Data-driven intensity normalization in PET group comparisons page 1 of 36 Data-driven intensity normalization of PET group comparison studies is superior to global mean normalization. Per Borghammer 1 , Joel Aanerud 1 , and Albert Gjedde 1,2 . 1 PET Center, Aarhus University Hospitals, Denmark 2 Center of Functionally Integrative Neuroscience (CFIN), Aarhus University, Denmark February 13 th , 2009 Corresponding author Per Borghammer, M.D., Ph.D. PET Centre, Aarhus University Hospitals Aarhus C, Denmark 8000 Email: [email protected] Phone: +0045 8949 4378 Fax: +0045 8949 4400 Short Title: Data-driven intensity normalization in PET group comparisons Key Words: Parkinson's disease, energy metabolism, CBF, normalization, proportional scaling

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Data-driven intensity normalization in PET group comparisons
page 1 of 36
Data-driven intensity normalization of PET group
comparison studies is superior to global mean
normalization.
Per Borghammer1, Joel Aanerud1, and Albert Gjedde1,2.
1PET Center, Aarhus University Hospitals, Denmark
2Center of Functionally Integrative Neuroscience (CFIN), Aarhus University, Denmark
February 13th, 2009
Corresponding author
Per Borghammer, M.D., Ph.D.
PET Centre, Aarhus University Hospitals
Aarhus C, Denmark 8000
Email: [email protected]
Phone: +0045 8949 4378
Fax: +0045 8949 4400
Short Title: Data-driven intensity normalization in PET group comparisons
Key Words: Parkinson's disease, energy metabolism, CBF, normalization,
proportional scaling
Data-driven intensity normalization in PET group comparisons
page 2 of 36
ABSTRACT
Background: Global mean (GM) normalization is one of the most commonly used
method of normalization in PET and SPECT group comparison studies of
neurodegenerative disorders. It requires that no between-group GM difference is
present, which may be strongly violated in neurodegenerative disorders. Importantly,
such GM differences often elude detection due to the large intrinsic variance in
absolute values of cerebral blood flow or glucose consumption. Alternative methods
of normalization are needed for this type of data.
Materials & Methods: Two types of simulation were performed using CBF images
from 49 controls. Two homogeneous groups of 20 subjects were sampled repeatedly.
In one group, cortical CBF was artificially decreased moderately (simulation I) or
slightly (simulation II). The other group served as controls. Ratio normalization was
performed using five reference regions: (1) Global mean; (2) An unbiased VOI; (3)
Data-driven region extraction (Andersson); (4-5) Reference cluster methods
(Yakushev et al.) Using voxel-based statistics, it was determined how much of the
original signal was detected following each type of normalization.
Results: For both simulations, global mean normalization performed poorly, with
only a few percent of the original signal recovered. Global mean normalization
moreover created artificial increases. In contrast, the data-driven reference cluster
method detected 65-95% of the original signal.
Conclusion: In the present simulation, the reference cluster method was superior to
GM normalization. We conclude that the reference cluster method will likely yield
more accurate results in the study of patients with early to moderate stage
neurodegenerative disorders.
Data-driven intensity normalization in PET group comparisons
page 3 of 36
INTRODUCTION:
Positron emission tomography (PET) has been used to investigate the cerebral blood
flow (CBF) and cerebral metabolic rate of glucose (CMRglc) in a range of brain
disorders. However, the absolute values of CBF and CMRglc exhibit large intra- and
inter-individual variation. The coefficient of variance (COV; SD/mean) is most often
in the order of 15% in healthy elderly subjects (Leenders et al., 1990), but can be as
high as 30% in neurodegenerative disorders such as Parkinson’s disease (PD) (Huang
et al., 2007) and Alzheimer’s disease (AD) (Fukuyama et al., 1994). For this reason,
ratio normalization of regional tracer uptake to a reference region has been a standard
data preprocessing step for more than two decades, most commonly using the global
mean as the reference value (Fox et al., 1988). When using this approach, signals of a
much smaller magnitude can be detected using the modest sample sizes typical of
PET studies, and it also obviates the necessity for invasive blood sampling.
Global mean normalization has the fundamental requirement that no between-group
differences exist in the global mean. This prerequisite generally is violated in studies
of neurodegenerative disorders (Eidelberg et al., 1990; Minoshima et al., 1995a).
Importantly, a difference in global mean can be well below detection threshold in
statistical group comparisons. Indeed, to reliably detect a global mean decrease of
10% in PET data (α=0.05, power=0.90, COV 15-30%, two-sided test), sample sizes
of 50-200 subjects per group are needed. However, an undetected difference of this
magnitude robustly introduces bias into an analysis employing global mean
normalization, with the subsequent creation of artificial hypermetabolism in
conserved regions (Borghammer et al., 2008a)). To avoid the potential bias of global
mean normalization, alternative reference regions have been proposed, most
Data-driven intensity normalization in PET group comparisons
page 4 of 36
commonly normalization to an a priori defined brain region spared by the disease
processes. Thus, the pons and cerebellum have been widely used for normalization of
PET and single photon emission computed tomography (SPECT) studies of AD
(Minoshima et al., 1995b; Soonawala et al., 2002) and PD (Pizzolato et al., 1988). In
contrast to a priori defined regions, Andersson proposed a data-driven method to
define the normalization reference region a posteriori (Andersson, 1997). And
recently, Yakushev and colleagues reported a simple and elegant way to define a
conserved region suitable as normalization reference region in comparisons of
patients with mild AD and controls (Yakushev et al., 2009). This method provided
very high accuracy in discriminating mild AD patients from normal controls, and has
general applicability to comparisons of patients with neurodegenerative disorders with
normal controls (see methods section for description of the methods).
Several comparative studies of the performance of different normalization methods
were published (for reviews, see (Arndt et al., 1996; Gullion et al., 1996)). Most of
these studies utilized real data from activation tasks, such as finger tapping (Strother
et al., 1995), memory tasks (Arndt et al., 1996; McIntosh et al., 1996), or visual
stimulation (Andersson, 1997). Using real data, however, is problematic, since the
extent and magnitude of the true signal is de facto unknown. Moreover, it is unclear,
whether results obtained from these activation studies, characterized by spatially
restricted, but large changes, are directly applicable to the case of neurodegenerative
disorders that often are characterized by more widespread decreases - often of a
smaller peak magnitude than in activation studies. Indeed, in a previously published
study, we simulated isolated cortical decreases of 11% in most of the neocortex with
interspersed smaller clusters of 23% decrease (Borghammer et al., 2008a). This
Data-driven intensity normalization in PET group comparisons
page 5 of 36
manipulation decreased the global mean value only by 8-10%, which was below
detection threshold in a 20 “patients” versus 20 controls comparison. Global mean
normalization of these simulation experiments resulted in the detection of few voxels
with 11% decrease, and only some of the 23% decreased voxels were identified. We
suggest that this simulation is similar to studies of early-moderate stage patients with
neurodegenerative disorders, in which the regional decreases often are too small for
the global mean decrease to be detected by standard statistical tests. Thus, global
mean normalization have often been employed in studies of early-stage AD (Kawachi
et al., 2006; Perneczky et al., 2007) and PD (Eidelberg et al., 1994; Ghaemi et al.,
2002; Huang et al., 2007; Imon et al., 1999), and many of these studies reported no or
only slight decreases – as was the case in our simulation studies.
