EVOLUTION & DEVELOPMENT 3:4, 241–250 (2001) © BLACKWELL SCIENCE, INC. 241 Conservation of a DPP/BMP signaling pathway in the nonbilateral cnidarian Acropora millepora Gabrielle Samuel, a,b David Miller, b and Robert Saint a, * a Centre for the Molecular Genetics of Development and Dept. of Molecular Biosciences, Adelaide University, Adelaide SA 5005, Australia; and b Biochemistry and Molecular Biology, James Cook University, Townsville, QLD 4811, Australia *Author for correspondence (email: [email protected]) SUMMARY Members of the TGF- superfamily of signaling molecules are widespread in metazoans, but the evolutionary origin of particular subclasses of signaling mechanisms is poorly defined. The DPP/BMP class, for example, is implicated in dor- sal-ventral patterning, neural patterning, and limb development. Here we report the presence of several components of a DPP/ BMP-specific signal transduction cascade in a nonbilateral animal, the coral Acropora millepora . The discovery of these components, a putative type I receptor and two putative re- ceptor-activated Smads, suggests that DPP/BMP signaling predates both dorsal-ventral pattern formation and limb devel- opment. We postulate that an ancestral role in neuroepithelial patterning may account for the high level of conservation be- tween DPP/BMP signaling components found in this nonbi- lateral animal and the more complex triploblastic organisms of the arthropod and chordate phyla. INTRODUCTION One of the key factors in both the rapid diversification and the increase in complexity of the Metazoa is likely to have been a corresponding increase in the diversity of intercellular signal- ing systems. This implied link is illustrated in vertebrates, which use many more specific signaling molecules than do in- vertebrates. However, most or all of the broad classes of sig- naling pathways are also present in Drosophila melanogaster or Caenorhabditis elegans, so the complexity found in verte- brates appears to have arisen from genome-wide duplications that are likely to have occurred in the chordate lineage (Hol- land 1998). While these observations indicate an ancient or- igin for these metazoan signaling systems, the evolutionary origins of the broad classes of signaling systems are un- known. To understand the origins of animal body plans and devel- opmental mechanisms, it is important to establish in what type of organism conserved functions requiring specific cellular signal- ing systems first arose (Holland 1999). A few signaling systems appear to be present in the simplest metazoans, the sponges (re- viewed by Muller 1997; Suga et al. 1999) and hence predate tissue-level organization. The Cnidaria can be regarded as the nearest-neighbor phylum of the triploblastic Metazoa and therefore are likely to be highly informative with respect to the evolution of signaling systems and developmental mechanisms. As a representative cnidarian, the coral Acropora mille- pora has many advantages over its more studied relatives (reviewed in Miller and Ball 2000), notably that it belongs to the basal class (the Anthozoa) and therefore may be expected to better reflect ancestral character states than members of the other classes. With only two cell layers, endoderm (or gas- troderm) and ectoderm, and an apparently small number of morphologically distinguishable cell types, cnidarians might be expected to have only limited intercellular signaling re- quirements, perhaps a few more than sponges. Of the signal- ing classes described by Gerhart and Kirschner (1997) as be- ing important to multicellularity, few have been shown to be possessed by cnidarians. G-protein coupled receptors have been reported (Vibede et al. 1998), as have integrins (Brower et al. 1997), while the presence of a probable FGF receptor (Genbank #AF070966) implies that cnidarians are likely to also make use of transmembrane tyrosine kinase signaling pathways. In addition to these classes, which have also been identified in sponges, the Cnidaria have various types of sep- tate (gap) junctions (e.g., Green and Flower 1980) and key components of nuclear hormone receptor-mediated (Kos- trouch et al. 1998) and Wnt (Hobmayer et al. 2000) signaling pathways have also been cloned. To date little is known about transmembrane serine/thre- onine signaling in the Cnidaria. The ligands for these recep- tors are secreted proteins of the transforming growth factor (TGF- ) family. The receptor complex for these ligands consists of type I and type II receptor proteins. Binding of the ligand to this receptor complex results in phosphorylation of receptor-mediated (R-)Smads by the intracellular serine/thre- onine kinase domain of the type I receptor molecule. When phosphorylated, these R-Smad molecules form hetero-oligo- mers with “common” (or co-) Smads, enter the nucleus, and

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
EVOLUTION & DEVELOPMENT
3:4, 241–250 (2001)
©
BLACKWELL SCIENCE, INC.
241
Conservation of a DPP/BMP signaling pathway in the nonbilateral
cnidarian
Acropora millepora
Gabrielle Samuel,
a,b
David Miller,
b
and Robert Saint
a,
*
a
Centre for the Molecular Genetics of Development and Dept. of Molecular Biosciences, Adelaide University, Adelaide SA 5005, Australia; and
b
Biochemistry and Molecular Biology, James Cook University, Townsville, QLD 4811, Australia
*Author for correspondence (email: [email protected])
SUMMARY
Members of the TGF-
�
superfamily of signalingmolecules are widespread in metazoans, but the evolutionaryorigin of particular subclasses of signaling mechanisms is poorlydefined. The DPP/BMP class, for example, is implicated in dor-
sal-ventral patterning, neural patterning, and limb development.Here we report the presence of several components of a DPP/BMP-specific signal transduction cascade in a nonbilateral
animal, the coral
Acropora millepora
. The discovery of these
components, a putative type I receptor and two putative re-ceptor-activated Smads, suggests that DPP/BMP signalingpredates both dorsal-ventral pattern formation and limb devel-opment. We postulate that an ancestral role in neuroepithelialpatterning may account for the high level of conservation be-tween DPP/BMP signaling components found in this nonbi-lateral animal and the more complex triploblastic organismsof the arthropod and chordate phyla.
