CHAPTER 1 PROJECT CONCEPTION – LITERATURE SURVEY 1.0 Introduction As an introduction, a few concepts and definitions concerning the project, “Refined Glycerine from RBD Palm Oil” will bw given in this part. Before the plant is designed, some literature study on the feasibility of this project has been performed. The feasibility study will be covered in this chapter too. 1.1 Process Background

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

CHAPTER 1
PROJECT CONCEPTION – LITERATURE SURVEY
1.0 Introduction
As an introduction, a few concepts and definitions concerning the project,
“Refined Glycerine from RBD Palm Oil” will bw given in this part. Before the plant
is designed, some literature study on the feasibility of this project has been
performed. The feasibility study will be covered in this chapter too.
1.1 Process Background
1.1.1 Introduction to Glycerine
Glycerine, Glycerin or Glycerol is a 1,2,3-propanetriol, a trihydric alcohol.
The term “glycerol” applies only to the pure chemical compound 1,2,3-propanetriol.
“Glycerine” or “glycerin”, is a purified commercial product whose principal
component is glycerol and normally containing about 95% or more glycerol.
Several grades of glycerine are available commercially. They differ somewhat n

Chapter I– Project Conception - Literature Survey I- 2
their glycerol content and in other characteristics such as colour, odour and trace
impurities.
The name comes from the Greek word glykys meaning sweet. Glycerol was
first discovered in 1779 by Scheele, who had heated a mixture of litharge and olive
oil and then extracted it with water. Upon evaporating the water, he obtained a
viscous sweet tasting solution later identified by Chevreul, Plouze, Berthelot and
others as a concentrated solution of trihyric alcohol, glycerol.
H H H CH2-OH
| | | |
H---C---C---C---H or CH-OH
| | | |
OH OH OH CH2-OH
Glycerol occurs in combined form in all animal and vegetable fats and oils.
It is rarely found in the free state in these fats but is usually present as a triglyceride
combined with such fatty acids as stearic, oleic, palmitic and lauric and these are
generally mixtures or combinations of glycerides of several fatty acids. Such oils as
coconut, palm kernel, cottonseed, soybean and olive yield larger amounts of
glycerine than do such animal fats as tallow and lard. Beginning in the 1980s, two
important sources of raw materials for the glycerine production come from the palm
oil industry. The fruit from oil palm produce two distinctive oils, the palm oil which
is mainly a C16 and C18 oil, and the palm kernel oil, which is a C12 and C14 oil. These
two oils have overtaken tallow and coconut oil as the raw materials for
oleochemicals at this present moment and with their continued dominance in
production, will continue to be the primary feed stocks.
Traditionally, glycerol has been recovered as a by-product from animal or
vegetable oils that have been saponified in the process of manufacturing soaps.
More recently it has been commercially produced by chemical synthesis from
propylene and from sugar. Until 1949 all glycerine was obtained from glycerides in
fats and oils from two sources:
(1) From soap manufacture
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 3
Fat is boiled with caustic soda slution and salt. The fatty acid
constituents of the fat combine with the caustic soda to form soap,
which is obtained as an upper layer. The lower aqueos layer, referred
to as spent lye contain glycerine, water, salt and unchanged caustic
(2) From oil splitting or hydrolysis without added alkali, of fats and oils
This is a method for preparing fatty acids which are the reduced to
the corresponding fatty alcohols. The glycerine is the obtained in the
sweet water. Crude glycerine recovered from this is referred to as
saponification crude. The spent lyes resulting from current
soapmaking processes generally contain from 10-15% glycerol while
sweet water from hydrolysis of fats contain up to 20% glycerol
In its common liquid form, glycerol is non-poisonous, colourless, odourless
and sweet tasting and has a high viscosity. It is miscible in water and forms a
solution in any proportion. It is also soluble alcohol but only partially soluble in
common organic solvents such as ether and ethyl acetate. It resists freezing. It is
hygroscopic, which favors as a humectant to retain moisture in cosmetics. Because
it is soluble in water and alcohol, its versatility is a major benefactor in its purported
growth and popularity within the manufacturing sector. It is invaluable as a natural
source ingredient with emollient like properties which can soften and soothe the skin
and it assist the outer epidermis is retaining moisture. This helps to explain why it is
one of the most popular cosmetic additives used today. Glycerine also has a wide
range of application areas on cosmetic and food products, tobacco and paints.
