Review Contributions of Myc to tumorigenesis Werner Lutz a , Javier Leon b , Martin Eilers a ; * a Institute for Molecular Biology and Tumor Research, University of Marburg, IMT, Mannkop¡ Str. 2, 35033 Marburg, Germany b Dpto. de Biologia Molecular, Facultad de Medicina, Universidad de Cantabria, 39011 Santander, Spain Received 28 August 2001; received in revised form 11 December 2001; accepted 13 December 2001 Abstract Despite intensive research, the mechanisms by which deregulation of myc gene expression contributes to tumorigenesis are still not fully resolved and many aspects are still enigmatic. Several recent reviews, including one published in this series a few months ago, have summarized recent progress in our understanding of the biochemistry of Myc proteins [Eisenmann, Genes Dev. (2001) in press; Amati et al., Biochim. Biophys. Acta 1471 (2001) 135^145]. Also, the evidence documenting a central role of Myc proteins in human tumorigenesis has been extensively reviewed [Henriksson and Lu «scher, Cancer Res. 68 (1996) 109^182]. In this article, we will argue that current progress allows us to present testable hypotheses on how Myc affects specific properties of transformed cells. ß 2002 Elsevier Science B.V. All rights reserved. Keywords : Myc ; Tumoriogenesis ; Mechanism ; Contribution 1. A brief account of Myc biochemistry Current models view Myc proteins as transcription factors that exert their biological function through their ability to both activate and repress target genes [1^3]. Early suggestions that Myc proteins have di- rect roles in DNA replication have not been substan- tiated, although there is some evidence suggesting an association between Myc and cdc6 and orc-1, pro- teins involved in the initiation of DNA replication (e.g. [4]). A dimeric complex with a partner protein, termed Max, mediates transcriptional activation by Myc [5]. Complex formation with Max has been formally proven to be required for most biological activities of Myc [6,7] and essentially all Myc protein in a cell is complexed with Max [8]; unlike expression of c-Myc, expression of max is constitutive or subjected to only minor variation [8]. The heterodimeric Myc/ Max complex binds to speci¢c DNA sequences with a core CACGTG sequence. Many genes that are ac- tivated by Myc/Max complexes are now known, ei- ther from array projects, educated guesses or from directed searches [9^12]. In some cases, in vivo bind- ing of Myc proteins to promoter or enhancer sequen- ces of these genes has been con¢rmed by chromatin immunoprecipitation (e.g. [13^15]). Max can also interact with other bHLH-LZ pro- teins of the Mad family, namely Mad1, Mxi1, Mad3 and Mad4. Other Mad-related proteins more dis- tantly related are Mnt/Rox and Mga (for review, see [16]). More recently, further complexity has been added to this already intricate network of inter- acting proteins by the discovery of novel Max-related proteins, Mlx and Mondo, which interact with some Mad proteins but cannot interact with Max [17^19]. 0304-419X / 02 / $ ^ see front matter ß 2002 Elsevier Science B.V. All rights reserved. PII:S0304-419X(02)00036-7 * Corresponding author. Fax : +49-6421-286-5196. E-mail address : [email protected] (M. Eilers). Biochimica et Biophysica Acta 1602 (2002) 61^71 www.bba-direct.com

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Review
Contributions of Myc to tumorigenesis
Werner Lutz a, Javier Leon b, Martin Eilers a;*a Institute for Molecular Biology and Tumor Research, University of Marburg, IMT, Mannkop¡ Str. 2, 35033 Marburg, Germany
b Dpto. de Biologia Molecular, Facultad de Medicina, Universidad de Cantabria, 39011 Santander, Spain
Received 28 August 2001; received in revised form 11 December 2001; accepted 13 December 2001
Abstract
Despite intensive research, the mechanisms by which deregulation of myc gene expression contributes to tumorigenesis arestill not fully resolved and many aspects are still enigmatic. Several recent reviews, including one published in this series a fewmonths ago, have summarized recent progress in our understanding of the biochemistry of Myc proteins [Eisenmann, GenesDev. (2001) in press; Amati et al., Biochim. Biophys. Acta 1471 (2001) 135^145]. Also, the evidence documenting a centralrole of Myc proteins in human tumorigenesis has been extensively reviewed [Henriksson and Lu«scher, Cancer Res. 68 (1996)109^182]. In this article, we will argue that current progress allows us to present testable hypotheses on how Myc affectsspecific properties of transformed cells. ß 2002 Elsevier Science B.V. All rights reserved.
Keywords: Myc; Tumoriogenesis ; Mechanism; Contribution
1. A brief account of Myc biochemistry
Current models view Myc proteins as transcriptionfactors that exert their biological function throughtheir ability to both activate and repress target genes[1^3]. Early suggestions that Myc proteins have di-rect roles in DNA replication have not been substan-tiated, although there is some evidence suggesting anassociation between Myc and cdc6 and orc-1, pro-teins involved in the initiation of DNA replication(e.g. [4]).
A dimeric complex with a partner protein, termedMax, mediates transcriptional activation by Myc [5].Complex formation with Max has been formallyproven to be required for most biological activitiesof Myc [6,7] and essentially all Myc protein in a cell
is complexed with Max [8]; unlike expression ofc-Myc, expression of max is constitutive or subjectedto only minor variation [8]. The heterodimeric Myc/Max complex binds to speci¢c DNA sequences witha core CACGTG sequence. Many genes that are ac-tivated by Myc/Max complexes are now known, ei-ther from array projects, educated guesses or fromdirected searches [9^12]. In some cases, in vivo bind-ing of Myc proteins to promoter or enhancer sequen-ces of these genes has been con¢rmed by chromatinimmunoprecipitation (e.g. [13^15]).
Max can also interact with other bHLH-LZ pro-teins of the Mad family, namely Mad1, Mxi1, Mad3and Mad4. Other Mad-related proteins more dis-tantly related are Mnt/Rox and Mga (for review,see [16]). More recently, further complexity hasbeen added to this already intricate network of inter-acting proteins by the discovery of novel Max-relatedproteins, Mlx and Mondo, which interact with someMad proteins but cannot interact with Max [17^19].
0304-419X / 02 / $ ^ see front matter ß 2002 Elsevier Science B.V. All rights reserved.PII: S 0 3 0 4 - 4 1 9 X ( 0 2 ) 0 0 0 3 6 - 7
* Corresponding author. Fax: +49-6421-286-5196.E-mail address: [email protected] (M. Eilers).