INTRODUCTION
One of the key factors in both the rapid diversification and theincrease in complexity of the Metazoa is likely to have been acorresponding increase in the diversity of intercellular signal-ing systems. This implied link is illustrated in vertebrates,which use many more specific signaling molecules than do in-vertebrates. However, most or all of the broad classes of sig-naling pathways are also present in
Drosophila melanogaster
or
Caenorhabditis elegans
, so the complexity found in verte-brates appears to have arisen from genome-wide duplicationsthat are likely to have occurred in the chordate lineage (Hol-land 1998). While these observations indicate an ancient or-igin for these metazoan signaling systems, the evolutionaryorigins of the broad classes of signaling systems are un-known.
To understand the origins of animal body plans and devel-opmental mechanisms, it is important to establish in what type oforganism conserved functions requiring specific cellular signal-ing systems first arose (Holland 1999). A few signaling systemsappear to be present in the simplest metazoans, the sponges (re-viewed by Muller 1997; Suga et al. 1999) and hence predatetissue-level organization. The Cnidaria can be regarded asthe nearest-neighbor phylum of the triploblastic Metazoaand therefore are likely to be highly informative with respectto the evolution of signaling systems and developmentalmechanisms.
As a representative cnidarian, the coral
Acropora mille-pora
has many advantages over its more studied relatives(reviewed in Miller and Ball 2000), notably that it belongs to
the basal class (the Anthozoa) and therefore may be expectedto better reflect ancestral character states than members ofthe other classes. With only two cell layers, endoderm (or gas-troderm) and ectoderm, and an apparently small number ofmorphologically distinguishable cell types, cnidarians mightbe expected to have only limited intercellular signaling re-quirements, perhaps a few more than sponges. Of the signal-ing classes described by Gerhart and Kirschner (1997) as be-ing important to multicellularity, few have been shown to bepossessed by cnidarians. G-protein coupled receptors havebeen reported (Vibede et al. 1998), as have integrins (Broweret al. 1997), while the presence of a probable FGF receptor(Genbank #AF070966) implies that cnidarians are likely toalso make use of transmembrane tyrosine kinase signalingpathways. In addition to these classes, which have also beenidentified in sponges, the Cnidaria have various types of sep-tate (gap) junctions (e.g., Green and Flower 1980) and keycomponents of nuclear hormone receptor-mediated (Kos-trouch et al. 1998) and Wnt (Hobmayer et al. 2000) signalingpathways have also been cloned.
To date little is known about transmembrane serine/thre-onine signaling in the Cnidaria. The ligands for these recep-tors are secreted proteins of the transforming growth factor
�
(TGF-
�
) family. The receptor complex for these ligandsconsists of type I and type II receptor proteins. Binding of theligand to this receptor complex results in phosphorylation ofreceptor-mediated (R-)Smads by the intracellular serine/thre-onine kinase domain of the type I receptor molecule. Whenphosphorylated, these R-Smad molecules form hetero-oligo-mers with “common” (or co-) Smads, enter the nucleus, and
242 EVOLUTION & DEVELOPMENT
Vol. 3, No. 4, July–August 2001
regulate specific target genes (reviewed in Hogan 1996). In-hibitory (anti-) Smads are alternative dimerization partnersof the R-Smads.
Several classes of transmembrane serine/threonine kinasesignaling systems are recognized on the basis of the ligandsubclass (reviewed in Newfeld et al. 1999; Raftery and Suth-erland 1999). The system responsive to Decapentaplegic (DPP)in
Drosophila
is well understood at the molecular level and de-fines a specific class of TGF-
�
signaling system that includesthe vertebrate bone morphogenetic proteins (BMPs). The DPP/BMP system has a wide variety of roles, but is central to dors-oventral (D/V) axis specification in both
Drosophila
and verte-brates (Irish and Gelbart 1987; Dale et al. 1992). Elegant cross-phylum rescue experiments using DPP/BMP4 (Padgett et al.1993; Sampath et al. 1993) led to the assumption that despitean axis inversion during evolution, roles of these molecules inD/V axis specification reflect conservation of an ancestralfunction. One hypothesis that arises from these observations isthat DPP/BMP4 signaling may have evolved in the context ofspecifying the D/V axis. As
Acropora
has only a single bodyaxis, this hypothesis can be tested by determining whether theDPP/BMP4 signaling pathway predated bilaterality, indicat-ing that roles in D/V patterning were secondarily acquired.
Here we show that key components of the DPP/BMP4 sig-naling pathway, a TGF-
�
-superfamily receptor and two Smads,are present in the nonbilateral animal
Acropora
millepora
. Onthe basis of sequence comparisons, these correspond to a type IDPP receptor and receptor-mediated Smads. Phylogenetic anal-yses indicate that the two
Acropora
Smads are likely to haveresulted from an independent duplication event, and do notcorrespond to the distinct Smad subtypes known in other or-ganisms. We conclude that the DPP/BMP signaling pathwaywas in place prior to the divergence of the Cnidaria from theline leading to the higher Metazoa, and that it has been sub-stantially conserved during evolution.
