite, bromide, nitrate, iodide and thiocyanatewas repeatedly injected, with the aforementioned continuousincrease in retention displayed by analyte anions no longerobserved.
In order to fully investigate the column coating stability, astandard mixture of nitrite, chloride, bromide and nitrate wasinjected repeatedly and continuously over a 30-h period. Toreduce run times and to allow the maximum number of repeatinjections over this period, a 5 cm monolithic DDMAU coatedcolumn was used, together with a 1 mM phthalic acid eluent (pH3.0) delivered at 2.0 mL/min. In this experiment, detection wasachieved using direct conductivity. The total run time for eachinjection was 6 min due to the late elution of a system peak at4 min resulting from the use of the phthalate eluent. However,over the 30-h period, a total of 288 repeat injections were car-ried out, equivalent to >6200 column volumes. The resultant 288chromatograms are shown overlaid as Fig. 2. As can be seen,the stability of the adsorbed coating was clear over this period.
To further assess the coating stability, a further set of repeatinjections was carried out using elevated column temperature.At a column temperature set at 40 ◦C, a total of 170 repeat injec-tions were carried out, with re-injection intervals set at 20 min(total = 57 h), equalling an approximate 11,000 column volumes.Over this period, the retention time reproducibility was found tovIt
Fstandard mixture of nitrite, chloride, bromide and nitrate on a 5 cm DDMAUcoated column, using a 1 mM phthalic acid eluent (pH 3.0) at a flow-rate of2.0 mL/min and direct conductivity detection.
ary less than 2 standard deviations of the mean retention time.n total, the column showed no significant signs of reduced reten-ion times with usage equalling over 17,000 column volumes.
ig. 2. Overlaid chromatograms of 288 consecutive injections of a 50 mg/L
C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119 115
3.3. Effect of pH on anion retention
3.3.1. pH study using 10 cm monolithic columnA series of eight phosphate buffered mobile phases cover-
ing the pH range from 3.0 to 8.0 were prepared, and individualsample standards of iodate, bromate, nitrite, bromide, nitrate,iodide and thiocyanate injected at each pH. The resultant chro-matograms showed large decreases in anion retention betweenthe pH range 3.0 and 6.0, with only iodide and thiocyanate show-ing any significant retention at pH > 6.5. Under acidic conditions,pH 3.0, certain anions showed extremely strong interaction withthe modified stationary phase. For example, thiocyanate wasretained in excess of 240 min. This compares to a retention timeof only approximately 5 min at pH 8. This impressive reduc-tion in retention also caused a significant improvement in peakshapes. This pH data reflects precisely the anticipated shield-ing effect of the dissociated terminal carboxylate groups on theDDMAU molecules, thereby reducing the electrostatic repul-sion experienced by analyte anions, allowing easier access tothe ammonium sites of the DDMAU molecules. The magni-tude of the pH effect shown is also significantly greater thanthat reported earlier with the dodecyldimethylaminoacetic acidcoated monoliths [19,20]. As the major difference between thetwo surfactants is the distance between the two charged sites, itwould suggest that the shielding effect of the terminal carboxylicacid group is magnified as this distance is increased, as mightb
pfbuf
bTafg
Fbpc
Fig. 4. Graph showing the relationship between retention and mobile phasepH using a 10 cm DDMAU coated monolithic column. Eluent: 10 mM sodiumphosphate.
that expected for the terminal carboxylic acid group. The unusualbehaviour of nitrite under acidic conditions is clearly due to theformation of nitrous acid (pKa = 3.15). Chromatograms obtainedfor mobile phases of pH > 6.5–7.0 showed poor resolution of theiodate to nitrate group of anions, although as can be seen fromFig. 4, considerable reductions in the retention of iodide andthiocyanate could still be obtained through the use of eluents atand above this pH range.