BBACAN 87512 11-4-02
Biochimica et Biophysica Acta 1602 (2002) 61^71www.bba-direct.com
Mad and Max interact via their leucine zippers, soMax^Myc and Max^Mad interactions are mutuallyexclusive. The Max/Mad complexes bind to the sameE-boxes as Myc^Max but repress rather than acti-vate transcription.
Recent work has focused on trying to understandmechanisms of transactivation by the Myc/Max com-plex and repression by Mad/Max complexes, respec-tively. The N-terminal domains of Mad proteins re-cruit the co-repressors mSin3A and mSin3B, whichin turn recruit a multi-subunit complex containingthe histone deacetylases HDAC1 and HDAC2 [1].Conversely, activation of endogenous target genesby Myc correlates with the recruitment of TRRAP,a component of at least two histone acetylase com-plexes [20,21]. Accordingly, Myc directs histone ace-tylation at target genes in vivo, strongly suggestingthat Myc and Mad regulate transcription at least inpart through recruitment of histone acetylases anddeacetylases, respectively [13,14].
Somewhat paradoxically, Myc proteins also havethe ability to repress transcription and several modelshave been suggested to account for this property.For example, Myc/Max complexes may induce thetranscription of transcriptional repressor proteins;in this model, transcriptional repression then occursas an indirect consequence of gene activation byMyc. Alternatively, Myc has been suggested to asso-ciate with several partner proteins other than Maxand to inhibit transcriptional activation by these pro-teins. For example, Myc associates with the zinc ¢n-ger proteins Sp-1 and Miz-1, which are involved inactivation of the p21cip1 and p15ink4b promoters, bothof which are negatively regulated by Myc [22^24].For Miz-1, binding to Myc prevents its associationwith p300, an obligatory co-factor for Miz-1-medi-ated activation of p15ink4b [22]. Most likely, therefore,repression of at least some genes will turn out to be abona ¢de direct biochemical activity of Myc bymechanisms unrelated to its E-box binding andtransactivation functions.
2. Models of transformation
Recent reviews have stressed the fact that a num-ber of distinct genetic programs have to be disruptedfor a tumor to emerge, including a reduction of
growth-factor requirement, inhibition of apoptosis,extension of lifespan and the ability to stimulate an-giogenesis [25]. Indeed, the function of individualoncogenes can often be assigned to speci¢c processes,e.g. to extension of lifespan or inhibition of apopto-sis. While initial studies stressed the ability of deregu-lated Myc proteins to stimulate cell proliferation, it isbecoming increasingly apparent that deregulation ofMyc function may contribute to various propertiesof tumor cells, defying a simple assignment to a sin-gle functional category.
3. Reduction of growth-factor requirements
One common feature of many transformed cells istheir reduced dependence on external growth factorsand cells expressing deregulated Myc genes share thisproperty [26]. Using conditional Myc-estrogen recep-tor (MycER) chimeras, it has been shown that acti-vation of Myc often allows cells to enter S-phase andundergo mitosis in the absence of external factors[27]. In many established cell lines, Myc-induced pro-liferation in the absence of external growth factors islimited solely by Myc-induced apoptosis [28]. Con-versely, c-myc3=3 rat ¢broblasts [29] and c-myc3=3
murine B-cells [30] show a dramatically reducedgrowth rate with respect to parental cells, mostlydue to G1 delay; thus, expression of c-myc appearsto be rate-limiting for proliferation in at least somecells.
In response to activation of Myc and also in trans-genic systems, in which Myc is expressed in a deregu-lated manner, at least three distinct genetic pro-grams, which are highly growth-factor-dependent innormal cells, are activated. Activation of any one ofthese programs may account for the ability of de-regulated Myc to reduce the growth-factor depen-dence. These programs are:
b Activation of cyclin E/Cdk2 kinase [31,32].
b Activation of E2F-dependent transcription [33].
b Activation of cellular growth, resulting in an in-crease in cell mass [34,35].
The molecular pathway leading to activation of
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^7162
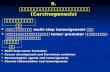
cyclin E/Cdk2 kinase by Myc in quiescent ¢broblastsis well analyzed and at least four direct target genesof Myc appear to be involved (summarized in Fig.1). Myc regulates cyclin E/Cdk2 kinase by suppress-ing the function of of the cdk2 inhibitor p27 [32,36];earlier suggestions implying Cdc25A as the mediatorof Myc action [37] are not compatible with the fail-ure of Myc to regulate cyclin E/Cdk2 kinase in p27-de¢cient cells (where p130 substitutes for p27 as in-hibitor) [36]. Initially, activation of cyclin D2, Cdk4and possibly other Myc-target genes induces seques-tration of p27 into cyclin D2/Cdk4 complexes; thisappears to be su⁄cient to generate a small amountof active cyclin E/Cdk2 kinase [10,38,39]. This cata-lyzes phosphorylation of p27 at threonine 187; phos-phorylated p27 is then recognized by an E3 ligasecomplex, which includes Cul-1 as a sca¡old protein.Recognition of phosphorylated p27 by the E3 ligaseis facilitated by Cks proteins. Both Cul-1 and one ofthe Cks proteins, Cks2, are target genes of Myc[10,40]. In addition, transcriptional repression ofthe p15ink4b and p21cip1 genes by Myc may furthercontribute to lowering the total inhibitor level underspeci¢c physiological circumstances (e.g. when p53 isactivated) [24,41].
From the use of p27-de¢cient cells, it is clear thatthe ability of Myc to activate E2F-dependent tran-
scription is independent of its ability to regulate cy-clin E/Cdk2 kinase [36]. Also, ectopic expression ofcyclin D2 does not substitute for Myc, implying thatother targets of Myc are involved. Potential candi-dates are the E2F2 and E2F3 genes [42]; thus, inMyc expressing cells, the amount of E2F proteinsmay exceed the amount of pocket proteins requiredto repress them. Also, the Id-2 gene is a target fortranscriptional activation by Myc; Id-2 protein bindsto, and inactivates, the retinoblastoma protein, whichwould result in the activation of E2F-dependenttranscription [43].