MATERIALS AND METHODS
Clone identification, isolation, and sequence analysis
To PCR amplify a section of a coral receptor type I gene, the DNAof an early
A. millepora
�
ZAPII cDNA library (Brower et al. 1997)was used as a template for PCR amplification, employing degener-ate antisense and sense primers that were designed from conservedresidues within kinase domains of different type I receptors (sensestrand, REC1 5
�
-CAYGAAYATHBTNGGNTTYAT-3
�
correspond-ing to residues 5
�
H (E/D)NI(L/A)GFI 3
�
; antisense strand, REC25
�
-CNATRTACSGNGGNCTYCA-3
�
corresponding to residues 5
�
RYM(A/P)PEV 3
�
). The PCR reaction was conducted using Taqpolymerase (Perkin Elmer), a final primer concentration of 1
�
m
eachand the following conditions: {94
�
C for 30 sec; 37
�
C for 30 sec; Rampfor 2 min to 72
�
C; 72
�
C for 1 min}
�
3;{94
�
C for 30 sec; 45
�
C for 30sec; 72
�
C for 1 min}
�
35; 72
�
C for 5 min.PCR products were separated in a 2% agarose gel. DNA frag-
ments of the expected size were isolated from gel slices and used asa template in an additional PCR reaction, which was performed un-der identical conditions to those described above, and the productsseparated in a 2% agarose gel. The DNA fragments of interest wereisolated and gel-extracted using the Qiagen gel extraction kit. Thislinear DNA was subcloned into the pGEM-T easy vector (Promega)and transformed into the bacterial strain DH5
�
. Recombinant cloneswere detected by Pst1/Not1 digestion of Qiagen minipreps and se-quence determined using the bacterial promoter T7 and SP6 primersaccording to the manufacturer’s instructions (ABI PRISM BigDyeTerminator kit).
For the amplification of a smad gene the procedure was as statedabove, except that the primers were designed from conserved aminoacid residues within the MHI and MH2 domains of receptor-regu-lated Smad proteins (sense, SMA1 primer 5
�
-CCNCAYGTNATNTAYYT-3
�
corresponding to residues 5
�
PHVIYC 3
�
; antisense, SMA2primer 5
�
-CCCCANCCYAANACRAA-3
�
corresponding to residues5
�
FVKGWG 3
�
). The PCR conditions were as follows: 94
�
C for 2min; {94
�
C for 45 sec; 45
�
C for 45 sec; 72
�
C for 2 min}
�
29; 72
�
Cfor 10 min.
To obtain full-length transcripts of each of the genes isolated byPCR, the early embryonic
A. millepora
cDNA library (see above)was screened using filter lifts (Amersham Pharmacia: Hybond mem-brane protocol [Buckinghamshire, UK]). The DNA was immobi-lized via UV cross-linking. Each set of lifts was probed with PCR-generated fragments that had been labeled with
32
P using a randompriming kit (Amersham). Hybridization proceeded overnight at50
�
C in 2
�
SSPE:1% SDS:0.5% blotto. Following hybridization,filters were washed 2
�
20 min at 50
�
C in 2
�
SSPE:0.1% SDS.Positive clones were detected by autoradiography and used to pre-pare filters for a repeat screening. These 2
�
positive clones were iso-lated and excised into the pBluescript vector using the Stratageneprotocol for
�
ZAP DNA libraries. Recombinant clones were de-tected by EcoR1/ Xho1 digestion of purified DNA (Qiagen mini-prep [Chatsworth, CA, USA]) and the sequence determined as de-scribed above.
Phylogenetic analyses
Maximum likelihood analyses were conducted in MolPhy version2.3 (Adachi and Hasegawa 1996), using the relative substitution ma-trix of the Dayhoff model and the local rearrangement search mode.
RESULTS
Identification of a type I DPP/BMP receptor
Degenerate PCR primers were designed on the basis of align-ments of the conserved kinase domains of vertebrate andinvertebrate type I transmembrane serine/threonine kinases.When an early embryonic
A. millepora
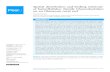
cDNA library was usedas template, these primers generated a 360-bp PCR productcontaining an open reading frame clearly corresponding to partof a type I receptor. This product was used as a probe to screen50,000 plaques of the cDNA library, resulting in the isola-tion of a single clone. Figure 1 shows the nucleotide and pre-dicted amino acid sequences of the clone.
Samuel et al.