The objective of the present paper was to conduct a formal comparison of different
types of normalization. Specifically, we investigated the case in which the signal
consisted of modest decreases in spatially widespread regions. For this purpose, we
employed simulated data to control the magnitude and extent of the true signal with
absolute certainty. In all analyses, we used ratio normalization (Fox et al., 1988), but
the reference region utilized for the normalization procedure differed. In short, we
normalized to (1) the global mean, (2) the mean of an unbiased volume of interest
(VOI) defined a priori, and (3) the mean of reference regions extracted by two
different data-driven methods (Andersson, 1997; Yakushev et al., 2009). The
normalized data were then analyzed by standard voxel-based statistical procedures
and it was determined how well each type of normalization facilitated the detection of
the true signal present in the simulated data.
Data-driven intensity normalization in PET group comparisons
page 6 of 36
MATERIALS AND METHODS:
The full details of subject recruitment, MR and PET scanning protocols, and data
preprocessing were published previously (Borghammer et al., 2008a) and only a brief
account is given here.
Subjects
We utilized PET CBF scans from 49 healthy volunteers (32 male/17 female; age 34-
72 y), all of whom had participated in previous protocols. Written informed consent
had been obtained from all study subjects. The studies had been approved by the
official science ethics committee, and were in accordance with the declaration of
Helsinki.
Scanning procedures
MRI. A high resolution T1-weighted MR was acquired for most subjects with a 3.0 T
Signa Excite GE Magnet using a 3DIR-fSPGR sequence (256x256, TE1=min full,
TI=450, slice thickness=1.5 mm.) A few subjects were scanned with GE MR 1.5-T
Echo Speed tomograph (3D-SPGR, 256x256, 1 Splap, NEX: 1, slice thickness
1.5mm).
PET. Each subject underwent one dynamic 21-frame [15O]H2O emission recording
with arterial blood sampling. Recordings were performed in a quiet room with
subjects resting in a supine position with open eyes in a quiet, darkened room. The
scans were acquired in 3D mode with the ECAT EXACT HR 47 (CTI/Siemens)
whole-body tomograph. Images were reconstructed as 128 × 128 matrices of 2 × 2
mm voxels using filtered back-projection with a 0.5 cycles−1 ramp filter, followed by
Data-driven intensity normalization in PET group comparisons
page 7 of 36
Gaussian filter, resulting in an isotropic resolution of 7 mm. Tissue attenuation scans
were performed using a rotating 68Ge source.
Data analysis
Parametric maps of CBF were calculated using the single step, two-compartment,
weighted-integration method (Ohta et al., 1996), and were co-registered, via the
subjects’ individual MR images, to common stereotaxic space (Talairach and
Tournoux, 1988), using a combination of linear and non-linear transformations
(Brabner, 2006).
Simulation I.
From the pool of 49 CBF images in common space, we randomly sampled two groups
of 20 subjects each, based on the following criteria: The groups should display no
between-group differences in sex- and age-distribution, or in global CBF values on an
unpaired t-test. If any of these t-tests yielded a p-value below 0.50, the groups were
discarded and two new groups were sampled. This sampling technique ensured the
creation of highly homogeneous groups.
The CBF maps of one of the groups were then systematically manipulated, while the
other group was left unaltered. In short, we designed a specific image volume in
standard space consisting of voxels with three possible values: 1, 0.89, or 0.77
(Figure 1A). In this volume, a total of 27.4% of all intra-cerebral voxels were
assigned the value 0.89, 12.4% had the value 0.77, while the remaining voxels had the
value 1. Importantly, all voxels with values 0.89 or 0.77 were situated in the cerebral
cortex. By multiplying each CBF map (in standard space) with this volume, we
Data-driven intensity normalization in PET group comparisons
page 8 of 36
artificially decreased most of the cortical voxels of the CBF map to either 89% or
77% of original values, while leaving the remaining cortical and all subcortical voxels
unchanged. The magnitudes of the decreases were decided by pilot trials. The specific
aim was to introduce cortical decreases, which would not produce detectable
decreases in the global CBF (due to the large variation in quantitative data). The
entire sampling procedure and subsequent manipulation was repeated four times to
produce a total of four sets of two groups. The full details of the simulations are given
in (Borghammer et al., 2008a).
Simulation II.
While simulation I was meant to emulate a heterogeneous, moderate involvement of
the cortex, we performed an additional simulation to investigate the case, in which a
weaker (and homogeneous) signal was present. We produced a second image volume
almost identical to the first one, except that the voxels, which in simulation I had a
value of 0.77, were now also assigned the value of 0.89 (Figure 2A). This produced a
volume with a homogeneous 11% decrease in large cortical regions. All subsequent
steps of simulation II was identical to simulation I (see above). Simulation II was
performed only twice. In general, the manipulations gave rise to only slightly visible
alterations in the pattern of the raw CBF images (see Supplementary Figure 1 on the
publishers website).
Normalization.
All trials of simulation I and II were all analyzed using five different types of
normalization: (1) Global mean normalization was performed by dividing each voxel
value by the mean of all intracerebral voxels (excluding extra-cerebral and ventricular
Data-driven intensity normalization in PET group comparisons
page 9 of 36
voxels) (Fox et al., 1988). The mean value was extracted by a whole-brain mask. (2)
Ratio normalization to the mean of an unbiased reference region. In the present
simulated data, we left the white matter (WM) unaltered, so we chose this region for
unbiased a priori normalization. The WM mask was conservative and included only
voxels at some distance from GM voxels to exclude spill over effect from the GM.
Details on the definition of the whole-brain and WM were provided previously
(Borghammer et al., 2008b). (3) The data was analyzed with the data-driven method
originally proposed by Andersson (Andersson, 1997). In brief, the method works
iteratively. In the first iteration, a standard voxel-based statistical analysis with global
mean normalization is performed. In the second iteration, a new normalization
reference region is constructed by masking the output t-map from the first iteration,
i.e. excluding all extreme voxels (t < -2 and t > 2). The resultant mask now includes
all voxels with t-values close to zero, and is used to again normalize the original, non-
normalized data. Another voxel-based statistical analysis is performed. The output t-
map from the second iteration forms the basis for the normalization mask of the third
iteration, and so on. For all analyses in the present study, we performed four such
iterations accepting the fourth iteration as the final result. (4) We also analyzed the
data with the recently proposed reference cluster method (Yakushev et al., 2009).
This method is very similar to the Andersson method, but involves only two
iterations. First, a standard global mean normalized voxel-based analysis is
performed. In the second iteration, a new normalization mask is likewise defined on
the basis of the output t-map from the first iteration. However, the t-map is
thresholded differently, i.e. only “hypermetabolic” voxels with t-values > 2 (p<0.05)
are included. Normalization of the original non-normalized data with the new mask
(created from the thresholded output t-map from the first iteration) is then carried out,
Data-driven intensity normalization in PET group comparisons
page 10 of 36
and subsequent voxel-based analysis is performed to produce the final results. The
validity of this method has the fundamental requirement that the seemingly
hypermetabolic region identified in the first iteration, is in fact a conserved region, in
which no between-group changes are present. It is assumed that the “hypermetabolic”
region has been artificially inflated by biased global mean normalization, due to
isolated cortical decreases in one group. (5) Finally, it could be argued that a more
conservative thresholding (i.e. p<0.001) of the output t-map from the first Yakushev
iteration would identify an even more conserved region, see (Yakushev et al., 2009).