3.3.2. Fast separations using a 2.5 cm DDMAU monolithiccolumn
The excessive retention of the polarisable anions (iodideand thiocyanate) under acidic conditions suggested the use ofa shorter modified monolithic column may be appropriate whenwishing to determine these anions. Therefore, a short 2.5 cmreversed-phase monolith column was modified in the same wayas the above 5 and 10 cm columns and evaluated for columnselectivity and efficiency. It was found that the reduced capac-ity of the shorter column meant optimal resolution of all sevenanions in the test mixture was obtained using a more acidic elu-ent pH (pH 4.0) than used with the longer column. However,peak shapes and resolution of the anions was found to be morethan satisfactory on the short monolith, which also offered theadvantage of even lower column backpressures. Peak efficien-cies were calculated for both the 10 and the 2.5 cm column andatcceciorwtbi
e expected.Fig. 3 shows the chromatograms obtained using eluents of
H 3.0 and 6.0. The chromatograms show that the run timeor the separation of the early eluting anions, namely iodate,romate, nitrite, bromide and nitrate, can be reduced from justnder 40 min to under 2 min simply by increasing the eluent pHrom 3.0 to 6.0.
The precise relationship between pH and anion retention cane seen in the plots of retention factor, log k against pH in Fig. 4.he obtained pH dependences showed a non-linear relationshipnd clear indications of an expected sigmoidal curved responseor most anions, with the first derivative analysis of the data sug-esting the stationary phase pKa to between 4.5 and 5.5, close to
ig. 3. Overlaid chromatograms of a 1.0 mM mixture of iodate, bromate, nitrite,romide, nitrate, iodide and thiocyanate, obtained using 10 mM sodium phos-hate buffer mobile phase at pH 3.0 (lower trace) and pH 6.0 (upper trace). Otheronditions: flow rate, 2.0 mL/min; wavelength of detection, 214 nm.
re listed in Table 2. As can be seen from the values shown,he average N/m values for the 10 cm column were ∼42,000ompared with ∼29,000 for the 2.5 cm column (N = 725), cal-ulated using N = 5.55t2
r /W250%. Although the shorter column
xhibited a reduced efficiency in comparison with the longerolumn, the baseline resolution of the test anions was still read-ly achieved. The efficiencies obtained were lower than thosebtainable when using the monolithic columns uncoated foreversed-phase separations, although this is generally the casehen comparing reversed-phase and ion exchange based separa-
ions, due to slower adsorption kinetics. In addition, there coulde efficiency effects related to the structure of the adsorbed coat-ng in this work, since it is well established that loss in efficiency
116 C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119
Table 2Comparison of efficiency values for all seven analyte anions using reversed-phase monolithic columns of different lengths (10 and 2.5 cm) coated withDDMAU
Analyte anion Efficiency using10 cm Monolith (N/m)
Efficiency using 2.5 cmMonolith (N/m)
Iodate 44910 29360Bromate 54290 31240Nitrite 53920 27920Bromide 27810 31000Nitrate 39650 23400Iodide 49270 30520Thiocyanate 22200 27960
due to slow mass transfer between surface adsorbed micelles andthe aqueous phase is often seen in micellar liquid chromatogra-phy [34]. However, despite the above observations, it was clearthat for both columns the peak efficiencies shown were eas-ily comparable to previous anion separations using zwitterionicsurfactant coated particle packed columns [8,10,35,36] and sev-eral commercially available polymeric anion exchange columns.Fig. 5 shows the separation of the test mixture on the newly mod-ified 2.5 cm monolithic column.
However, as is clear from Fig. 5, despite the much loweroverall column capacity, the selectivity of the DDMAU modi-fied phase still resulted in the excessive retention of iodide andthiocyanate relative to the other test anions. It was clear thatto obtain complete resolution of all anions in the mixture, inreasonable run times, a gradient approach would be required.