Coral DPP/BMP pathway genes
243
The predicted polypeptide, referred to here as Bmpr1-Am, has the characteristic domain structure of a TGF-
�
typeI receptor (Fig. 1), including the characteristic pattern of cys-teine residues [CXCX
3–5
CX
4–25
CX
4–5
CX
13–16
GCX
7–19
CX
11–13
]in the region corresponding to the extracellular domain, anda GS box in the predicted intracellular domain (shown un-
derlined in Fig. 1). In addition there is no evidence for thepresence of an extended C-terminal tail of the type character-istic of type II receptors. The features present in Bmpr1-Amthat are common to both type I and II TGF-
�
receptors in-clude an N-terminal signal sequence, an extracellular cys-teine box [CCX
4
CN] (shown boxed in Fig. 1), a hydrophobic
Fig. 1. (A) Schematic do-main representation of theA. millepora Bmpr1-Am re-ceptor. The signal sequence,GS domain, transmembraneregion (TM), kinase domain,and cysteine box are indicat-ed by labeled boxes, and theextracellular cysteines arerepresented by vertical linesbelow a “c.” The diagram isnot drawn to scale. (B) Nu-cleotide sequence and de-duced amino acid sequenceof A. millepora bmpr1-AmcDNA. The putative hydro-phobic signal sequence andtransmembrane domain areindicated by a bold under-line; the cysteine box is in-dicated by a box, as are theadditional extracellular cys-teine residues. The bordersof the kinase domain are rep-resented by arrows. The GSbox in the predicted intracel-lular domain is indicated bythe nonbold underline.
244 EVOLUTION & DEVELOPMENT
Vol. 3, No. 4, July–August 2001
region representing the single-pass transmembrane domain,and a serine/threonine kinase domain (see Fig. 1).
Comparison of Bmpr1-Am with the databases revealed thatit is a DPP/BMP-specific receptor. Alignment of the polypep-tide with related type I receptors shows the expected low levelof identity in the extracellular ligand-binding domain (data notshown) and much higher conservation in the intracellular ki-nase domain. Within the kinase domain, Bmpr1-Am is mostsimilar to the
Xenopus
Bmpr 1a receptor (66.1% identity);identity with
Drosophila
Tkv and Sax is somewhat lower(60.2% and 57.4% identity, respectively). Figure 2 shows analignment of the kinase domains of a representative range oftype I receptors with the Bmpr1-Am polypeptide, and Fig. 3summarizes phylogenetic analyses based on a more extensiverange of related molecules, including type II receptors. Notethat only the kinase domain was used in phylogenetic analy-ses, as unambiguous alignment is only possible for this partof the protein.
As in published phylogenetic analyses (see, for example,Newfeld et al. 1999), when type II receptors were used to de-fine the out-group, our analyses resolved type I TGF-
�
recep-tors into three clades. The Bmpr1-Am sequence falls basal tothe clade constituting the DPP/BMP subclass of type I TGF-
�
receptor sequences (Fig. 3). The position of the Bmpr1-Am se-quence in the analyses highlights the similarity between thecoral protein and the
Drosophila
DPP receptor Thickveins(Tkv) and the vertebrate type 1a and 1b proteins (the BMP2/4receptors).
Interestingly, the Bmpr1-Am polypeptide has a higherlevel of identity with these type I receptors than does any re-lated molecule from
C. elegans
(including the putative DPPreceptor, SMA-6) (Krishna et al. 1999). The evolutionary re-lationship of the
C. elegans
receptors is reflected in the rela-tionship of their ligands; both the SMA-6 ligand DBL-1 andthe DAF-1 ligand DAF-7 show relatively low levels of iden-tity with the DPP/BMP class. These observations are consis-
Fig. 1. Continued.
Samuel et al.
Coral DPP/BMP pathway genes
245
tent with the DPP pathway having undergone a high degreeof secondary modification in
C. elegans
. The analyses alsohighlight the similarity between the
D. melanogaster
Saxo-phone and sponge sALK3 sequences, which extends to theL45 loop (see discussion).
Acropora millepora
has two distinct smad1/5 genes
As
A. millepora
possesses a gene encoding a protein with ahigh level of similarity to a type I TGF-
�
receptor, we rea-soned that it would also possess genes encoding componentsof the signal transduction pathway for this receptor. Applica-tion of a similar strategy to that described above allowed theamplification of an approximately 1-kb PCR product corre-sponding to
A. millepora
R-Smad cDNAs. After cloning andsequence analysis, it became clear that this product was het-erogeneous and represented two distinct (but related) Smad
cDNAs, designated A and B. These were used (independently)to screen the
A. millepora
cDNA library (50,000 plaques).Screening yielded eight cDNA clones corresponding to probeA (inserts of three distinct sizes, 1635, 1904, and 2114 bp) andnine corresponding to probe B (inserts of 2038 and 2457 bp).For both genes, the different size classes of cDNA differedonly in the length of the putative 3
�
-UTRs.Three distinct subfamilies of Smads are recognized: the
Mad family (receptor-mediated Smads, generally viewed asdedicated to one ligand), the Med family (common, or co-Smads, involved in multiple ligand signaling), and the Dadfamily (inhibitory Smads, antagonists involved in multipleligand signaling). Comparison of the
A. millepora
sequenceswith the databases indicated that both genes encoded pro-teins that are most closely related to the Smad1/5 group (i.e.,R-Smads specific to the DPP/BMP signaling pathway), andhence they were designated
smad1/5a-AM
and
smad1/5b-AM
.
Fig. 2. Sequence alignmentof the predicted A. milleporaBmpr1-Am kinase domainwith DPP/BMP type I kinasedomains from other organ-isms. Sequences aligned usingCLUSTALW. Gaps were in-troduced to maximize align-ment and are shown by dashes.Asterisks (*) show identicalresidues; dots (.) show con-served residues; Dm D. mel-anogaster; Ce C. elegans.