1.1.2 Grades
The crude glycerine can be refined by redistillation to obtain refined
glycerine which is more than 99% pure. When the crude glycerine is refined, the
value added is about 80%.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 4
Several grades of refined glycerine, such as high-gravity, dynamite, and USP
are marketed; specifications vary depending on the consumer and the intended use:
(1) USP-grade glycerine is water white, meeting the requirements of the
USP. It is classified as GRAS (Generally Recognized As Safe for
human use) by the US Department of Agriculture and is suitable for
use in foods, pharmaceuticals, and cosmetic, or when the highest
quality is demanded or the end product is designed for human
consumption.
(2) The BP grade is similar to the USP
(3) The CP grade designates a grade of glycerine that is about the same
as USP but with the specifications varying slightly as agreed by
buyer and seller
(4) The high gravity grade is a pale-yellow glycerin for industrial use
(5) The dynamite grade is more yellow
1.1.3 Palm Oil: An Overview
1.1.3.1 History of Palm Oil
Palm oil is produced from the fruit of the oil palm or also known as Elaeis
Guinnesis tree, which originated in West Guinea. The palm bears its fruit in
bunches varying in weight from 10 to 40 kg. The individual fruit, ranging from 6 to
20 gm, are made up of an outer skin (the exocarp), a pulp (mesocarp) containing the
palm oil in a fibrous matrix; a central nut consisting of a shell (endocarp); and the
kernel, which itself contains an oil, quite different to palm oil, resembling coconut
oil. Palm oil is obtained from flesh surrounding the seed through cooking, mashing
and pressing. The flesh is oily, and oil can be recovered by very simple means, so
that it is probable that palm oil has been recovered and used for human food for tens
of thousands of years.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 5
Because of its economic importance as an high-yielding source of edible and
technical oils, the oil palm is now grown as a plantation crop in most countries with
high rainfall (minimum 1 600 mm/yr) in tropical climates within 10° of the equator.
The tree was introduced into other parts of Africa, South East Asia and Latin
America during the 15th century while in Asia, it was first introduced at 1848 into
the botanical gardens at Bogor in Java as an ornamental plant. Its commercial
exploitation started in Sumatra after 1910 and in Malaya in the 1920s.
Palm oil is available in a variety of forms; crude palm oil, palm olein, palm
stearin, RBD palm oil, double-fractionated palm olein and palm mid-fraction.
1.1.3.2 Malaysia Palm Oil
Today palm oil is a well known commodity throughout the world’s food and
oleo-chemical industries. Oil palm growth in Malaysia started in 1950s when
Malaysia has decided to diversify significantly away from rubber, the principal
export crop. Commercial cultivation of palm oil did not begin until 1960’s. This was
due to government was opening up huge jungle track for the FELDA scheme for
landless farmers. By 1975, more than 640,000 hectares were planted with palm oil
and the hectarage continue to surge so much until recently Malaysia had more than
2.8 million hectares under palm oil. Consistent with the rapid expansion in palm oil
cultivation, production of palm oil also rose dramatically from the 1960’s. Apart
from the expansion in hectarage, yields have also risen steadily over the years, as
plantations introduce better yielding and better quality fruits as extraction method.
A principal agent of this development was the government Federal Land
Development Agency (FELDA) which undertook jungle clearance and the settling
of smallholders. Until today, FELDA continues to be a major successful participant
in the palm oil industry. For the user of Malaysian palm oil products there is a
technical backup service provided by the Palm Oil Research Institute of Malaysia Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 6
(PORIM), which has a well equipped and staffed Research Centre near Kuala
Lumpur, and Technical Advisory Officers stationed in Europe, America, Asia and
Africa.