However, since a very stringent threshold would necessarily lead to identification of a
much smaller reference region, this method could potentially be vulnerable to random
noise in the data set, with resultant detection of too extensive regional decreases (i.e.
false positive decreases, not to be confused with the false positive increases detected
by biased global mean normalization). We investigated this potential problem by
performing an additional Yakushev type normalization, in which the output t-maps
were threholded at t>3.6 (p<0.001). The two Yakushev methods are referred to as
Yakushev2 and Yakushev3.6, respectively.
Statistical analysis.
The global values of all CBF images prior to, and subsequent to manipulations were
extracted, and group comparisons were performed using unpaired t-tests.
Prior to voxel-based analysis, the coregistered, normalized CBF maps were blurred
with a Gaussian filter to a resultant full-width-at-half-maximum (FWHM) of
14x14x14 mm. We analyzed the data with univariate statistics using the freely
available software package FMRISTAT written by Worsley and colleagues (available
Data-driven intensity normalization in PET group comparisons
page 11 of 36
at www.math.mcgill.ca/keith/fmristat). This method is nearly identical to the
commonly used SPM methodology. In the analysis, we used the mixed effect model
analysis method as advocated in Section Four of Worsley et al. (Worsley et al., 2002),
with spatial smoothing of the standard deviation image to increase the degrees of
freedom. Appropriate linear contrasts were defined to reveal group differences in
CBF. FMRISTAT assigns a t-value to each voxel in the brain and examines the map
for significant focal changes (p < 0.05, corrected for false discovery rate (Genovese et
al., 2002)), based on 3D Gaussian Random Field Theory (Worsley, 1996).
To estimate each normalization method’s ability to facilitate detection of the true
signal, the number of voxels identified in each voxel-based analysis was compared to
the actual number of truly decreased voxels present in the manipulation image
volume. This is presented as a percentage in the results section, i.e. 100 x (number of
voxels detected within the pattern of truly decreased voxels / total number of truly
decreased voxels in the manipulation image volume). For simulation I, we separately
identified the percentage of severely (77%) and moderately (89%) decreased voxels
identified by the voxel-based analysis. When a normalization method gave rise to
artificial increases, the number of detected voxels displaying increases is presented as
a percentage of the number of detected voxels showing decreases.
RESULTS
Global differences. There were no age-, sex-, or global CBF differences between
groups before the manipulation in any of the trials in simulations I and II (p > 0.5 in
all t-tests). The global CBF values prior to, and after the manipulation are presented in
Table 1. In simulation I, the four manipulated groups had their global CBF decreased
Data-driven intensity normalization in PET group comparisons
page 12 of 36
8-10% as a consequence of the manipulation, but this was below detection threshold
when comparing to the control groups (p>0.10). In both trials of simulation II, the
manipulation resulted in a 5% decrease of the gCBF, which was also below detection
threshold when comparing to controls (p>0.31).
Voxel-based analyses. Figures 1 and 2 visually illustrate the extent of the significant
clusters detected in simulation I and II by the different types of normalization. Table
2 summarizes the percentage of the true signal recovered by the five normalization
methods. In short, for both simulations I and II the methods performed as follows
(listed worst to best): Global mean normalization; Andersson normalization; ratio
normalization to white matter; Yakushev2; Yakushev3.6. Significant artificial increases
were detected only following global mean normalization (Table 3). Finally, although
the Yakushev3.6 method detected most of the true signal in all simulations, it also had
the propensity to detect false significant decreases, i.e. voxels, which had not been
artificially decreased by the manipulation step. This is illustrated in Figures 1F and
2F (yellow arrows).
DISCUSSION
The present study yielded two important findings. (1) Standard global mean
normalization performed very poorly and detected only a few percent of the voxels
subjected to an 11% decrease. Moreover, only GM normalization consistently created
artificial increases. (2) By contrast, the reference cluster method developed by
Yakushev et al performed extraordinarily well. A central part of the present
simulations was to induce cortical decreases, which only slightly decreased the global
mean values, i.e. by 8-10% in simulation I, and by 5% in simulation II. Nevertheless,
Data-driven intensity normalization in PET group comparisons
page 13 of 36
the reference cluster method correctly identified 65-95% of the slightly decreased
(11%) voxels, and more than 91% of the severely decreased (23%) voxels (Table 2).
In the following paragraphs the different normalization methods will be discussed.
White matter and Andersson normalization.
WM normalization can be considered a gold standard normalization procedure in the
present simulation, since it was left unaltered by the manipulation. Indeed, the clusters
detected after WM normalization were most often 300-500% larger then clusters
detected subsequent to GM normalization (Table 2). Nevertheless, only 33% in
average of the slightly decreased voxels (11%) were identified. In itself, this is not
very informative since the absolute number of voxels detected is dependent on the
power of the individual study, i.e. sample size and data variance. Nevertheless, it
illustrates that in a typical CBF PET comparison of two groups of 20 subjects, gold
standard ratio normalization to an unbiased region does not guarantee the reliable
detection of widespread, low-magnitude decreases.
The data-driven method developed by Andersson (Andersson, 1997) generally
performed better than GM normalization, but not quite as well as WM normalization
for this kind of data. This was actually to be expected. The following idealized
example illustrates how the iterative Andersson method can be trapped. Consider a
group comparison in which one group displays heterogeneous decreases, i.e. one third
of the brain is decreased by 20%, one third by 10%, while the remaining third is
unchanged. The decreases are homogenous across tissue types, so the global mean is
decreased by 10%. The first Andersson iteration, i.e. standard GM normalization
yields a t-map, in which the unchanged region appears hypermetabolic (t>2), while
Data-driven intensity normalization in PET group comparisons
page 14 of 36
only the 20% decreased region will be identified as hypometabolic (t<-2). These
regions are excluded in the second Andersson iteration, which retains only the
apparently unchanged region (t-values close to zero). However, this region was in
reality decreased by 10%, and the subsequent iterations will be identical to the first
one. Thus, the Andersson method is trapped. In this idealized case, the performance of
Andersson normalization would be identical to GM normalization. In the present
study, it actually performed much better – almost equal to WM normalization. Indeed,
the final normalization mask (Andersson iteration three) was somewhat similar to our
a priori defined WM mask, although some overlap with the truly decreased regions
were detected. All in all, Andersson normalization is surely preferable to standard
GM normalization, and seems recommendable, when no a priori expectations of the
data is available, which precludes the use of VOI normalization, or the Yakushev
method. A comparison of the normalization masks employed in the WM, Andersson,
and Yakushev is provided as supplementary material (Supplementary Figure 2).
It should be noted that we used only four iterations, whereas ten iterations were used
in the original study by Andersson. However, there was hardly any difference in the
final results between the third and fourth iteration in any of the six simulation trials,
so we concluded that the “ceiling” was reached by the fourth iteration in our data sets.