In addition to the above e¡ects on cell prolifera-tion, Myc also regulates cell growth. For example,enforced expression of the Drosophila c-myc orthologin wing imaginal discs results in increased cell size[35]. A similar e¡ect has been observed for mamma-lian c-Myc in murine B-cells [34,44] and developinglimb of the chicken (M. Ros and J. Leo¤n, unpub-lished). Consistent with these observations, manyMyc-target gene products are involved in proteinsynthesis and ribosome biogenesis (e.g. [9^12]). Spe-ci¢c metabolic pathways are also targeted by Myc.For example, Myc induces glycolytic enzymes, in-cluding lactate dehydrogenase, providing a possiblemolecular explanation for the enhanced rates of gly-colysis observed in many tumor cells [45]. Little
Fig. 1. Pathway depicting cyclin E/Cdk2 regulation by Myc in quiescent ¢broblasts. Target genes of Myc that a¡ect cyclin E/Cdk2 ki-nase activity are depicted in blue. Active kinase complexes are depicted in green, inactive complexes in red. According to this model,Myc stimulates both sequestration of p27 in cyclin D2/Cdk4 complexes and degradation of p27.
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^71 63
doubt exists that stimulation of cell growth and me-tabolism contributes to cell proliferation; however,how precisely enhanced expression of metabolicgenes leads to enhanced £ux through metabolic path-ways and how this, in turn, could contribute to de-regulation of cell proliferation or tumorigenesis, islargely unknown.
Thus, the present array of candidate target geneso¡ers several hypotheses as to how Myc might func-tion to reduce the requirement for external growthfactors and enhance proliferation in speci¢c cellsand/or tumors. A few genetic studies demonstratinga requirement for a speci¢c target gene in Myc-in-duced proliferation or tumorigenesis have been per-formed. Id-2 and cyclin D2 have been shown to berequired for Myc to stimulate proliferation of mouseembryo ¢broblasts [39,43,46] ; conversely, depletionof p27 has been shown to rescue the proliferationdefect of c-myc3=3 ¢broblasts [40]. In addition,lack of E2F2 or E2F3 has been reported to limitthe ability of cells to enter S-phase in response toinfection with very high titers of adenoviruses ex-pressing Myc [47]; whether this re£ects a physiolog-ical situation, however, remains to be determined.
To our knowledge, only a single study analyzingMyc-induced tumorigenesis in genetically de¢cientanimals has been published. This study showed thatcyclin D1 is not required for MMTV-Myc inducedmammary tumorigenesis [48]. Therefore, while thereis some certainty that the results presented describeevents that are relevant in ¢broblasts in culture, thereis yet little evidence as to how relevant they are intumorigenesis. For example, regulation of p27 levelsis highly signi¢cant during the development of manytumors; whether there is any correlation betweenderegulation of Myc and p27 levels in human tumorsis not clear. At least in human neuroblastoma, this isnot the case [49].
4. Immortalization
Most ‘primary’ cells have a ¢nite lifespan in cul-ture. In contrast, being ‘immortal’ is a hallmark ofmany tumor cells and, as a consequence, the processof ‘immortalization’ is of considerable interest.
The lifespan of primary human cells in culture islargely dictated by telomere length, since expression
of the catalytic subunit of human telomerase, htert,can signi¢cantly extend lifespan of human primarycells in culture (for review, see [50]). The htert pro-moter is a target for transactivation by Myc andactivation of conditional MycER proteins can inducehtert expression and telomerase activity in some pri-mary cells (e.g. [51]). Primary human cells can beconverted into tumor cells by the introduction ofRas, htert and the SV40 early region [52]; whetherMyc can replace htert in this process remains un-tested. Activation of telomerase activity is likely tobe an important step in the genesis of many, if not allhuman tumors; for example, telomerase activity canserve as a prognostic marker in childhood neuroblas-toma and correlates strongly with ampli¢cation ofNMYC (e.g. [53]). It is possible, therefore, that acti-vation of the htert promoter (or at least facilitationof htert expression) by Myc contributes to the gen-esis of human tumors. The hypothesis is hard to test,since telomere length is not a major regulatory factorin rodent tumorigenesis.
In order to achieve immortalization of human cellsin culture, mutations in the retinoblastoma pathwayare required in addition to maintenance of telomer-ase activity (e.g. [52,54]). However, manipulation ofthe culture conditions (for example the use of feederlayers) suggests that these ‘telomere independent’mechanisms of life span regulation re£ect stress re-sponses to the inadequate culture conditions on plas-tic rather than a counting mechanism of cell divisionsper se [55]. If so, deregulated expression of Myc alsointerferes with such stress responses, since Myc canextend the lifespan of primary human ¢broblastseven under conditions when ectopic expression ofhtert cannot [56].
This situation may be analogous to the situation inprimary rodent ¢broblasts, since the ¢nite lifespan ofprimary mouse cells in culture is not dictated by telo-mere length: arrest in culture is very rapid and oc-curs without signi¢cant shortening of the very longtelomeres. Again, senescence appears to be a stressresponse to the inappropriate tissue culture condi-tions or unusual environment and thus is likely tobe a tissue culture artifact [57]. However, since thegenetic circuitry controlling senescence under suchconditions involves a number of bona ¢de oncogenesand tumor suppressor genes, it is likely that relatedstress situations occur in vivo and that it is important
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^7164
for an organism that cells respond to them in anappropriate manner. In particular, high-level expres-sion of oncogenic alleles of Ras accelerates the ap-pearance of senescence in culture, which may possi-bly re£ect physiological processes that protect fromtumorigenesis by Ras in vivo [58,59].
The genetic circuitry underlying this process is wellunderstood (see Fig. 2). Two genetic pathways a¡ectthis process: ¢rst, both oncogenic Ras and prolongedculture of primary cells induces expression ofp19ARF, a negative regulator of mdm-2 (for review,see [60]). Induction of ARF stabilizes p53, thus lead-ing to cell cycle arrest and contributing to inductionof apoptosis. Loss of either ARF or p53 allowstransformation of primary cells by Ras and immor-talizes primary MEFs; therefore, this pathway is nec-essary for senescence to occur (e.g. [61]).