3.4. Application of combined pH and flow gradients
One of the principal advantages of using a monolithic col-umn rather than a particulate column is the high flow throughporosity which results in relatively low backpressures at moder-ate to high eluent flow-rates. This makes monolithic columnssuitable for programmed flow gradients, as has been shownrecently with dodecyldimethylaminoacetic acid modified mono-liths [19,20]. The ability to maintain relatively high efficiencies
Fnmb2
at higher flow-rates often seen with monolithic columns (pre-dominantly when used in reversed-phase mode), and shown asa relatively flat C-term in a van Deemter plot, would suggestthe use of a constant elevated flow-rate would be suitable toreduce the overall run times. However, as shown previouslyusing short dodecyldimethylaminoacetic acid coated columns[20], the effects of flow-rate upon efficiency are not always uni-form for early and late eluting anions, and so resolution of earlyeluting anions was compromised if a constant high flow-ratewas applied. Therefore, the application of a flow gradient, fromlower to higher flow-rates, maintained efficiency and resolutionof early eluting anions whilst speeding the elution of those morestrongly retained.
In addition to applying such flow gradients, it is also clearfrom Section 3.3 that increasing the eluent pH had the effect ofsignificantly lowering anion retention, although eluent pH valuesabove pH 4 or 5 (for the short monolith) resulted in the co-elutionof the less retained anions (namely iodate, bromate, nitrite, bro-mide and nitrate). Therefore, combining a delayed eluent pHgradient and an eluent flow gradient (so-called double gradientIC), would facilitate the baseline separation of the five afore-mentioned faster eluting anions, while significantly shorteningthe retention times of the later eluting analytes (namely iodideand thiocyanate).
Initial gradient conditions investigated utilised two phosphatebased eluents of equal overall concentration, the first preparedtp(ioatbc<figpitFdtotatauc2rlet
ig. 5. Chromatogram of a 1.0 mM mixture of iodate, bromate, nitrite, bromide,itrate, iodide and thiocyanate obtained obtained using a DDMAU coated 2.5 cmonolithic column and a mobile phase comprised of 10 mM sodium phosphate
uffer at pH 4.0, a flow-rate of 2.0 mL/min and a wavelength of detection of14 nm.
o pH 4.0 (A), the second to pH 8.0 (B). A linear gradient wasrogrammed to switch from 100% A to 100% B over 3 minbetween t = 1.5 and t = 4.5 min), whilst simultaneously linearlyncreasing the mobile phase flow-rate from 2.0 to 6.0 mL/minver the same time period. As expected, the combined gradientpproach resulted in a very significant decrease in the retentionimes of both iodide and thiocyanate, whilst maintaining theaseline resolution of the five early eluting anions. Under theseonditions, the total time of analysis was shortened from >50 to5 min, coupled with a visible improvement in the peak shapeor thiocyanate. This chromatogram is shown as the lower tracen Fig. 6(a). Unfortunately, it was noted that the above combinedradient resulted in a large, sharp system peak in between theeaks for iodide and thiocyanate. This peak was also presentn blank injections of Milli-Q water alone, and so was thoughto be associated with the change in column capacity with pH.or example, the effective column anion exchange capacity willecrease as the column pH increases due to the dissociation ofhe carboxylic acid groups. Therefore, at this point (close to pKaf the acid group) retained anions will be released from the sta-ionary phase. These will include a large amount of the eluentnions, in this case phosphate. This will cause a disturbance inhe system baseline, the magnitude of which is related to thebsorbance of the eluent anions. This observation was verifiedsing an acetate eluent, which resulted in a much more signifi-ant system peak due to its more significant UV absorbance at14 nm. In an attempt to both investigate further and possiblyeduce or move the system peak, the final pH of the gradient wasowered from pH 8.0 to 6.0. This resulted in both the system peakluting later, and also being reduced in size (see Fig. 6(a), upperrace); however, overall run times were increased.