In 1966, Malaysia had surpassed Africa’s total palm oil production.
According to Oil Palm Review, published by the Tropical Development and
Research Institute in the United Kingdom, over 3 million tonnes of palm oil was
produced by Malaysia alone in 1983, compared with a total of about 1.3 million
tonnes of African production. Malaysia currently accounts for 51 % of world palm
oil production and 62% of world exports, and therefore also for 8% and 22% of the
world’s total production and exports of oils and fats. As the biggest producer and
exporter of palm oil and palm oil products, Malaysia has an important role to play in
fulfilling the growing global need for oils and fats in general.
1.1.3.3 Palm Oil Composition
Palm oil contains an equal proportion of saturated fatty acids and unsaturated
fatty acids. Fatty acids are the major oleochemicals derived from animal and
vegetable oils and fats. Fatty acids consist of the elements carbon (C), hydrogen
(H), and oxygen (O) arranged as a carbon chain skeleton with a carboxyl (--COOH)
group at one end. Fatty acids appear in chain lengths between C6 and C22, with the
vast majority in C18.
The difference between fatty acid types is mainly due to their degree of
saturation and their chain length. Saturated fatty acids, for examples, palmitic and
stearic acids have all the hydrogen that the carbon atoms can hold.
Monounsaturated fatty acids have only one double bond, e.g. oleic acids.
Polyunsaturated fatty acids have more than one double bond, such as linoleic acids
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 7
(two double bonds) and linolenic acids (three double bonds). Fatty acids are
generally found combined with glycerol in lipids such as triglycerides.
Table 1.1: Fatty Acids Found in Palm Oil and Palm Kernel Oil in Weight
Percentage
Group Fatty Acids Carbon Chain
Identification
Palm Oil (%wt)
Saturated Fatty
Acids
Caproic C6 -
Caprylic C8 -
Capric C10 -
Lauric C12 0.2
Myristic C14 1.101
Palmitic C16 44.044
Stearic C18 4.505
Arachidic C20 0.4
Unsaturated Fatty
Acids
Oleic C18:1 39.34
Linoleic C18:2 10.01
Linolenic C18:3 0.4
Molecular formula is a notation that indicates the type and number of atoms
in a molecule. Below is the table that shows the molecular formula for all the fatty
acids contained in the palm oil.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 8
Table 1.2: Molecular Formula of Fatty Acids
Trivial Name IUPAC Name Notation Molecular Formula
Caproic Acid Hexanoic Acid C6:0 C6H12O2
Caprylic Acid Octanoic Acid C8:0 C8H16O2
Capric Acid Decanoic Acid C10:0 C10H20O2
Lauric Acid Dodecanoic Acid C12:0 C12H24O2
Myristic Acid Tetradecanoic Acid C14:0 C14H28O2
Palmitic Acid Hexadecanoic Acid C16:0 C16H32O2
Stearic Acid Octadecanoic Acid C18:0 C18H36O2
Oleic Acid 9-octadecanoic Acid C18:1 C18H34O2
Linoleic Acid 9, 12-octadecadienoic Acid C18:2 C18H32O2
Linolenic
Acid
9, 12, 15-octadecatrienoic
Acid
C19:3 C18H30O2
Arachidic
Acid
Eicosanoic Acid C20:0 C20H40O2
1.1.3.4 Structural Formula
Structural formula is a diagram that shows how the atoms in a molecule are
bonded together. Atoms are represented by their element symbols and covalent
bonds are represented by lines. Most structural formulas do not show the actual
shape of the molecule (they’re like floor plans that show the layout but not the 3D
shape of a house).