246 EVOLUTION & DEVELOPMENT
Vol. 3, No. 4, July–August 2001
The deduced polypeptide sizes of the Smad1/5 a and b pro-teins are 444 and 440 amino acids, respectively (Fig. 4).
Receptors and co-Smads possess an N-terminal (MH1) do-main required for DNA binding, a C-terminal (MH2) domaininvolved in oligomerization and transcription activation, and aproline- and serine-rich linker region (Massague 1998). Ingeneral, the linker region is sufficiently variable to prevent
meaningful alignments between species; the MH1 and MH2domains are conserved, the latter more so than the former.
The coral Smad sequences have a high level of identity(approximately 78% identity overall; 89% and 87% in theMH1 and MH2 domains, respectively), and both are mostclosely related to vertebrate Smad1/5 proteins (Fig. 4). In ad-dition, indicative of receptor-mediated Smads, they contain
Fig. 3. Phylogenetic relationships of the type I TGF-� receptors. The maximum likelihood analysis was conducted on the alignment ofthe kinase domains only. Numbers in parentheses are GenPep identification numbers. The type II receptors D. melanogaster Punt (Dm-Punt, 784876), C. elegans DAF-4 (CeDAF-4, 542467), and human TGF-� type II receptor (Human T�RII, 4507469) were used to rootthe tree. The A. millepora Bmpr1 (Bmpr1-Am) protein sequence was compared with: BMP type 1b receptors from quail (6164918), hu-man (4502431), and zebrafish (4586516); the type 1a receptors from quail (6164916), human (4757854), and mouse (547779); the Xenopuslaevis BMP receptor (Xenopus1, 2446990); D. melanogaster Thickveins (DmTkv, 2133655), Saxophone (DmSax, 2133654), and ATR1(DmATR1, 436960, product of the baboon gene); the human TGF-� and activin receptors ALK1 (3915750), ALK2 (462447), T�R-1(547777), and AtR-1B (547775); C. elegans DAF-1 (CeDAF-1, 118230) and SMA-6 (CeSMA-6, 4219016); and the sponge receptorssALK1 (5596340), sALK2 (5596342), sALK3 (5596344), and sALK4 (5596346). The three brackets on the right-hand side of the figureindicate the three clades of receptors described by Newfeld et al. (1999). Numbers above branches indicate the percentage of 2000 boot-strap replicates supporting the topology shown.
Samuel et al. Coral DPP/BMP pathway genes 247
the characteristic S/TSXS sequence at the C-terminus of theMH2 domain (Fig. 4). This is the site of phosphorylation bythe type I receptor during ligand-induced activation (Macias-Silva et al. 1996). By contrast, the co- and inhibitory Smadshave a maximum of one serine residue in this region (New-feld et al. 1999). Interestingly, in the case of Smad1/5a-AM,
this motif is followed by an alanine residue. There are fewprecedents for this, and the functional significance of thisresidue C-terminal to the phosphorylation site is unknown.
To better understand the relationship between the A.millepora Smads and their counterparts in bilateral animals,phylogenetic analyses were conducted using only the MH1
Fig. 4. Alignment of the pre-dicted complete amino acidsequences of A. milleporaSmad1/5a and b (Smad1/5a-Am and Smad1/5b-Am) alongwith their orthologs fromother organisms. Sequencesaligned using CLUSTALW.Gaps were introduced tomaximize alignment and areshown by dashes. Asterisks(*) show identical residues,and dots (.) show conservedresidues. The MH1 domainis overlined and the MH2domain is doubly overlined.Dm D. melanogaster; Ce C. elegans.
248 EVOLUTION & DEVELOPMENT Vol. 3, No. 4, July–August 2001
and MH2 domain data (Fig. 5). In these analyses, the A. mille-pora Smads were basal to the clade consisting of DPP/BMP-responsive Smads and are well resolved from the TGF-�-responsive Smads (Hsmad2 and Hsmad3). The analyses alsoindicate that Smad1/5a-AM and Smad1/5b-AM are likely toreflect a cnidarian-specific duplication event (i.e., both arederived from an ancestral cnidarian Smad1/5 gene) and donot correspond to different subtypes from higher animals.Smad1/5b-AM has higher levels of identity with Smads inhigher animals than does Smad1/5a-AM, suggesting that theformer may more closely reflect the ancestral state. (For ex-ample, in the MH2 domain, Smad1/5b has 86.1% identitywith human Smad1; the corresponding figure for Smad1/5ais 81.2%.)
DISCUSSION
The data presented here indicate the presence of a receptorserine/threonine kinase-mediated signaling pathway of theDPP/BMP type in Acropora millepora, a representative non-
bilateral animal, as evidenced by the presence of the speci-ficity-conferring components of the DPP/BMP signaling sys-tem. Members of the TGF-� receptor family of proteins haverecently been identified in the sponge Ephydatia fluviatilis(Suga et al. 1999), so the presence of related signal transduc-tion pathway components in a cnidarian is perhaps not sur-prising. However, the sponge proteins are divergent mem-bers of receptor types responsive to other classes of ligands(see below), while the level of identity between each of theA. millepora proteins (a DPP/BMP receptor and two recep-tor-mediated Smads) and the corresponding components of theDPP/BMP pathway in Drosophila melanogaster and chordatesis unexpected and striking. While the sponge receptors appearto predate the origins of the DPP/BMP signaling system, the A.millepora proteins are clearly of the DPP/BMP type.