C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119 117
Fig. 6. (a) Overlaid chromatograms of a 1.0 mM mixture of iodate, bromate,nitrite, bromide, nitrate, iodide and thiocyanate obtained using initial flow-ratesof 2.0 mL/min and a starting mobile phase pH of 4.0, with a dual gradient from1.5 to 4.5 min. Gradient details: (lower trace)—100% pH 4.0 to 100% pH 8.0,2.0–6.0 mL/min; (upper trace)—100% pH 4.0 to 100% pH 6.0, 2.0–6.0 mL/min.(b) As above using a starting mobile phase pH of 4.0, with a pH gradient (100%pH 4.0 to 100% pH 6.0) and a flow gradient (2.0–6.0 mL/min) from (lower trace)1.5 to 4.5 min, and (upper trace) 1.5 to 2.0 min.
To regain the <5 min total run time, whilst maintaining a min-imal system disturbance, it was possible to simply increase therate of the applied double gradient. Fig. 6(b) shows the decreasein overall retention achieved through maintaining the same 4.0 to6.0 pH gradient, but applying the gradient over a shorter period,namely 100% A to 100% B over just 0.5 min (between t = 1.5 andt = 2.0 min), whilst once again simultaneously linearly increas-ing the eluent flow-rate from 2.0 to 6.0 mL/min over the sametime period.
3.5. Analytical performance of the dual gradient separation
3.5.1. ReproducibilityMultiple injections (n = 6) were made of a 1.0 mM mixture
of iodate, bromate, nitrite, bromide, nitrate, iodide and thio-
Table 3Mean, standard deviation and % RSD values of retention times of all sevenanalytes (n = 6), using a dual gradient from 1.5 to 4.5 min
Analyte anion Mean retentiontime (min)
Standard deviation ofretention time (min)
% RSD
Iodate 0.5248 0.0166 3.16Bromate 0.9633 0.0367 3.81Nitrite 1.0923 0.0384 3.51Bromide 1.5238 0.0467 3.06Nitrate 1.9567 0.0362 1.85Iodide 4.5387 0.0338 0.74Thiocyanate 5.5227 0.0079 0.14
cyanate, employing a combined eluent (100% 10 mM sodiumphosphate at pH 4 to 100% 10 mM sodium phosphate at pH6) and flow (2.0–6.0 mL/min) gradient from 1.5 to 4.5 min. Inbetween each successive injection, the chromatographic sys-tem was re-equilibrated for 20 min at 2.0 mL/min. The resultantretention time reproducibility is shown in Table 3. As can beseen from the retention time data shown the % RSD values for allseven anions was below 4%, with particularly impressive repro-ducibility shown for the later eluting anions (<1% for iodide andthiocyanate).
3.5.2. Detection limitsTo determine the limits of detection (L.O.D.) for iodide and
thiocyanate upon application of the dual gradient, a 0.02 mMmixture of the analytes was injected in triplicate, as was a sam-ple blank (100% Milli-Q water), and the heights of the analytepeaks and the distance between the upper and lower amplitudesof the baseline noise for the blank injections compared. Theresults calculated using L.O.D. = 3 × baseline noise can be seenin Table 4.
3.5.3. LinearityA range of low concentration standards (five various concen-
trations between 0.005 and 1.0 mM) of the seven anion test mix-ture were prepared, and analysed in triplicate, again using a dualgp1to
TLa
A
IBNBNIT
t
radient program (2.0–6.0 mL/min, and 10 mM sodium phos-hate as eluent from 100% pH 4 to 100% pH 6) applied between.5 and 4.5 min of the chromatographic run time. Table 5 con-ains the R2 values for the linear trendlines for all seven anionsf interest.
able 4imits of detection for anions employing a dual gradienta applied between 1.5nd 4.5 min of the chromatographic run time
nalyte anion L.O.D. (�M) L.O.D. (mg/L)
odate 0.72 0.13romate 5.10 0.65itrite 0.97 0.04romide 1.85 0.15itrate 0.97 0.06
odide 6.66 0.83hiocyanate 3.84 0.22
a 2.0–6.0 mL/min, and 10 mM sodium phosphate as eluent from 100% pH 4o 100% pH 6. UV absorbance at 214 nm.