Applicability and caveats of the reference cluster method
The Yakushev reference cluster method performed very well and has potential
applicability in several cases: (a) group-comparisons of control subjects to patients, in
whom isolated uni-directional changes exist (i.e. isolated decreases or increases), (b)
intervention studies, in which the treatment induces regional uni-directional changes
Data-driven intensity normalization in PET group comparisons
page 15 of 36
in some brain regions while leaving other regions unchanged, (c) group-comparisons
of control subjects to patients, in whom bi-directional changes exist (i.e. both
decreases and increases), but only for cases in which one direction dominates. This is
the case in PD, in which many human PET studies report absolute decreases in CBF
and CMRglc in widespread cortical regions. Although no absolute subcortical
increases were ever convincingly reported in the patient studies, such absolute
subcortical increases were reported in autoradiography studies of animal models of
PD - in isolated small basal ganglia structures (pallidum, thalamic subnuclei,
pedunculo-pontine nucleus; see (Borghammer et al., 2008a) for references and full
discussion). Thus, for application of the Yakushev method to PD, these subcortical
structures should be excluded from the final normalization reference region.
Some caveats must be mentioned. The accuracy of the reference cluster method is
sensitive to the specific t-threshold and the optimal t-threshold surely varies between
different applications. In our simulation, the ground truth was known, so the optimal
threshold could potentially be determined from an ROC-analysis. However, this is not
the case for real data. A too stringent threshold leads to the definition of a small
normalization region, which is inherently susceptible to random noise. In this
scenario, the Yakushev method will be overly optimistic, i.e. lead to identification of
false decreases. In the present simulation, the propensity to detect false decreases was
only a major concern at the restricted threshold of t>3.6. Based on the present study,
we recommend that a threshold of not more than t>2 (p<0.05 uncorrected) is used in
most data sets, particularly in studies of early-stage disease, in which between-group
GM differences are smallest. Further simulation studies are needed for a full
description of the stability and performance of the Yakushev method, so care should
Data-driven intensity normalization in PET group comparisons
page 16 of 36
be taken in the interpretation of results based on this method. Yet, this particular
caveat applies to all normalization methods. Indeed, the present study confirms that
whereas the Yakushev method may lead to the detection of false positive decreases
(type I error), the gold standard WM normalization method lead to a marked
underestimation of the true pattern (type II error). In the study of neurodegenerative
disorders, investigators should therefore ideally utilize more than one type of
normalization, i.e. VOI- and Yakushev normalization, and interpret the results
accordingly. This also applies when automatic procedures are employed for
differential diagnostic purposes, i.e. the false positive decreases detected after
Yakushev normalization may create problems for correctly categorizing individual
scans in accordance with disease entities. However, in a recent study, the Yakushev
method exhibited the highest accuracy in correctly diagnosing patients with AD when
compared to VOI normalization (Yakushev et al., 2009), so it seems that the type I
error created by Yakushev normalization presents less of a problem than the type II
error created by unbiased VOI normalization.
On absolute values and Parkinson’s disease.
As mentioned in the introduction, samples sizes of 50-200 subjects pr group are
needed to detect a 10% decrease in the GM, and as many as 180-750 subjects per
group would be needed to detect a the 5% decrease present in simulation II (α=0.05,
power=0.90, COV 15-30%). The typical PET or SPECT group comparison involves
sample sizes of 10-40 subjects pr group, making the detection of a 5-10% decrease in
the GM mean improbable. This is likely part of the explanation, why GM
normalization is one of the most commonly used methods. However, as mentioned
above, GM normalization could well be the worst possible method of normalization
Data-driven intensity normalization in PET group comparisons
page 17 of 36
for this type of data, whereas VOI methods and particularly the reference cluster
method would likely yield more correct results. These considerations have the very
serious implication that a large number of studies of neurodegenerative disorders may
have yielded incomplete results, i.e. the extent of true hypometabolism has been
underestimated. Moreover, artefactual hypermetabolism has been reported in regions,
which were merely conserved.
In support of this conclusion, the following examples from the Parkinson’s disease
(PD) literature will serve as an illustration. Global mean CBF and CMRglc values
were reported in at least 23 comparisons of PD and healthy controls. Of these
comparisons, seven reported significant GM decreases in PD patients (Bohnen et al.,
2007; Globus et al., 1985; Hu et al., 2000; Imon et al., 1999; Karbe et al., 1992; Kuhl
et al., 1984; Sasaki et al., 1992), thirteen reported non-significant decreases in PD
(Abe et al., 2003; Agniel et al., 1991; Berding et al., 2001; Bes et al., 1983; Eidelberg
et al., 1994; Ghaemi et al., 2002; Huang et al., 2007; Kitamura et al., 1988; Leenders
et al., 1985; Montastruc et al., 1987; Otsuka et al., 1991; Perlmutter and Raichle,
1985; Playford et al., 1992), and only three studies reported small non-significant GM
increases in the PD groups (Arahata et al., 1999; Huang et al., 2007; Otsuka et al.,
1991). Moreover, nine additional studies disclosed absolute decreases in regional
(mostly cortical) values, but did not explicitly report global mean values (Eberling et
al., 1994; Eidelberg et al., 1990; Kondo et al., 1994; Mito et al., 2005; Otsuka et al.,
1996; Peppard et al., 1992; Piert et al., 1996; Vander Borght et al., 1997; Wolfson et
al., 1985). No study reported significant absolute increases, except one very early
PET study of only four PD patients, which reported increases almost everywhere
(Rougemont et al., 1984). Taken together, this evidence strongly suggests that cortical
Data-driven intensity normalization in PET group comparisons
page 18 of 36
absolute CBF and CMRglc is decreased in PD, and therefore by extension the global
mean is also decreased – even if undetected in the individual study.
Nevertheless, most normalized PET and SPECT studies of PD employed GM
normalization (Eidelberg et al., 1994; Huang et al., 2007; Imon et al., 1999; Nagano-
Saito et al., 2004), and these reported extensive subcortical increases in the
cerebellum, white matter, thalamus, pallidum, and striatum, with concomitant cortical
decreases. In contrast, all 16 studies of PD, which utilized VOI normalization (to the
cerebellum or pons), reported no subcortical increases, and quite extensive cortical
decreases (see (Borghammer et al., 2008a) for references). This shift of pattern is
predicted by the present simulation studies (compare Figure 1B and 1D), indicating
that PD is characterized by widespread cortical decreases of activity with relative
preservation of subcortical regions. Therefore, we predict that a reevaluation of
populations of early-stage PD patients with the preferable normalization techniques
presented in this study, will demonstrate more widespread cortical decreases in PD,
and no concurrent widespread subcortical increases.
Other types of normalization – ANCOVA & SSM.
In this study, we only considered standard ratio normalization, which assumes a
proportional relationship between regional and global values. However, a covariance
adjustment using linear regression (ANCOVA) was developed for cognitive
activation studies, upon demonstration that an additive model better approximates the
relationship between regional and global values in cognitive activation PET studies
(Friston et al., 1990). The fundamental requirement in ANCOVA normalization is
that homogeneous regression coefficients exist between groups. However, covariance
Data-driven intensity normalization in PET group comparisons
page 19 of 36
adjustment with global mean as a covariate can reveal heterogeneous regression
coefficients between groups of subjects (Devous et al., 1993; Gullion et al., 1996),
which can be a serious limitation to the use of ANCOVA as an approach to removing
intersubject variation in global mean values. Moreover, previous comparisons of ratio
and ANCOVA normalization in cognitive activation studies (Arndt et al., 1996;
McIntosh et al., 1996; Strother et al., 1995) reported that the two methods perform
equally well. We previously reported that GM ratio normalization and ANCOVA
normalization yield approximately the same results in studies of healthy aging
(Borghammer et al., 2008b), i.e., they both create artificial increases in subcortical
regions and detect smaller clusters of cortical decreases than VOI ratio normalization
to white matter mean. Similarly, ANCOVA normalization of PD data (Eckert et al.,
2005) leads to the detection of widespread subcortical increases, which is also seen
after GM normalization, but never when using the cerebellum as the reference region.