Second, the concomitant loss of several pocketproteins abolishes induction of senescence by Ras.Introduction of oncogenic Ras into pRb/p107-doubleknockout or into pRb/p107/p130 triple knockoutcells fails to induce senescence, even though bothp53 and p19ARF remain both intact and p21 is in-duced [62,63]. Most likely, therefore, the Cdk4 path-way that controls pocket protein function is a paral-lel pathway that is independently necessary, but not
su⁄cient to induce senescence in MEFs. This view issupported by observations showing that the loss ofp15ink4b, p18ink4c or both also facilitates transfor-mation by Ras alone [64]. However, the e¡ects re-ported are not as strong as those seen after loss ofARF or p53. Therefore, it is also possible that pock-et proteins are downstream of p53 and p21; for ex-ample because of the ability of p21 to halt prolifer-ation through its ability to block phosphorylation ofpocket proteins (see Fig. 1).
Ectopic expression of Myc immortalizes primarymouse embryo ¢broblasts and co-operates with on-cogenic alleles of Ras in transformation [65]. Whatmay be the underlying mechanisms?
Paradoxically, as mentioned above for Ras, ec-topic expression of Myc induces expression ofp19ARF, thus leading to stabilization of p53 [66].Primary MEFs that are immortalized by Myc havesustained mutations of p53 or have lost expression ofARF. This suggests that induction of ARF limits theimmortalizing functions of Myc as well as Myc-in-duced lymphomagenesis in mice [67]. How can Mycbe immortalizing despite its ability to induce ARF?
Before discussing possible models, it is worth re-membering that MEFs are by no means clonal cells ;thus it is formally possible that expression of Mycselects out a population of cells from the pool thathave already sustained ARF mutations and allowstheir transformation by Ras. In the absence of aclear and validated mechanistic model for Myc’s ac-tion, this remains a possibility. However, two modelsthat do not invoke a selection of a cell populationfrom the pool of mouse embryo ¢broblasts appearplausible.
4.1. Model I
In one simple model Myc immortalizes primaryrodent cells by enhancing the chance of mutationsoccurring at either the p19ARF or the p53 locusbefore cells become senescent (see Fig. 3). The modelpredicts that Myc acts in an irreversible manner andtherefore once either mutation has occurred, Mycshould no longer be required. How could Myc func-tion in this way? It may a¡ect mutation rates at thep19ARF or p53 loci; this would imply that deregu-lated expression of Myc is mutagenic. However,there is only limited evidence for this (see below).
Fig. 2. Genetic control of senescence in primary mouse embryo¢broblasts. High-level expression of oncogenic alleles of Ras ac-celerates senescence; however, in the absence of oncogenic Ras,other stimuli may enhance ARF expression during prolongedpassages in culture.
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^71 65
Alternatively, Myc may not a¡ect mutation rates perse but extend the time (number of passages) in whichmutations can accumulate at either the p53 or ARFlocus before cells arrest and become senescent. Inthis latter view, at least some of the mechanisms bywhich Myc stimulates cell proliferation would alsooccur in primary cells (e.g. repression of p21,p15ink4b, induction of Id2 and cyclin D2); via theregulation of these targets, Myc would delay the on-set of senescence. Since MEFs are cultivated inhighly stressful and potentially mutagenic environ-ments, this delay may well be su⁄cient to select forARF- or p53-mutant cells. The ¢ndings that bothcyclin D2- and Id2-de¢cient MEFs are resistant toimmortalization by Myc strongly argues that such ascenario is plausible [39,43,46].
One prediction from such a model is that the ac-tion of Myc is irreversible implying that Myc-in-duced tumorigenesis is irreversible in vivo. This hasbeen tested several times and there are clear examplesof reversible tumor formation, for example in lym-phoid cells [68]. However, recent data using a tetra-cycline-regulated allele of Myc targeted to the breastepithelium show that re-repression of Myc once tu-mors had established led to tumor regression in onlya subset of tumors [69]. Further analysis revealedthat the non-regressing tumors had sustained Kras2mutations, suggesting that once these mutations hadoccurred Myc was dispensable. Oncogenic mutationof Kras2 did not substitute for Myc function (e.g. by
transcriptionally activating endogenous Myc genes),since several target genes of Myc were downregulatedwhen Myc was repressed. Therefore, the datastrongly suggest that, in analogy to the proposedmodel in MEFs, deregulated expression of Myc isrequired only transiently during mammary tumori-genesis to facilitate the emergence of cell clonesthat carry mutations in Ras. Whether the emergingtumors have mutations in either ARF or p53 has notbeen reported; it is not entirely clear, therefore,whether the genes that are mutated to facilitate emer-gence are identical in both systems.
4.2. Model 2
An alternative model would maintain that Mycinterferes with p53-dependent transcriptional activa-tion in primary MEFs at a level distinct from ARFbut upstream of p21 and other target genes of p53(Fig. 3). Thus, Myc may dampen p53 transcriptionalresponses and thereby facilitate the emergence of im-mortal clones. In this view, immortalization of MEFsby Myc is the sum of two opposing interactions ofMyc with p53: activation of p53 function via ARFand dampening of transcriptional activation by p53via a mechanistically unknown pathway. At present,there are two pieces of evidence to suggest the exis-tence of such a pathway.
First, at least two genes that are targets for tran-scriptional activation of p53, namely p21 and gadd45,
Fig. 3. Alternate models for the functions of Myc during immortalization of MEFs. See text for details.
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^7166
are well established targets of transcriptional repres-sion by Myc. Indeed, it has formally been shown thatMyc interferes with p53-dependent induction ofgadd45 [70]; our own data show the same is truefor p21 and other target genes of p53 (J.L. andM.E., unpublished).
Second, further evidence for such a model hasbeen provided by the analysis of K562 cells thatharbor both a temperature-sensitive allele of p53and an inducible allele of Myc [71]. These cells lackARF and therefore mimic the situation of many hu-man leukemias, which have both wt53 and high lev-els of Myc. In these cells, a shift in temperature in-duces both cell cycle arrest and subsequentapoptosis. Activation of Myc in arrested cells sup-presses cell cycle arrest by ts53 and this has beentraced to its ability to repress induction of p21 ; sim-ilar observations have been made in other cell lines.Surprisingly, however, Myc also reduces p53-inducedapoptosis in this situation, unlike what is seen inARF-positive cells. The p53-target genes which me-diate apoptosis in this system have not been identi-¢ed; however, array analyses show that several targetgenes of p53 are repressed upon induction of Myc.Together, these data raise the possibility that Mycmay dampen p53-mediated signal transduction,maybe by interfering with the function and/or ex-pression of co-activators of p53.