118 C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119
Table 5Linearity data obtained using dual gradient run for all seven analyte anions
Conc. injected (mM) Peak area
Iodate Bromate Nitrite Bromide Nitrate Iodide Thiocyanate
0.005 0.1202 0.0259 0.1587 0.0345 0.2507 0.0464 0.43580.01 0.2304 0.0550 0.3159 0.0768 0.4541 0.0851 0.75630.02 0.4759 0.1154 0.6798 0.1970 1.1338 0.2035 0.82660.1 2.9230 0.6417 4.7869 1.2704 6.2387 1.1771 1.91941.0 22.8666 4.9983 41.7237 10.3507 51.8039 8.8884 11.8667
R2 0.9992 0.9992 0.9997 0.9994 0.9996 0.9989 0.9991
All data shown averaged from three repeat runs.
Fig. 7. Column outlet pH determined during application of pH gradients (pH 4.0–6.0 over 1.5–4.5 min) using the DDMAU coated 2.5 cm monolithic column, overlaidwith pH profiles obtained without column. Flow-rate = 4 mL/min.
3.6. Column capacity
Finally, to fully understand the pH dependent capacitybehaviour of the modified monoliths and to additionally deter-mine the effective capacity of the coated column (this willobviously be less than the total amount of DDMAU coated), itwas necessary to monitor the pH of the column eluate during theapplication of the above pH gradients. Using pre-programmedgradient profiles, ranging from convex to linear to concave gradi-ents, a flow through pH electrode cell was utilised to monitor therate of pH change within the column itself. The results obtainedshowed the column exhibited a considerable buffering capacity,from which the ion exchange capacity of the column could bereadily calculated, and used to compare the amount of DDMAUcoated on newly modified monoliths. To distinguish betweencolumn buffering, from which effective capacity could be cal-culated, and system dwell volume (Vdwell) a modified version ofan experiment referred to in recent literature by Hendriks et al.was employed [37]. The column in the chromatographic set-upwas replaced by a union, leaving the sample injector connecteddirectly to a UV detector and an eluent pH probe. A step gra-dient was run at 0.5 min from an eluent of low pH (pH 4) toan eluent of higher pH (pH 6) using a single uniform flow-rate.The experiment was carried out in duplicate, at two differentflow-rates, 2.0 and 4.0 mL/min. The pH of the eluent was moni-tored over time, and the resulting graphs were used to determineac
the column and thus its effective anion exchange capacity. Thisresult is shown graphically as Fig. 7. From the data shown aneffective column capacity of 119 �mol of DDMAU was calcu-lated. The point of inflection shown on the curve obtained whenthe column was attached corresponds in time to the appearanceof the small system peak within the chromatogram.
4. Conclusions
The ability to control effective column capacity through theutilisation of a stationary phase weak acid or basic group is auseful tool for manipulation of column selectivity and reten-tion times. Here, a stable coated monolith with a terminal weakacid group protecting an inner strong anion exchange site wasinvestigated for just that purpose. The DDMAU column not onlyexhibits excellent selectivity and efficiency, but also has such apH dependent capacity, allowing for the application of what iseffectively a capacity gradient for the rapid elution of otherwisestrongly retained anions. On top of this, the fact the columnsinvestigated were monolithic in structure, meant a combinedcapacity and flow gradient approach could be demonstrated, withresultant large decreases in run times whilst still maintainingbaseline resolution of all the UV absorbing anions investigated.
Acknowledgements
system Vdwell of 7.03 mL. Repeating the experiment with theolumn attached allowed calculation of the buffering capacity of
The authors would like to thank the referees for their usefulsuggestions and comments on the above work.
C.O. Rıordain et al. / J. Chromatogr. A 1109 (2006) 111–119 119
References
[1] P.N. Nesterenko, J. High. Resolut. Chromatogr. 14 (1991) 767.[2] P.N. Nesterenko, J. Chromatogr. 605 (1992) 192.[3] W. Hu, T. Takeuchi, H. Haraguchi, Anal. Chem. 65 (1993) 2204.[4] H.A. Cook, G.W. Dicinoski, P.R. Haddad, J. Chromatogr. A 997 (2003)
13.[5] T. Jonsson, P. Appelblad, LC–GC Eur. 17 (2004) 40.[6] W. Hu, P.R. Haddad, K. Hasebe, K. Tanaka, P. Tong, C. Khoo, Anal.