Although functional analyses in Acropora have not beenundertaken, Bmpr1-Am and the Acropora Smads identifiedhere are likely to mediate signaling by a molecule closely re-lated to DPP. Phylogenetic analyses resolve type I receptorsinto three distinct classes (Newfeld et al. 1999). These threereceptor classes have distinct Smad specificities, determinedby the interaction of a small region of the L45 loop of the re-ceptor kinase domain (Chen et al. 1998; Chen and Massague1999) with two residues in the L3 loop of the R-Smad MH2domain (Lo et al. 1998). In the case of Bmpr1-Am, two of thethree specificity-conferring residues are identical to the DPP/BMP subtype, and the third is a conservative substitution (Serfor Thr). Both Smad1/5a-AM and Smad1/5b-AM have thetwo residues that define R-Smads responsive to DPP/BMP-mediated signaling (His403, Asp406 in Smad1/5a-AM, andHis400 and Asp403 in Smad1/5b-AM); indeed, the L3 loopsequences in the two coral proteins are identical with thoseof Drosophila Mad and many of the vertebrate Smad1/5types.
The sponge type I receptors sALK-3 and sALK-4 have L45sequences that are intermediate between consensus sequencesof TGF-� and activin-responsive receptors (Chen and Mas-sague 1999), while the receptors sALK-1 and sALK-2 havehighly divergent sequences unrelated to all other type I re-ceptors (presumably reflecting sponge-specific derivation).This is consistent with the ancestral type I receptor being ofthe activin/TGF-�-type and the DPP/BMP type arising afterthe Porifera/(Cnidaria bilateral Metazoa) split.
Analysis of the A. millepora Smads also places them withinthe DPP/BMP signaling pathway group. Comparisons betweenMH1 and MH2 domain sequences identifies the A. milleporaSmads as belonging to the Smad1/5 subfamily, a family of DPP/BMP-responsive Smads. Curiously, the C. elegans DPP/BMPsignaling components show surprising divergence. Althoughmaximum likelihood analyses (Fig. 5) are consistent withC. elegans SMA-2 being orthologous with D. melanogasterMAD, the branch length indicates that the SMA-2 sequenceis highly diverged. Sequence identities are higher between A.
Fig. 5. Phylogenetic analysis of Smad sequences. The maximumlikelihood analysis was conducted on the alignment of the con-served MH1 and MH2 domains only. Numbers in parentheses areGenPep identification numbers. In these analyses, the inhibitorySmads Drosophila Dad (2541864) and human Smad6 (6502523)were used to root the tree. Other sequences compared with the A.millepora sequences (Smad1/5a-Am and Smad1/5b-Am) werethe co-Smads D. melanogaster Medea (3004861), C. elegans DAF-3(2226360), and human DPC4 (4885457), as well as the following: Hu-man Smad1 (5174509), Smad2 (5174511), Smad3 (2351035), andSmad5 (5174515); Rat (Rattus rattus) Smad1 (6981172); XenopusSmad1 (1381671) and Smad1.1 (1763545); Halocynthia roretzi Smad1/5 (4519908); D. melanogaster MAD (1170853); and C. elegans Dwar-fin SMA-2 (1173452). Numbers above branches indicate the percent-age of 2000 bootstrap replicates supporting the topology shown.
Samuel et al. Coral DPP/BMP pathway genes 249
millepora and D. melanogaster/vertebrate Smads than be-tween SMA-2 and the latter. The situation in C. elegans islikely to reflect secondary modification of the pathway.
Rather than being orthologs of distinct vertebrate R-Smadsubclasses, Smad1/5a-AM and Smad1/5b-AM reflect a du-plication event that postdated the divergence of cnidariansand higher metazoans. Since this split occurred at least 540Mya (Grotzinger et al. 1995), it is perhaps not surprising thatseveral gene classes have undergone duplication events inthe Cnidaria since that time. There are precedents for thepresence of duplicated R-Smads in Acropora. In Hydra, thepaired-like genes, prdla and prdlb, are both likely to havearisen from the precursor of Drosophila aristaless (Gauchatet al. 1998), and Cnnos1 and Cnnos2 are both related to Droso-phila nanos (Mochizuki et al. 2000).
Even so, the significance of the presence of two R-Smadsin Acropora is unclear. As they have identical L3 sequences,both are likely to transmit signals originating from BMP (ratherthan activin or TGF-�) molecules. The Acropora R-Smads arealso unlikely to have different intrinsic DNA-binding proper-ties, as there are only minor differences in the DNA-bindingregion (see Fig. 4) and the residues likely to make contacts(R75, Q77 and K82) (Shi et al. 1998) are identical in the twoproteins. Other possible explanations for the presence of twoR-Smads include differing protein/protein interaction speci-ficities. The differences in the C-terminal region of the MH2domain, which is involved in oligomerization (Shi et al.1997), suggest that the two R-Smads may interact differentlywith co-Smads.