5. Resistance to anti-mitogenic factors
Proliferation of cells in vivo is not only controlledby positively acting growth factors, but is also re-strained by anti-mitogenic factors. One of the mostprominent examples for such a factor is transforminggrowth factor L (TGF-L) and a large body of evi-dence suggests that inactivation of TGF-L signalingcontributes to tumorigenesis of colon, skin andbreast, among others (for review, see [72]).
Several genetic factors can lead to resistanceagainst TGF-L signaling; for example, mutations inreceptor proteins and in Smad proteins, which trans-mit the TGF-L signal to the nucleus, have been re-ported in human tumors. However, loss of TGF-Lresponsiveness can also occur when the TGF-L sig-naling pathway itself, is intact [73]. In one of thesecases, array analysis strongly suggests that the emer-
gence of resistance correlates speci¢cally with a lossof downregulation of the endogenous c-myc gene,which normally occurs in response to addition ofTGF-L. Indeed, ectopic expression of c-myc renderskeratinocytes resistant to the anti-mitogenic e¡ects ofTGF-L ; in part, this is due to Myc’s ability to sup-press induction of the p15ink4b and p21cip1 genes byTGF-L [74,75]. Repression of p15ink4b is mediated bythe Myc-associated protein, Miz-1, which is part ofthe signaling pathway that mediates induction of thep15ink4b gene by TGF-L ; deregulated expression ofMyc prevents Miz-1 from functioning properly inthe pathway [22,23]. Similarly, repression of p21cip1
appears to be caused by complex formation betweenMiz-1 and Sp-1 [24].
It should be pointed out that TGF-L may also be acritical part of the mechanism that protects primarycells from transformation by oncogenic Ras, at leastin primary keratinocytes (e.g. [76]). In these cells,expression of oncogenic Ras induces expression ofTGF-L and secretion of TGF-L is required for in-duction of premature senescence by Ras. Thus, ‘im-mortalization’ by Myc (which to our knowledge hasnot formally been demonstrated in keratinocytes)may re£ect its ability to disrupt TGF-L signaling inthis cell type.
6. Genomic instability
In order for a tumor cell to emerge, several muta-tions have to accumulate in a single cell ; in addition,most human tumors are characterized by alterationsin chromosome numbers. Thus, the genome of tumorcells may be either transiently or irreversibly unsta-ble. The ¢nding that Myc-induced tumorigenesis canbe irreversible at least in some cases [69] raises thequestion as to whether Myc proteins contribute totumorigenesis by promoting the accumulation of sec-ondary mutations or by destabilizing checkpointmechanisms that control chromosome number andploidy.
We are not aware of studies that test whether de-regulated expression of Myc a¡ects the appearanceof point mutations, although assays to ask this ques-tion are available. There is limited evidence to sup-port the suggestion that deregulated expression ofMyc can promote gene ampli¢cation. For example,
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^71 67
challenge of mammalian cells with the DNA damag-ing drug N-(phosphonoacetyl)-L-aspartate (PALA)requires ampli¢cation of the CAD gene for resistanceto emerge and even transient activation of Myc facil-itates the emergence of resistant clones upon PALAtreatment [77]. Similarly, several genes including cy-clin D2 have been demonstrated to be unstable incells expressing deregulated Myc [78]. Surprisingly,both CAD and cyclin D2 are targets for transcrip-tional activation by Myc. It is possible, therefore,that the binding of Myc recruits co-activators thatincrease the likelihood of creating genetic havoc atthe binding site. Similarly, there are several pieces ofevidence suggesting that Myc can override check-point mechanisms that control chromosome numberand ploidy. For example, exposure of ¢broblasts ex-pressing constitutive Myc to nocodazole led to theaccumulation of polyploid cell populations, sincecells exiting mitosis did not arrest but instead under-went multiple S-phases [79,80]. A similar e¡ect ofMyc is observed in murine keratinocytes [81]. Fur-ther, deregulation of Myc enhances the phenotypesof p53 loss when cells are challenged by taxol, lead-ing to an almost complete loss of G2 arrest [79,82].
How does Myc act in these circumstances? Onemechanism by which Myc overrides checkpoints isalmost certainly an indirect consequence of its mito-genic properties. For example, cells expressing Mycoverride an arrest imposed by physiological levels ofthe cdk inhibitor p21, which mediates cell cycle arrestby p53 [32]. Thus, these cells will not respond toactivation of p53 (for example in a tsp53 situation)with a cell cycle arrest (e.g. [83]). Similarly, chromo-somal abnormalities are observed in cells expressinghigh levels of cyclin E, suggesting that enhanced pro-liferation per se may be mutagenic [84].
It is possible that all observed e¡ects of Myc ongenomic stability are indirect consequences of its mi-togenic properties. The strongest suggestion for alter-native pathways comes from the observation thatMyc suppresses induction of the DNA-damage in-ducible gadd45 gene in response to several stress sig-nals [70]. Gadd45-de¢cient cells are highly unstablesuggesting that expression of gadd45 is required formaintenance of correct chromosome number and ge-nomic stability in the G2 phase [85]. Conversely, ec-topic expression of gadd45 is su⁄cient to establish acell cycle arrest at the G2/M boundary [86]. Expres-
sion of gadd45 is induced by a number of di¡erentsignaling pathways, including p53- and BRCA-de-pendent pathways, and all seem to be susceptible toinhibition by Myc. If Gadd45 and potentially relatedproteins are an integral part of mammalian check-point responses, it will be important to ¢nd outwhich aspect of checkpoint functions are disruptedby Myc. It is worth pointing out that, in yeast,checkpoints not only function to regulate the cellcycle, but have multiple functions that lead to anintegrated repair response. Depending on how Mycinterferes with gadd45 induction, it may thereforenot only a¡ect cell cycle arrest, but may also interferewith its repair functions.
7. Angiogenesis
All mechanisms discussed so far suggest that Mycacts early in tumor formation. However, several ob-servations suggest that deregulation of Myc can alsoa¡ect later stages of tumorigenesis, most notably an-giogenesis. For example, the reversible activation ofconditional alleles of Myc in keratinocytes in vivoinduces hyperproliferation, dedi¡erentiation and an-giogenesis in a reversible manner, suggesting thatthese events may be directly a¡ected by Myc [87].Similarly, angiogenesis occurs early in the genesisof myc-induced avian lymphomas [88].