Chem. 71 (1999) 1617.[7] T. Umemura, S. Kamiya, A. Itoh, K. Chiba, H. Haraguchi, Anal. Chim.
Acta 349 (1997) 231.[8] E. Twohill, B. Paull, J. Chromatogr. A 973 (2002) 103.[9] W. Jiang, K. Irgum, Anal. Chem. 71 (1999) 333.
[10] P.N. Nesterenko, P.R. Haddad, Anal. Sci. 16 (2000) 565.[11] W. Jiang, K. Irgum, Anal. Chem. 73 (2001) 1993.[12] P.N. Nesterenko, A.I. Elefterov, D.A. Tarasenko, O.A. Shpigun, J. Chro-
matogr. A 706 (1995) 59.[13] W. Jiang, K. Irgum, Anal. Chem. 74 (2002) 4682.[14] M.G. Kiseleva, P.A. Kebets, P.N. Nesterenko, Analyst 126 (2001) 2119.[15] H.A. Cook, W. Hu, J.S. Fritz, P.R. Haddad, Anal. Chem. 73 (2001)
3022.[16] W. Hu, Langmuir 15 (1999) 6241.[17] J.M. Patil, T. Okada, Anal. Commun. 36 (1999) 9.[18] W. Hu, P.R. Haddad, K. Tanaka, K. Hasebe, Anal. Bioanal. Chem. 375
(2003) 259.[19] C. O Rıordain, P. Nesterenko, B. Paull, J. Chromatogr. A 1070 (2005)
71.
[20] C. O Rıordain, P. Nesterenko, B. Paull, Chem. Commun. (2005) 215.[21] Y. Chevalier, Y. Storet, S. Pourchet, P. Le Perchec, Langmuir 7 (1991)
848.[22] C. Brenner, G. Jan, Y. Chevalier, H. Wroblewski, Anal. Biochem. 224
(1995) 515.[23] P. Hatsis, C.A. Lucy, Analyst 127 (2002) 451.[24] P. Hatsis, C.A. Lucy, Anal. Chem. 75 (2003) 995.[25] SRC Environmental Fate Database, Syracuse Research Corporation,
http://www.syrres.com/esc/est kowdemo.htm, 2004.[26] S. Manne, J.P. Cleveland, H.E. Gaub, G.D. Stucky, P.K. Hansma, Lang-
muir 10 (1994) 4409.[27] S. Manne, H.E. Gaub, Science 270 (1995) 1480.[28] H.N. Patrick, G.G. Warr, S. Manne, I.A. Aksay, Langmuir 15 (1999)
1685.[29] V. Subramanian, W.A. Ducker, Langmuir 16 (2000) 4447.[30] N.E. Baryla, J.E. Melanson, M.T. McDermott, C.A. Lucy, Anal. Chem.
73 (2001) 4558.[31] M.H. Guermouche, D. Habel, S. Guermouche, Fluid Phase Equil. 147
(1998) 301.[32] D. Habel, S. Guermouche, M.H. Guermouche, Analyst 118 (1993)
1511.[33] J.P. Berry, S.G. Weber, J. Chromatogr. Sci. 25 (1987) 307.[34] D.P. Thomas, J.P. Foley, J. Chromatogr. A 1060 (2004) 195.[35] W. Hu, P.R. Haddad, Trends Anal. Chem. 17 (1998) 73.[36] W. Hu, P.R. Haddad, H. Cook, H. Yamamoto, K. Hasebe, K. Tanaka,
J.S. Fritz, J. Chromatogr. A 920 (2001) 95.[37] G. Hendricks, J.P. Franke, D.R.A. Uges, J. Chromatogr. A 1089 (2005)
193.
Related Documents