For these reasons, we chose not to employ ANCOVA normalization in the present
study.
Statistical Parametric Mapping (SPM) (Friston, 1994) is the most commonly used
voxel-based statistical approach, but some studies have been performed with
alternative studies, such as the principal component network analysis known as the
scaled subprofile model (SSM) (Moeller et al., 1987; Strother et al., 1995).
Importantly, this method performs a preprocessing step that resembles GM
normalization. Nevertheless, it has been claimed that SSM removes irrelevant global
scaling factors, without introducing bias into the analysis (Ma et al., 2008; Strother et
al., 1995). Yet, in our previous simulation studies we found the SSM to perform very
similarly to standard GM normalization in SPM style analysis, i.e. artificial
Data-driven intensity normalization in PET group comparisons
page 20 of 36
subcortical increases were robustly created, whereas smaller clusters of cortical
decreases were detected, than seen following unbiased VOI ratio normalization
(Borghammer et al., 2008a; Borghammer et al., 2008b). Therefore, SSM was not
considered further in the present study.
Limitations of the simulation.
Any simulation should be a reasonable approximation of reality, since generalization
of the findings may otherwise be compromised. We do not claim that the present
simulations accurately portray any specific neurodegenerative disorder. Nevertheless,
we argue that the simulations were a realistic general approximation for the following
reasons: (1) The simulated pattern of modest (11%) and severe (23%) cortical
decreases were based on a t-map from an actual PD versus Controls comparison (see
(Borghammer et al., 2008a) for details). (2) Previous studies of AD and PD display
heterogeneous t-values, i.e. the classical regions, which are also the first to appear,
consistently exhibit the highest t-values (Soonawala et al., 2002; Yakushev et al.,
2009). At later disease stages, new regions appear but these display lower t-values
than the primary regions. Thus, simulation I is probably the most realistic emulation
of neurodegenerative disorders, whereas simulation II may be less realistic. (3)
Several previous studies of AD compared GM normalization to alternative VOI
normalization. They unanimously reported that normalization to regions known to be
spared in AD (pons, motor cortex, cerebellum) resulted in the detection of much more
extensive, but fairly isolated cortical decreases than seen following GM normalization
(Minoshima et al., 1995a; Soonawala et al., 2002; Yakushev et al., 2009). And even
more widespread cortical decreases were detecting using the cluster reference method
in AD (Yakushev et al., 2009). (4) Many researches claim that GM is not affected at
Data-driven intensity normalization in PET group comparisons
page 21 of 36
early stages of neurodegenerative disorders (Ma et al., 2008). However, as reviewed
above, most quantitative studies of PD reported absolute decreases in cortical and
global CBF and CMRglc. Absolute increases were never convincingly reported
anywhere in the brain. Moreover, it is counterintuitive to assume that the GM
decreases reported at later disease stages are not preceded by smaller (undetected)
decreases at earlier disease stages, especially when considering that the absolute
increases , which would be necessary to balance the often reported absolute decreases,
have themselves never been reported. (5) Finally, it is impossible to know whether
our simulation was a realistic emulation of the true extent and magnitude of cortical
decreases in early stage PD. However, some VOI based SPECT CBF studies
employed cerebellum ratio normalization. Assuming that the cerebellum is fairly
conserved in PD, the relative between-group differences in different regions would be
similar to the true absolute differences. One study compared 20 healthy controls to 17
early-stage PD patients without cognitive impairment. Relative decreases of 8-12%
were detected in the four brain lobes (Derejko et al., 2006). This is comparable to the
present simulation I.
We specifically chose not to alter the subcortical regions, since much evidence
indicates that subcortical regions are relatively conserved in conditions such as
Alzheimer’s disease (Buchert et al., 2005), Parkinson’s disease (Berding et al., 2001;
Hu et al., 2000), healthy aging (Kalpouzos et al., 2007), and hepatic encephalopathy
(Borghammer et al., 2008b). A few studies reported absolute thalamic and striatal
decreases. However, many GM normalized studies detected relative increases in these
and other subcortical regions, suggesting that any decrease in subcortical regions is of
Data-driven intensity normalization in PET group comparisons
page 22 of 36
a smaller magnitude than the decrease in the GM. For simplicity, we chose not to
perturb the subcortical regions in our simulation.
Finally, we used CBF images from healthy controls, which have a slightly poorer
signal/noise ratio than FDG-PET images. We also filtered our CBF images to a
resultant FWHM of 14mm, whereas FDG-PET images often employ filters of 10-12
mm. However, the aim of our study was to investigate how different normalization
methods affect the detection of large, widespread clusters of change in signal.
Therefore, the results should be robust irrespective of filter size used, and should
generalize to both FDG-PET (smaller filters) and SPECT-CBF studies (larger filters).
Summary
We repeatedly performed simulations of group-comparisons, in which one group had
isolated cortical decreases. We contend this to be a realistic simulation of
neurodegenerative disorders in general. Ratio normalization to five different reference
regions were compared, and standard global mean normalization was found to
perform very poorly in the detection of the true signal. Furthermore, it robustly
created artificial increases in conserved regions. In contrast, the data-driven reference
cluster method correctly identified most of the true signal without creating artificial
increases. We conclude that many neurodegenerative disorders, such as Alzheimer’s
disease, Parkinson’s disease, and other neurodegenerative disorders should be
reevaluated using data-driven normalization methods. We predict that more
widespread cortical decreases will be detected in these disorders, even at early
disease-stages. This would have important implications for our understanding of the
neuropathological mechanisms behind these disorders.
Data-driven intensity normalization in PET group comparisons
page 23 of 36
ACKNOWLEDGEMENTS
This work was supported by the Danish National Science Foundation, Medical
Research Council of Denmark, and the Danish Parkinson Foundation. The authors
wish to thank the reviewers for providing many helpful comments.
DISCLOSURE / CONFLICT OF INTEREST
None.
REFERENCES
Abe, Y., Kachi, T., Kato, T., Arahata, Y., Yamada, T., Washimi, Y., Iwai, K., Ito, K., Yanagisawa, N., Sobue, G., 2003. Occipital hypoperfusion in Parkinson's disease without dementia: correlation to impaired cortical visual processing. J Neurol Neurosurg Psychiatry 74, 419-422. Agniel, A., Celsis, P., Viallard, G., Montastruc, J.L., Rascol, O., Demonet, J.F., Marc-Vergnes, J.P., Rascol, A., 1991. Cognition and cerebral blood flow in lateralised parkinsonism: lack of functional lateral asymmetries. J Neurol Neurosurg Psychiatry 54, 783-786. Andersson, J.L., 1997. How to estimate global activity independent of changes in local activity. Neuroimage 6, 237-244. Arahata, Y., Hirayama, M., Ieda, T., Koike, Y., Kato, T., Tadokoro, M., Ikeda, M., Ito, K., Sobue, G., 1999. Parieto-occipital glucose hypometabolism in Parkinson's disease with autonomic failure. J Neurol Sci 163, 119-126. Arndt, S., Cizadlo, T., O'Leary, D., Gold, S., Andreasen, N.C., 1996. Normalizing counts and cerebral blood flow intensity in functional imaging studies of the human brain. Neuroimage 3, 175-184. Berding, G., Odin, P., Brooks, D.J., Nikkhah, G., Matthies, C., Peschel, T., Shing, M., Kolbe, H., van Den Hoff, J., Fricke, H., Dengler, R., Samii, M., Knapp, W.H., 2001. Resting regional cerebral glucose metabolism in advanced Parkinson's disease studied in the off and on conditions with [(18)F]FDG-PET. Mov Disord 16, 1014-1022.