Several mechanisms have been suggested to con-tribute to these observations: for example, suppres-sion of anti-angiogenic factors including thrombo-spondin and activin A by c-Myc or N-Myc,respectively, has been demonstrated [89,90]. Finally,the ¢nding that there is an overlap between hypoxia-regulated elements and myc-regulated elements in thepromoters of glycolytic enzymes suggests additionalpotential links between Myc- and hypoxia-inducedsignals.
8. Summary
Recent progress has clari¢ed several key aspects ofthe biochemistry of Myc proteins, and strongly rein-forced the notion that Myc’s biological e¡ects aredue to its ability to both positively and negativelya¡ect gene transcription. Current evidence is compat-
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^7168
ible with the notion that an early and critical contri-bution of Myc in tumorigenesis is the deregulation ofcell growth and proliferation, leading to a clonal ex-pansion of premalignant cells.
In our view, two key issues have now become ac-cessible.
First, work in ¢broblasts has identi¢ed severalgenes that appear to be candidates for mediators ofMyc’s mitogenic and growth-promoting functions.One might therefore predict, that at least some ofthese targets are rate-limiting for Myc-induced tu-morigenesis, and this seems to us an important andunresolved question.
Second, Myc’s role in tumorigenesis may well gobeyond its ability to de-regulate cell proliferation.For example, its ability to immortalize cells may bedue to its ability to induce genomic instability and/orto interfere with p53-dependent signal transduction.While much work has focused on Myc’s mitogenicand growth-promoting properties, it now seems crit-ical to understand whether Myc can also promotethe accumulation of mutations and, if so, what thepathways are that mediate this e¡ect.
Finally, it is also clear that Myc has been proposedto control or a¡ect other processes, which may behighly relevant for its tumorigenic action. For exam-ple, ectopic expression of Myc can block di¡erentia-tion in several systems and this may be independentof its mitogenic e¡ects [91]. Thus the choice of topicscovered in this review is in part due to the preferenceof the authors, and we apologize to all our col-leagues, whose work was not covered in detail.
Acknowledgements
The co-operation between the two contributinglaboratories is ¢nanced by the European Commun-ity. We thank Bruno Amati for critical comments onthe manuscript.
References
[1] R.N. Eisenmann, Genes Dev. 15 (2001) 2023^2030.[2] B. Amati, S.R. Frank, D. Donjerkovic, S. Taubert, Biochim.
Biophys. Acta 1471 (2001) M135^M145.
[3] M. Henriksson, B. Lu«scher, Cancer Res. 68 (1996) 109^182.[4] M. Takayama, T. Taira, S.M. Iguchi-Ariga, H. Ariga, FEBS
Lett. 477 (2000) 43^48.[5] L. Kretzner, E.M. Blackwood, R.N. Eisenmann, Nature 359
(1992) 426^429.[6] B. Amati, T.D. Littlewood, G.I. Evan, H. Land, EMBO J.
13 (1993) 5083^5087.[7] B. Amati, M.W. Brooks, N. Levy, T.D. Littlewood, G.I.
Evan, H. Land, Cell 72 (1993) 233^245.[8] E.M. Blackwood, B. Lu«scher, R.N. Eisenman, Genes Dev. 6
(1992) 71^80.[9] M. Schuhmacher, F. Kohlhuber, M. Holzel, C. Kaiser, H.
Burtscher, M. Jarsch, G.W. Bornkamm, G. Laux, A. Polack,U.H. Weidle, D. Eick, Nucleic Acids Res. 29 (2001) 397^406.
[10] H.A. Coller, C. Grandori, P. Tamayo, T. Colbert, E.S.Lander, R.N. Eisenman, T.R. Golub, Proc. Natl. Acad.Sci. USA 97 (2000) 3260^3265.
[11] Q.M. Guo, R.L. Malek, S. Kim, C. Chiao, M. He, M. Ru¡y,K. Sanka, N.H. Lee, C.V. Dang, E.T. Liu, Cancer Res. 60(2000) 5922^5928.
[12] R.C. O’Hagan, N. Schreiber-Agus, K. Chen, G. David, J.A.Engelman, R. Schwab, L. Alland, C. Thomson, D.R. Ron-ning, J.C. Sacchettini, P. Meltzer, R.A. DePinho, Nat. Gen-et. 24 (2000) 113^119.
[13] C. Bouchard, O. Dittrich, A. Kiermaier, K. Dohmann, A.Menkel, M. Eilers, B. Lu«scher, Genes Dev. 15 (2001) 2042^2047.
[14] S.R. Frank, M. Schroeder, P. Fernandez, S. Taubert, B.Amati, Genes Dev. 15 (2001) 2069^2082.
[15] K.E. Boyd, P.J. Farnham, Mol. Cell. Biol. 19 (1999) 8393^8399.
[16] T.A. Baudino, J.L. Cleveland, Mol. Cell. Biol. 21 (2001)691^702.
[17] G. Meroni, S. Cairo, G. Merla, S. Messali, R. Brent, A.Ballabio, A. Reymond, Oncogene 19 (2000) 3266^3277.
[18] A.N. Billin, A.L. Eilers, K.L. Coulter, J.S. Logan, D.E.Ayer, Mol. Cell. Biol. 20 (2000) 8845^8854.
[19] A.N. Billin, A.L. Eilers, C. Queva, D.E. Ayer, J. Biol. Chem.274 (1999) 36344^36350.
[20] S.B. McMahon, M.A. Wood, M.D. Cole, Mol. Cell. Biol. 20(2000) 556^562.
[21] S.B. McMahon, H.A. Van Buskirk, K.A. Dugan, T.D. Co-peland, M.D. Cole, Cell 94 (1998) 363^374.
[22] P. Staller, K. Peukert, A. Kiermaier, J. Seoane, J. Lukas, H.Karsunky, T. Moroy, J. Bartek, J. Massague, F. Hanel, M.Eilers, Nat. Cell. Biol. 3 (2001) 392^399.
[23] J. Seoane, C. Pouponnot, P. Staller, M. Schader, M. Eilers,J. Massague, Nat. Cell. Biol. 3 (2001) 400^408.