Another difference between the two A. millepora R-Smadsis evident at the C-terminus of these proteins. The S/TSXSphosphorylation site is at the C-terminus of almost all knownR-Smads. Smad1/5a-AM has a C-terminal extension of a sin-gle alanine residue. The effect of this residue on phosphoryla-tion of the S/TSXS motif, and consequently on the interac-tion with the co-Smad and nuclear localization, is unknown.A precedent for an extension is provided by Xenopus Smad1.1,which carries a three-residue (LMD) C-terminal extension(GenBank accession #U77639) (Meersseman et al. 1997). Thisprotein was shown to be localized in the nucleus after overex-pression in COS cells (Meersseman et al. 1997), although thisdoes not preclude the possibility that the single amino acidextension has some effect on phosphorylation of the C-terminal region and nuclear localization.
Transmembrane serine/threonine kinase signaling sys-tems in animals presumably have their origins as mecha-nisms by which cells could regulate division and differentia-tion of other cells, so it is not surprising to find receptors ofthis type even in as simple a metazoan as a sponge. However,within the TGF-� family, the DPP/BMP ligands are a well-defined class, with well-established roles in axis specifica-tion and limb development in both Drosophila and verte-brates. What are the likely roles of the DPP/BMP signaling
system in Acropora, an animal that has only a single axis, theoral-aboral axis? One possibility is that the oral-aboral axiscorresponds to the dorsal-ventral axis of triploblastic meta-zoans, although no evidence supports this proposal. A sec-ond possibility is that there is a second axis at right angles tothe oral-aboral axis that corresponds to the dorsal-ventralaxis of triploblastic metazoans. If a second axis does exist, itwould have to form transiently during development and pro-vide few morphological clues to its existence.
In addition to its roles in axis specification and limb de-velopment, this signaling pathway performs a wide range ofother functions in both chordates and Drosophila (for exam-ple, see Spencer et al. 1982 and Immergluck et al. 1990). Onesuch role that may be ancestral is the antineurogenic functionof DPP. In Drosophila and chordates, the entire ectodermhas neurogenic potential; the dorsal or ventral position of thenerve chord is determined by the balance between the an-tineurogenic activity of DPP and the inhibitory effect of SOG/Chordin on DPP activity. In contrast with bilateral animals,cnidarians do not have a single dorsal or ventral nerve chord.Rather, the basic organization of the nervous system is one ormore interacting nets, with linear or circular condensationsinto multiple nerve tracts. Gerhart (2000) has suggested thatthe spacing of these nerve tracts may be generated by the cni-darian equivalent of the DPP/SOG system. Unfortunately, asanthozoans have the least condensed type of cnidarian ner-vous systems, Acropora is not an ideal system in which to in-vestigate this possibility. Nonetheless, a conserved role forthe DPP/BMP pathway in patterning neural epithelia remainsa likely explanation for the remarkable conservation of thispathway.
AcknowledgmentsThis work was supported by the Australian Research Council andan Overseas Postgraduate Research Award to G.S. The authors thankDr. M. J. H. van Oppen for assistance with the MolPhy software.
REFERENCES
Adachi, J., and Hasegawa, M. 1996. MOLPHY version 2.3 programs formolecular phylogenetics based on maximum likelihood. Institute of Sta-tistical Mathematics, Tokyo.
Brower, D. L., Brower, S. M., Hayward, D. C., and Ball, E. E. 1997. Molec-ular evolution of integrins: genes encoding integrin beta subunits froma coral and a sponge. Proc. Natl. Acad. Sci. 94: 9182–9187.
Chen, Y. G., Hata, A., Lo, R. S., Wotton, D., Shi, Y., Pavletich, N., et al.1998. Determinants of specificity in TGF-beta signal transduction.Genes Dev. 12: 2144–2152.
Chen, Y. G., and Massague, J. 1999. Smad1 recognition and activation bythe ALK1 group of transforming growth factor-beta family receptors.J. Biol. Chem. 274: 3672–3677.
Dale, L., Howes, G., Price, B. M. J., and Smith, J. C. 1992. Bone morpho-genetic protein 4: a ventralizing factor in early Xenopus development.Development 115: 573–585.
Gauchat, D., Kreger, S., Holstein, T., and Galliot, B. 1998. prdl-a, a genemarker for hydra apical differentiation related to triploblastic paired-like head-specific genes. Development 125: 1637–1645.
250 EVOLUTION & DEVELOPMENT Vol. 3, No. 4, July–August 2001
Gerhart, J. 2000. Inversion of the chordate body axis: are there alterna-tives? Proc. Natl. Acad. Sci. 97: 4445–4448.
Gerhart, J., and Kirschner, M. W. 1997. Cells, Embryos, and Evolution: To-ward a Cellular and Developmental Understanding of Phenotypic Vari-ation and Evolutionary Adaptability. Blackwell Science Inc., Malden.
Green, C. R., and Flower, N. E. 1980. Two new septate junctions in thephylum Coelenterata. J. Cell Sci. 42: 43–59.
Grotzinger, J. P., Bowring, S. A., Saylor, B. Z., and Kaufman, A. J. 1995.Biostratigraphic and geochronologic constraints on early animal evolu-tion. Science 270: 598–604.