Data-driven intensity normalization in PET group comparisons
page 24 of 36
Bes, A., Guell, A., Fabre, N., Dupui, P., Victor, G., Geraud, G., 1983. Cerebral blood flow studied by Xenon-133 inhalation technique in parkinsonism: loss of hyperfrontal pattern. J Cereb Blood Flow Metab 3, 33-37. Bohnen, N.I., Gedela, S., Kuwabara, H., Constantine, G.M., Mathis, C.A., Studenski, S.A., Moore, R.Y., 2007. Selective hyposmia and nigrostriatal dopaminergic denervation in Parkinson's disease. Journal of Neurology 254, 84-90. Borghammer, P., Cumming, P., Aanerud, J., Gjedde, A., 2008a. Artefactual subcortical hyperperfusion in PET studies normalized to global mean: Lessons from Parkinson's disease. Neuroimage. Borghammer, P., Jonsdottir, K.Y., Cumming, P., Ostergaard, K., Vang, K., Ashkanian, M., Vafaee, M., Iversen, P., Gjedde, A., 2008b. Normalization in PET group comparison studies--the importance of a valid reference region. Neuroimage 40, 529-540. Brabner, G., Janke, A.L., Budge, M.M., Smith, D., Pruessner, J., Collins, D.L., 2006. Symmetric Atlasing and Model Based Segmentation: An application to the Hippocampus in Older Adults. In: Larsen, R., Nielsen, M., Sporring, J. (Ed.), Medical Image Computing and Computer-assisted Intervention - MICCAI 2006. Springer-Verlag, Berlin, pp. 58-66. Buchert, R., Wilke, F., Chakrabarti, B., Martin, B., Brenner, W., Mester, J., Clausen, M., 2005. Adjusted scaling of FDG positron emission tomography images for statistical evaluation in patients with suspected Alzheimer's disease. Journal of Neuroimaging 15, 348-355. Derejko, M., Slawek, J., Wieczorek, D., Brockhuis, B., Dubaniewicz, M., Lass, P., 2006. Regional cerebral blood flow in Parkinson's disease as an indicator of cognitive impairment. Nuclear Medicine Communications 27, 945-951. Devous, M.D., Sr., Gullion, C.M., Grannemann, B.D., Trivedi, M.H., Rush, A.J., 1993. Regional cerebral blood flow alterations in unipolar depression. Psychiatry Research 50, 233-256. Eberling, J.L., Richardson, B.C., Reed, B.R., Wolfe, N., Jagust, W.J., 1994. Cortical glucose metabolism in Parkinson's disease without dementia. Neurobiol Aging 15, 329-335. Eckert, T., Barnes, A., Dhawan, V., Frucht, S., Gordon, M.F., Feigin, A.S., Eidelberg, D., 2005. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 26, 912-921. Eidelberg, D., Moeller, J.R., Dhawan, V., Sidtis, J.J., Ginos, J.Z., Strother, S.C., Cedarbaum, J., Greene, P., Fahn, S., Rottenberg, D.A., 1990. The metabolic anatomy of Parkinson's disease: complementary [18F]fluorodeoxyglucose and [18F]fluorodopa positron emission tomographic studies. Mov Disord 5, 203-213.
Data-driven intensity normalization in PET group comparisons
page 25 of 36
Eidelberg, D., Moeller, J.R., Dhawan, V., Spetsieris, P., Takikawa, S., Ishikawa, T., Chaly, T., Robeson, W., Margouleff, D., Przedborski, S., et al., 1994. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab 14, 783-801. Fox, P.T., Mintun, M.A., Reiman, E.M., Raichle, M.E., 1988. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J Cereb Blood Flow Metab 8, 642-653. Friston, K., 1994. Statistical Parametric Mapping. In: Thatcher, R.W., Hallet, M., Zeffiro, T., John, E.R., Huerta, M. (Eds.), Functional Neuroimaging. Academic Press, New York, pp. 77-93. Friston, K.J., Frith, C.D., Liddle, P.F., Dolan, R.J., Lammertsma, A.A., Frackowiak, R.S., 1990. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 10, 458-466. Fukuyama, H., Ogawa, M., Yamauchi, H., Yamaguchi, S., Kimura, J., Yonekura, Y., Konishi, J., 1994. Altered cerebral energy metabolism in Alzheimer's disease: a PET study. J Nucl Med 35, 1-6. Genovese, C.R., Lazar, N.A., Nichols, T., 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870-878. Ghaemi, M., Raethjen, J., Hilker, R., Rudolf, J., Sobesky, J., Deuschl, G., Heiss, W.D., 2002. Monosymptomatic resting tremor and Parkinson's disease: a multitracer positron emission tomographic study. Mov Disord 17, 782-788. Globus, M., Mildworf, B., Melamed, E., 1985. Cerebral blood flow and cognitive impairment in Parkinson's disease. Neurology 35, 1135-1139. Gullion, C.M., Devous, M.D., Sr., Rush, A.J., 1996. Effects of four normalizing methods on data analytic results in functional brain imaging. Biological Psychiatry 40, 1106-1121. Hu, M.T., Taylor-Robinson, S.D., Chaudhuri, K.R., Bell, J.D., Labbe, C., Cunningham, V.J., Koepp, M.J., Hammers, A., Morris, R.G., Turjanski, N., Brooks, D.J., 2000. Cortical dysfunction in non-demented Parkinson's disease patients: a combined (31)P-MRS and (18)FDG-PET study. Brain 123 ( Pt 2), 340-352. Huang, C., Tang, C., Feigin, A., Lesser, M., Ma, Y., Pourfar, M., Dhawan, V., Eidelberg, D., 2007. Changes in network activity with the progression of Parkinson's disease. Brain 130, 1834-1846. Imon, Y., Matsuda, H., Ogawa, M., Kogure, D., Sunohara, N., 1999. SPECT image analysis using statistical parametric mapping in patients with Parkinson's disease. J Nucl Med 40, 1583-1589. Kalpouzos, G., Chetelat, G., Baron, J.C., Landeau, B., Mevel, K., Godeau, C., Barre, L., Constans, J.M., Viader, F., Eustache, F., Desgranges, B., 2007. Voxel-based
Data-driven intensity normalization in PET group comparisons
page 26 of 36
mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. Karbe, H., Holthoff, V., Huber, M., Herholz, K., Wienhard, K., Wagner, R., Heiss, W.D., 1992. Positron emission tomography in degenerative disorders of the dopaminergic system. Journal of Neural Transmission. Parkinsons Disease and Dementia Section 4, 121-130. Kawachi, T., Ishii, K., Sakamoto, S., Sasaki, M., Mori, T., Yamashita, F., Matsuda, H., Mori, E., 2006. Comparison of the diagnostic performance of FDG-PET and VBM-MRI in very mild Alzheimer's disease. Eur J Nucl Med Mol Imaging 33, 801-809. Kitamura, S., Ujike, T., Kuroki, S., Sakamoto, S., Soeda, T., Iio, M., Terashi, A., 1988. [Cerebral blood flow and oxygen metabolism in patients with Parkinson's disease]. No To Shinkei 40, 979-985. Kondo, S., Tanaka, M., Sun, X., Okamoto, K., Hirai, S., 1994. [Cerebral blood flow and oxygen metabolism in patients with pure akinesia and progressive supranuclear palsy]. Rinsho Shinkeigaku. Clinical Neurology 34, 531-537. Kuhl, D.E., Metter, E.J., Riege, W.H., 1984. Patterns of local cerebral glucose utilization determined in Parkinson's disease by the [18F]fluorodeoxyglucose method. Ann Neurol 15, 419-424. Leenders, K.L., Perani, D., Lammertsma, A.A., Heather, J.D., Buckingham, P., Healy, M.J., Gibbs, J.M., Wise, R.J., Hatazawa, J., Herold, S., et al., 1990. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113 ( Pt 1), 27-47. Leenders, K.L., Wolfson, L., Gibbs, J.M., Wise, R.J., Causon, R., Jones, T., Legg, N.J., 1985. The effects of L-DOPA on regional cerebral blood flow and oxygen metabolism in patients with Parkinson's disease. Brain 108 ( Pt 1), 171-191. Ma, Y., Tang, C., Moeller, J.R., Eidelberg, D., 2008. Abnormal regional brain function in Parkinson's disease: truth or fiction? Neuroimage. McIntosh, A.R., Grady, C., Haxby, J.V., Maisog, J., Horwitz, B., Clark, C.M., 1996. Within-subject Transformations of PET Regional Cerebral Blood Flow Data: ANCOVA, Ratio, and Z-score Adjustments on Empirical Data. Human Brain Mapping 4, 93-102. Minoshima, S., Frey, K.A., Foster, N.L., Kuhl, D.E., 1995a. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr 19, 541-547. Minoshima, S., Frey, K.A., Koeppe, R.A., Foster, N.L., Kuhl, D.E., 1995b. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36, 1238-1248.
Data-driven intensity normalization in PET group comparisons
page 27 of 36
Mito, Y., Yoshida, K., Yabe, I., Makino, K., Hirotani, M., Tashiro, K., Kikuchi, S., Sasaki, H., 2005. Brain 3D-SSP SPECT analysis in dementia with Lewy bodies, Parkinson's disease with and without dementia, and Alzheimer's disease. Clinical Neurology and Neurosurgery 107, 396-403. Moeller, J.R., Strother, S.C., Sidtis, J.J., Rottenberg, D.A., 1987. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab 7, 649-658. Montastruc, J.L., Celsis, P., Agniel, A., Demonet, J.F., Doyon, B., Puel, M., Marc-Vergnes, J.P., Rascol, A., 1987. Levodopa-induced regional cerebral blood flow changes in normal volunteers and patients with Parkinson's disease. Lack of correlation with clinical or neuropsychological improvements. Mov Disord 2, 279-289. Nagano-Saito, A., Kato, T., Arahata, Y., Washimi, Y., Nakamura, A., Abe, Y., Yamada, T., Iwai, K., Hatano, K., Kawasumi, Y., Kachi, T., Dagher, A., Ito, K., 2004. Cognitive- and motor-related regions in Parkinson's disease: FDOPA and FDG PET studies. Neuroimage 22, 553-561. Ohta, S., Meyer, E., Fujita, H., Reutens, D.C., Evans, A., Gjedde, A., 1996. Cerebral [15O]water clearance in humans determined by PET: I. Theory and normal values. J Cereb Blood Flow Metab 16, 765-780. Otsuka, M., Ichiya, Y., Hosokawa, S., Kuwabara, Y., Tahara, T., Fukumura, T., Kato, M., Masuda, K., Goto, I., 1991. Striatal blood flow, glucose metabolism and 18F-dopa uptake: difference in Parkinson's disease and atypical parkinsonism. J Neurol Neurosurg Psychiatry 54, 898-904. Otsuka, M., Ichiya, Y., Kuwabara, Y., Hosokawa, S., Sasaki, M., Yoshida, T., Fukumura, T., Kato, M., Masuda, K., 1996. Glucose metabolism in the cortical and subcortical brain structures in multiple system atrophy and Parkinson's disease: a positron emission tomographic study. J Neurol Sci 144, 77-83. Peppard, R.F., Martin, W.R., Carr, G.D., Grochowski, E., Schulzer, M., Guttman, M., McGeer, P.L., Phillips, A.G., Tsui, J.K., Calne, D.B., 1992. Cerebral glucose metabolism in Parkinson's disease with and without dementia. Arch Neurol 49, 1262-1268. Perlmutter, J.S., Raichle, M.E., 1985. Regional blood flow in hemiparkinsonism. Neurology 35, 1127-1134. Perneczky, R., Hartmann, J., Grimmer, T., Drzezga, A., Kurz, A., 2007. Cerebral metabolic correlates of the clinical dementia rating scale in mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology 20, 84-88. Piert, M., Koeppe, R.A., Giordani, B., Minoshima, S., Kuhl, D.E., 1996. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson's disease. J Nucl Med 37, 1115-1122.
Data-driven intensity normalization in PET group comparisons
page 28 of 36
Pizzolato, G., Dam, M., Borsato, N., Saitta, B., Da Col, C., Perlotto, N., Zanco, P., Ferlin, G., Battistin, L., 1988. [99mTc]-HM-PAO SPECT in Parkinson's disease. J Cereb Blood Flow Metab 8, S101-108. Playford, E.D., Jenkins, I.H., Passingham, R.E., Nutt, J., Frackowiak, R.S., Brooks, D.J., 1992. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol 32, 151-161. Rougemont, D., Baron, J.C., Collard, P., Bustany, P., Comar, D., Agid, Y., 1984. Local cerebral glucose utilisation in treated and untreated patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 47, 824-830. Sasaki, M., Ichiya, Y., Hosokawa, S., Otsuka, M., Kuwabara, Y., Fukumura, T., Kato, M., Goto, I., Masuda, K., 1992. Regional cerebral glucose metabolism in patients with Parkinson's disease with or without dementia. Annals of Nuclear Medicine 6, 241-246. Soonawala, D., Amin, T., Ebmeier, K.P., Steele, J.D., Dougall, N.J., Best, J., Migneco, O., Nobili, F., Scheidhauer, K., 2002. Statistical parametric mapping of (99m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer's disease: normalizing to cerebellar tracer uptake. Neuroimage 17, 1193-1202. Strother, S.C., Anderson, J.R., Schaper, K.A., Sidtis, J.J., Liow, J.S., Woods, R.P., Rottenberg, D.A., 1995. Principal component analysis and the scaled subprofile model compared to intersubject averaging and statistical parametric mapping: I. "Functional connectivity" of the human motor system studied with [15O]water PET. J Cereb Blood Flow Metab 15, 738-753. Talairach, J., Tournoux, P., 1988. Co-planar stereotaxic atlas of the human brain. Thieme Verlag, New York. Vander Borght, T., Minoshima, S., Giordani, B., Foster, N.L., Frey, K.A., Berent, S., Albin, R.L., Koeppe, R.A., Kuhl, D.E., 1997. Cerebral metabolic differences in Parkinson's and Alzheimer's diseases matched for dementia severity. J Nucl Med 38, 797-802. Wolfson, L.I., Leenders, K.L., Brown, L.L., Jones, T., 1985. Alterations of regional cerebral blood flow and oxygen metabolism in Parkinson's disease. Neurology 35, 1399-1405. Worsley, K.J., Liao, C.H., Aston, J., Petre, V., Duncan, G.H., Morales, F., Evans, A.C., 2002. A general statistical analysis for fMRI data. Neuroimage 15, 1-15. Worsley, K.J., Marrett, S., Neelin, P., Vandal, A.C., Friston, K.J., and Evans, A.C., 1996. A Unified Statistical Approach for Determining Significant Signals in Images of Cerebral Activation. Human Brain Mapping 4, 58-73. Yakushev, I., Hammers, A., Fellgiebel, A., Schmidtmann, I., Scheurich, A., Buchholz, H.G., Peters, J., Bartenstein, P., Lieb, K., Schreckenberger, M., 2009. SPM-based count normalization provides excellent discrimination of mild
Data-driven intensity normalization in PET group comparisons
page 29 of 36
Alzheimer's disease and amnestic mild cognitive impairment from healthy aging. Neuroimage 44, 43-50.