[24] A.L. Gartel, X. Ye, E. Goufman, P. Shianov, N. Hay, F.Najmabadi, A.L. Tyner, Proc. Natl. Acad. Sci. USA 98(2001) 4510^4515.
[25] D. Hanahan, R.A. Weinberg, Cell 100 (2000) 57^70.[26] E.J. Keath, P.G. Caimi, M.D. Cole, Cell 39 (1984) 339^348.[27] M. Eilers, S. Schirm, J.M. Bishop, EMBO J. 10 (1991) 133^
141.[28] G.I. Evan, A.H. Wyllie, C.S. Gilbert, T.D. Littlewood, H.
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^71 69
Land, M. Brooks, C.M. Waters, L.Z. Penn, D.C. Hancock,Cell 69 (1992) 119^128.
[29] M.K. Mateyak, A.J. Obaya, S. Adachi, J.M. Sedivy, CellGrowth Di¡er. 8 (1997) 1039^1048.
[30] I.M. de Alboran, R.C. O’Hagan, F. Gartner, B. Malynn, L.Davidson, R. Rickert, K. Rajewsky, R.A. DePinho, F.W.Alt, Immunity 14 (2001) 45^55.
[31] P. Steiner, A. Philipp, J. Lukas, D. Godden-Kent, M. Paga-no, S. Mittnacht, J. Bartek, M. Eilers, EMBO J. 14 (1995)4814^4826.
[32] J. Vlach, S. Hennecke, K. Alevizopoulos, D. Conti, B. Ama-ti, EMBO J. 15 (1996) 6595^6604.
[33] P. Jansen-Du«rr, A. Meichle, P. Steiner, M. Pagano, K.Finke, J. Botz, J. Wessbecher, G. Draetta, M. Eilers, Proc.Natl. Acad. Sci. USA 90 (1993) 3685^3689.
[34] M. Schuhmacher, M.S. Staege, A. Pajic, A. Polack, U.H.Weidle, G.W. Bornkamm, D. Eick, F. Kohlhuber, Curr.Biol. 9 (1999) 1255^1258.
[35] L.A. Johnston, D.A. Prober, B.A. Edgar, R.N. Eisenman, P.Gallant, Cell 98 (1999) 779^790.
[36] R. Beier, A. Burgin, A. Kiermaier, M. Fero, H. Karsunky,R. Sa¡rich, T. Moroy, W. Ansorge, J. Roberts, M. Eilers,EMBO J. 19 (2000) 5813^5823.
[37] K. Galaktionov, X. Chen, D. Beach, Nature 382 (1996) 511^517.
[38] H. Hermeking, C. Rago, M. Schuhmacher, Q. Li, J.F. Bar-rett, A.J. Obaya, B.C. O’Connell, M.K. Mateyak, W. Tam,F. Kohlhuber, C.V. Dang, J.M. Sedivy, D. Eick, B. Vogel-stein, K.W. Kinzler, Proc. Natl. Acad. Sci. USA 97 (2000)2229^2234.
[39] C. Bouchard, K. Thieke, A. Maier, R. Sa¡rich, J. Hanley-Hyde, W. Ansorge, S. Reed, P. Sicinski, J. Bartek, M. Eilers,EMBO J. 18 (1999) 5321^5333.
[40] R.C. O’Hagan, M. Ohh, G. David, I.M. de Alboran, F.W.Alt, W.G. Kaelin Jr., R.A. DePinho, Genes Dev. 14 (2000)2185^2191.
[41] G.F. Claassen, S.R. Hann, Proc. Natl. Acad. Sci. USA 97(2000) 9498^9503.
[42] R. Sears, K. Ohtani, J.R. Nevins, Mol. Cell. Biol. 17 (1997)5227^5235.
[43] A. Lasorella, M. Noseda, M. Beyna, A. Iavarone, Nature407 (2000) 592^598.
[44] B.M. Iritani, R.N. Eisenman, Proc. Natl. Acad. Sci. USA 96(1999) 13180^13185.
[45] H. Shim, C. Dolde, B.C. Lewis, C.S. Wu, G. Dang, R.A.Jungmann, R. Dalla-Favera, C.V. Dang, Proc. Natl. Acad.Sci. USA 94 (1997) 6658^6663.
[46] I. Perez-Roger, S.-H. Kim, B. Gri⁄ths, A. Sewing, H. Land,EMBO J. 18 (1999) 5310^5320.
[47] G. Leone, R. Sears, E. Huang, R. Rempel, F. Nuckolls,C.-H. Park, P. Giangrande, L. Wu, H.I. Saavedra, S.J. Field,M.A. Thompson, H. Yang, Y. Fujiwara, M.E. Greenberg, S.Orkin, C. Smith, J.R. Nevins, Mol. Cell 8 (2001) 105^113.
[48] Q. Yu, Y. Geng, P. Sicinski, Nature 411 (2001) 1017^1021.[49] E. Bergmann, M. Wanzel, A. Weber, I. Shin, H. Christian-
sen, M. Eilers, Int. J. Cancer 95 (2001) 176^183.
[50] R.A. Weinberg, Nature 396 (1998) 23^24.[51] R.A. Greenberg, R.C. O’Hagan, H. Deng, Q. Xiao, S.R.
Hann, R.R. Adams, S. Lichtsteiner, L. Chin, G.B. Morin,R.A. DePinho, Oncogene 18 (1999) 1219^1226.
[52] W.C. Hahn, C.M. Counter, A.S. Lundberg, R.L. Beijersber-gen, M.W. Brooks, R.A. Weinberg, Nature 400 (1999) 464^468.
[53] E. Hiyama, K. Hiyama, T. Yokoyama, Y. Matsuura, M.A.Piatyszek, J.W. Shay, Nat. Med. 1 (1995) 249^255.
[54] M.A. Dickson, W.C. Hahn, Y. Ino, V. Ronfard, J.Y. Wu,R.A. Weinberg, D.N. Louis, F.P. Li, J.G. Rheinwald, Mol.Cell. Biol. 20 (2000) 1436^1447.
[55] R.D. Ramirez, C.P. Morales, B.S. Herbert, J.M. Rohde, C.Passons, J.W. Shay, W.E. Wright, Genes Dev. 15 (2001)398^403.
[56] J. Wang, L.Y. Xie, S. Allan, D. Beach, G.J. Hannon, GenesDev. 12 (1998) 1769^1774.