Hobmayer, B., Rentzsch, F., Kuhn, K., Happel, C. M., von Laue, C. C.,Snyder, P., et al. 2000. WNT signalling molecules act in axis formationin the diploblastic metazoan Hydra. Nature 407: 186–189.
Hogan, B. L. M. 1996. Bone morphogenetic proteins in development. Curr.Opin. Genet. Dev. 6: 432–438.
Holland, P. W. H. 1998. Major transitions in animal evolution: A develop-mental genetic perspective. Amer. Zool. 38: 829–842.
Holland, P. W. H. 1999. The future of evolutionary developmental biology.Nature 402 (Supp): C41–C44.
Immergluck, K., Lawrence, P. A., and Bienz, M. 1990. Induction across germlayers in Drosophila mediated by a genetic cascade. Cell 62: 261–268.
Irish, V., and Gelbart, W. M. 1987. The dpp gene is required for dorsal-ven-tral patterning of the Drosophila embryo. Genes Dev. 1: 868–879.
Kostrouch, Z., Kostrouchova, M., Love, W., Jannini, E., Piatigorsky, J.,and Rall, J. E. 1998. Retinoic acid X receptor in the diploblast, Tripeda-lia cystophora. Proc. Natl. Acad. Sci. 95: 13442–13447.
Krishna, S., Maduzia, L. L., and Padgett, R. W. 1999. Specificity of TGFbe-ta signaling is conferred by distinct type I receptors and their associatedSMAD proteins in Caenorhabditis elegans. Development 126: 251–260.
Lo, R. S., Chen, Y.-G., Shi, Y., Pavletich, N. P., and Massague, J. 1998. TheL3 loop: a structural motif determining specific interactions betweenSMAD proteins and TGF-receptors. EMBO J. 17: 996–1005
Macias-Silva, M., Abdollah, S., Hoodless, P. A., Pirone, R., Attisano, L.,and Wrana, J. L. 1996. MADR2 is a substrate of the TGFbeta receptorand its phosphorylation is required for nuclear accumulation and signal-ing. Cell 87: 1215–1224.
Massague, J. 1998. TGF-beta signal transduction. Ann. Rev. Biochem. 67:753–791.
Meersseman, G., Verschueren, K., Nelles, L., Blumenstock, C., Kraft, H.,Wuytens, G., et al. 1997. The C-terminal domain of Mad-like signal
transducers is sufficient for biological activity in the Xenopus embryoand transcriptional activation. Mech. Dev. 61: 127–140.
Miller, D. J., and Ball, E. E. 2000. The coral Acropora: what it can contrib-ute to our knowledge of metazoan evolution and the evolution of devel-opmental processes. BioEssays 22: 291–296.
Mochizuki, K., Sano, H., Kobayashi, S., Nishimiya-Fujisawa, C., andFujisawa, T. 2000. Expression and evolutionary conservation of nanos-related genes in Hydra. Dev. Genes & Evol. 210: 591–602.
Muller, W. E. G. 1997. Origin of metazoan adhesion molecules and adhe-sion receptors as deduced from cDNA analyses in the marine spongeGeodia cydonium: a review. Cell and Tissue Research 289: 383–395.
Newfeld, S. J., Wisotzkey, R. G., and Kumar, S. 1999. Molecular evolutionof a developmental pathway: phylogenetic analyses of transforminggrowth factor-beta family ligands, receptors and Smad signal transduc-ers. Genetics 152: 783–795.
Padgett, R. W., Wozney, J. M., and Gelbart, W. M. 1993. Human BMP se-quences can confer normal dorsal-ventral patterning in the Drosophilaembryo. Proc. Natl. Acad. Sci. 90: 2905–2909.
Raftery, L. A., and Sutherland, D. J. 1999. TGF-beta family signal transductionin Drosophila development: from Mad to Smads. Dev. Biol. 210: 251–268.
Sampath, T. K., Rashka, K. E., Doctor, J. S., Tucker, R. F., and Hoffmann,F. M. 1993. Drosophila transforming growth factor beta superfamilyproteins induce endochondral bone formation in mammals. Proc. Natl.Acad. Sci. 90: 6004–6008.
Shi, Y., Hata, A., Lo, R. S., Massague, J., and Pavletich, N. P. 1997. A struc-tural basis for mutational inactivation of the tumour suppressor Smad4.Nature 388: 87–93.
Shi, Y., Wang, Y. F., Jayaraman, L., Yang, H., Massague, J., and Pavletich,N. P. 1998. Crystal structure of a Smad MH1 domain bound to DNA: in-sights on DNA binding in TGF-signaling. Cell 94: 585–594.
Spencer, F. A., Hoffmann, F. M., and Gelbart, W. M. 1982. Decapentaple-gic: a gene complex affecting morphogenesis in Drosophila melano-gaster. Cell 28: 451–461.
Suga, H., Ono, K., and Miyata, T. 1999. Multiple TGF-beta receptor relat-ed genes in sponge and ancient gene duplications before the parazoan-eumetazoan split. FEBS Lett. 453: 346–350.
Vibede, N., Hauser, F., Williamson, M., and Grimmelikhuijzen, C. J. 1998.Genomic organization of a receptor from sea anemones, structurallyand evolutionarily related to glycoprotein hormone receptors frommammals. Biochem. Biophys. Res. Commun. 252: 497–501.
Related Documents