Data-driven intensity normalization in PET group comparisons
page 30 of 36
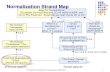
Figure 1. Illustration of the five types of normalization in Simulation I (trial 2). A.
The manipulation image volume used in simulation I. B. Global mean normalization
produced large artefactual increases in all four trials and detected very little of the true
signal. C. Andersson (AND) normalization. D. Ratio normalization to the mean of
white matter (WM). E. Yakushev normalization using a liberal t>2 threshold (YAK2).
F. Yakushev normalization using a restricted t>3.6 threshold (YAK3.6) detected most
of the true signal, but also some “false significant decreases” (yellow arrows). [Note:
the t-value scaling is extended in E-F, due to the very extreme t-values reported in the
two Yakushev normalizations. All slices are z = -1 (MNI space).]
0.770.89
A C DB E F“Truth”
3 5-3 -5.5
3 5-3.5 -7
Global AND WM YAK2 YAK3.6
Data-driven intensity normalization in PET group comparisons
page 31 of 36
Figure 2. The five types of normalization in Simulation II (trial 2). A. The
homogeneous manipulation image volume used in simulation II. B-F. Global mean
and Andersson normalization identified very little of the true signal. VOI
normalization identified slightly more. Both Yakushev methods recovered much more
of the original signal, but the YAK3.6 method identified even more false decreases
(yellow arrows) than in simulation I. [See Figure 1 for details and abbreviations. All
slices are z = 5 (MNI space).]
0.89
A C DB E F“Truth”
3 5-3 -5.5
3 5-3.5 -7
Global AND WM YAK2 YAK3.6
Data-driven intensity normalization in PET group comparisons
page 32 of 36
Supplementary Figure 1. A. The two groups of 20 healthy subjects sampled in Simulation II. The large inter-individual variation (SD/mean = 20%) in mean absolute CBF values is typical for absolute CBF and cerebral rate of glucose (CMRglc) data in the literature. B. In one group (right) a large part of the cerebral cortex was artificially decreased by 11%. However, no striking between-group differences are discernible from the visual impression of the raw absolute CBF images after manipulation. [CBF units: mL/100g/min. Slices are visualized by the view_slices feature of fMRIstat.]
Data-driven intensity normalization in PET group comparisons
page 33 of 36
Supplementary Figure 2. The figure illustrates the true signal (dark blue and light blue) and the extent of the normalization masks (green) used for simulation I, trial 2. Top row depicts the Yakushev normalization mask (i.e. all voxels t>2 from the standard GM normalization analysis). Bottom row left illustrates the a priori defined white matter mask. Bottom row right illustrates the final Andersson mask (i.e. all voxels -2<t<2 from the third Andersson analysis).
0.77 0.89
z= 0 z= 10 z= 22 z= 60z= -13 z= 50
Yakushev (t >2)
White matter
z= 0 z= 25 z= 50
Andersson (final mask)
z= 0 z= 25 z= 50
Mask extent
Data-driven intensity normalization in PET group comparisons
page 34 of 36
Table 1. Global CBF values in the control and manipulated groups.
Controls Manipulated Group
Before Manipulation After Manipulation
Trial Mean SD Mean SD p-value mean SD p-value
Simulation I
1 37.5 6.8 37.4 6.8 0.95 34.2 6.2 0.13
2 40.0 9.3 38.9 7.4 0.69 35.7 6.8 0.10
3 38.9 7.6 39.5 8.3 0.81 36.2 7.6 0.27
4 39.1 7.0 39.2 6.6 0.98 35.9 6.0 0.13
Simulation II
1 36.1 7.6 36.2 6.8 0.96 34.3 6.4 0.45
2 37.1 8.6 36.3 7.8 0.76 34.5 7.4 0.31
Global mean CBF in the control groups was compared with that in the manipulated groups prior to, and after the
manipulation (see methods). [CBF has units ml/100g/min. Unpaired two-sided t-tests were used.]
Data-driven intensity normalization in PET group comparisons
page 35 of 36
Table 2. The percentage of true signal recovered by the five normalization methods.
SIMULATION I SIMULATION II
1 2 3 4 Mean(SD) 1 2 Mean(SD)
Global 77 24.9% 27.2% 25.1% 21.8% 24.7±2.2%
89 5.9% 6.7% 6.0% 4.6% 5.7±0.8% 5.5% 0.4% 2.9±3.6%
AND 77 59.2% 56.7% 54.4% 47.5% 54.4±5.1%
89 20.9% 20.0% 19.6% 13.3% 18.4±3.4% 1.6% 0.8% 1.2±0.5%
WM 77 73.6% 66.5% 88.2% 69.4% 74.4±9.6%
89 28.4% 24.8% 54.0% 24.6% 33.0±14.1% 44.0% 4.6% 24.3±27.9%
YAK2 77 93.7% 95.8% 92.1% 91.5% 93.3±2.0%
89 74.8% 81.5% 71.0% 67.6% 73.7±6.0% 64.9% 68.2% 66.5±2.3%
YAK3.6 77 95.5% 97.6% 96.0% 96.4% 96.4±0.9%
89 80.8% 87.4% 85.6% 82.9% 84.2±2.9% 87.9% 95.2% 91.5±5.1%
For each normalization method, the two rows present the percentage of severe (77) and moderate (89)
decreases detected. In simulation II a uniform decrease to 89% of original values were used. [WM=white
matter. AND=Andersson normalization. YAK2=Yakushev normalization using liberal threshold of t>2.
YAK3.6=Yakushev using threshold of t>3.6.]
Data-driven intensity normalization in PET group comparisons
page 36 of 36
Table 3. Artificial increases detected following global mean normalization.
SIMULATION I SIMULATION II
1 2 3 4 Mean(SD) 1 2 Mean(SD)
Global 44.9% 29.9% 100.2% 38.9% 53.5±31.7% 91.7% 0% 45.8±64.8%
The extent of the artificial increases are presented as a percentage of the detected true decreases, i.e. 100 x (voxels
showing increases / voxels showing decreases).
Related Documents