[57] C.J. Sherr, R.A. DePinho, Cell 102 (2000) 407^410.[58] M. Serrano, H. Lee, L. Chin, C. Cordon Cardo, D. Beach,
R.A. DePinho, Cell 85 (1996) 27^37.[59] M. Serrano, E. Gomez Lahoz, R.A. DePinho, D. Beach, D.
Bar Sagi, Science 267 (1995) 249^252.[60] L. Chin, J. Pomerantz, R.A. DePinho, Trends Biochem. Sci.
23 (1998) 291^296.[61] T. Kamijo, F. Zindy, M.F. Roussel, D.E. Quelle, J.R.
Downing, R.A. Ashmun, G. Grosveld, C.J. Sherr, Cell 91(1997) 649^659.
[62] J. Sage, G.J. Mulligan, L.D. Attardi, A. Miller, S. Chen, B.Williams, E. Theodorou, T. Jacks, Genes Dev. 14 (2000)3037^3050.
[63] D.S. Peeper, J.H. Dannenberg, S. Douma, H. te Riele, R.Bernards, Nat. Cell. Biol. 3 (2001) 198^203.
[64] E. Latres, M. Malumbres, R. Sotillo, J. Martin, S. Ortega, J.Martin-Caballero, J.M. Flores, C. Cordon-Cardo, M. Bar-bacid, EMBO J. 19 (2000) 3496^3506.
[65] H. Land, L.F. Parada, R.A. Weinberg, Nature 304 (1983)596^602.
[66] F. Zindy, C.M. Eischen, D.H. Randle, T. Kamijo, J.L.Cleveland, C.J. Sherr, M.F. Roussel, Genes Dev. 12 (1998)2424^2433.
[67] C.M. Eischen, J.D. Weber, M.F. Roussel, C.J. Sherr, J.L.Cleveland, Genes Dev. 13 (1999) 2658^2669.
[68] D.W. Felsher, J.M. Bishop, Mol. Cell. 4 (1999) 199^207.[69] C.M. D’Cruz, E.J. Gunther, R.B. Boxer, J.L. Hartman, L.
Sintasath, S.E. Moody, J.D. Cox, S.I. Ha, G.K. Belka, A.Golant, R.D. Cardi¡, L.A. Chodosh, Nat. Med. 7 (2001)235^239.
[70] W.W. Marhin, S. Chen, L.M. Facchini, A.J. Fornace Jr.,L.Z. Penn, Oncogene 14 (1997) 2825^2834.
[71] E. Ceballos, M.D. Delgado, P. Gutierrez, C. Richard, D.Muller, M. Eilers, M. Ehinger, U. Gullberg, J. Leon, Onco-gene 19 (2000) 2194^2204.
[72] J. Massague, Y.G. Chen, Genes Dev. 14 (2000) 627^644.
[73] C.R. Chen, Y. Kang, J. Massague, Proc. Natl. Acad. Sci.USA 98 (2001) 992^999.
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^7170
[74] G.F. Claassen, S.R. Hann, Proc. Natl. Acad. Sci. USA 97(2000) 9498^9503.
[75] B.J. Warner, S.W. Blain, J. Seoane, J. Massague¤, Mol. Cell.Biol. 19 (1999) 5913^5922.
[76] R. Tremain, M. Marko, V. Kinnimulki, H. Ueno, E. Bot-tinger, A. Glick, Oncogene 19 (2000) 1698^1709.
[77] O.B. Chernova, M.V. Chernov, Y. Ishizaka, M.L. Agarwal,G.R. Stark, Mol. Cell. Biol. 18 (1998) 536^545.
[78] J.F. Mushinski, J. Hanley-Hyde, G.J. Rainey, T.I. Kuschak,C. Taylor, M. Fluri, L.M. Stevens, D.W. Henderson, S. Mai,Curr. Top. Microbiol. Immunol. 246 (1999) 183^189.
[79] Q. Li, C.V. Dang, Mol. Cell. Biol. 19 (1999) 5339^5351.
[80] D.W. Felsher, J.M. Bishop, Proc. Natl. Acad. Sci. USA 96(1999) 3940^3944.
[81] A. Gandarillas, D. Davies, J.M. Blanchard, Oncogene 19(2000) 3278^3289.
[82] X.Y. Yin, L. Grove, N.S. Datta, M.W. Long, E.V. Prochow-nik, Oncogene 18 (1999) 1177^1184.
[83] A.J. Wagner, J.M. Kokontis, N. Hay, Genes Dev. 8 (1994)2817^2830.
[84] C.H. Spruck, K.A. Won, S.I. Reed, Nature 401 (1999) 297^300.
[85] M.C. Hollander, M.S. Sheikh, D.V. Bulavin, K. Lundgren,L. Augeri-Henmueller, R. Shehee, T.A. Molinaro, K.E.Kim, E. Tolosa, J.D. Ashwell, M.P. Rosenberg, Q. Zhan,P.M. Fernandez-Salguero, W.F. Morgan, C.X. Deng, A.J.Fornace Jr., Nat. Genet. 23 (1999) 176^184.
[86] X.W. Wang, Q. Zhan, J.D. Coursen, M.A. Khan, H.U.Kontny, L. Yu, M.C. Hollander, P.M. O’Connor, A.J. For-nace Jr., C.C. Harris, Proc. Natl. Acad. Sci. USA 96 (1999)3706^3711.
[87] S. Pelengaris, T. Littlewood, M. Khan, G. Elia, G. Evan,Mol. Cell. 3 (1999) 565^577.
[88] K.A. Brandvold, P. Neiman, A. Ruddell, Oncogene 19(2000) 2780^2785.
[89] C.V. Ngo, M. Gee, N. Akhtar, D. Yu, O. Volpert, R. Auer-bach, A. Thomas-Tikhonenko, Cell Growth Di¡er. 11 (2000)201^210.
[90] S. Breit, K. Ashman, J. Wilting, J. Rossler, E. Hatzi, T.Fotsis, L. Schweigerer, Cancer Res. 60 (2000) 4596^4601.
[91] S.O. Freytag, Mol. Cell. Biol. 8 (1988) 1614^1624.
BBACAN 87512 11-4-02
W. Lutz et al. / Biochimica et Biophysica Acta 1602 (2002) 61^71 71
Related Documents