Table 1.3: Structural Formula of Fatty Acids
Name Structural Formula
Caproic
Acid
CH3(CH2)4COOH
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 9
Caprylic
Acid
CH3(CH2)6COOH
Capric Acid
CH3(CH2)8COOH
Lauric Acid
CH3(CH2)10COOH
Myristic
Acid
CH3(CH2)12COOH
Palmitic
Acid
CH3(CH2)14COOH
Stearic Acid
CH3(CH2)16COOH
Oleic Acid
CH3(CH2)7CH=CH(CH2)7COOH
Linoleic
Acid
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 10
CH3(CH2)4CH=CHCH2CH=CH (CH2)7COOH
Linolenic
Acid
CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH
Arachidic
Acid
CH3(CH2)18COOH
1.2 Product market survey
1.2.1 Introduction
One of the main considerations for planning a plant is the economics of the
industry. Before proceeding with the plans, economic survey is the first and most
important criteria to be observed. Glycerine or glycerol is one of the common basic
oleochemical products. Refined glycerine is used in the manufacture of many
pharmaceutical, food, and oral care products. The markets are renowned for their
complexity and unpredictability. It is because glycerine is produced primarily as co-
product, so the demand for the primary product influences the amount of glycerine
produced. At the same time, there are hundreds of end uses for glycerine. Thus, the
large number of uses and the complexity of supply markets make the future
glycerine pricing very difficult to predict.
1.2.2 Global Market
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 11
The production of glycerine as co-product comes from several process in
oleochemicals, soap and biodesel industries and also synthetically process. As the
co-product, sometime the demand of glycerine is really depend to the main
oleochemical product prices.
Figure 1.1: Total World Production of Glycerine
The demand for glycerine in the world has been increases since 1992. In 1992,
the world estimate consumption of glycerine was 638,000 MT. It has been increase
to 730,000 MT in 1995. In 1998 it has been rise to 800,000 MT. This represents a
growth rate of 4 ½ % between 1992 and 1995, and 3% between 1995 and 1998. The
demand is made up 30% for Europe, 30% North America and the rest of the world,
40%.
Figure 1.2: Geographic Demand for Glycerine
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 12
Before 90’s, the world consumption for glycerine is just below 600 k MT per
year. Most of the glycerine was produced by natural raw material. Countries in
Western Europe have a high demand for glycerine. This maybe caused by the
developing of their industries.
Table 1.4: World Glycerine Production, Consumption and Capacity, 1988(1,000 MT)
Production Consumption Refining Capacity
Natural Synthetic
North America 153 166 170 60
Western Europe 200 160 177 50
Asia/Pacific 150 137 189 40
Other 85 125 107 20
The high demand for glycerine comes from the developing country as the
increasing of its application in producing a basic oleochemical product. In addition,
nowadays most of the consumer want something that is seemed more environmental
friendly and come from the nature product. This brings to the increasing of glycerine
market in the world.
The demand for natural glycerine has also been increased since 1988. In
1988, the demand for natural glycerine is only 240 k MT, but it has been increased
to 300 k MT in 1995 and 341 k MT in year 2000. The increasing of natural glycerine
demand has increased the production of this oleochemical product from fats and
oils.
The increasing of its application in many industries also caused the increase
of glycerine demand. At this moment, there are more than 1500 known end uses for
glycerine. An approximate breakdown by major application area for Western Europe
is detailed below.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 13
Table 1.5: The Application of Glycerine in Western Europe
Percentage (%)
1978 1985 1993 1997
Pharmaceuticals
Cosmetic Toiletries
Esters
Polyols
Resins
Food and Drinks
Cellulose Film
Other Chemical Uses
Tobacco
Nitration
Paper
Resale
Others
-
-
-
8.0
17.0
-
8.0
-
-
9.0
-
-
-
11.5
9.7
15.4
12.2
9.9
5.9
4.8
4.5
3.5
3.4
1.8
14.6
2.8
9.4
14.5
13.1
10.6
7.1
7.0
5.8
3.5
3.3
0.7
1.1
18.5
5.4
9.8
16.1
10.7
11.7
5.7
8.5
3.1
8.2
3.6
0.2
0.9
17.3
4.2
(I) Supplier
Table 1.6: Global Market Supplier of Glycerine
PRODUCER CAPACITY*
Cognis, Cincinnati, Ohio 65
Colgate-Palmolive, Jeffersonville,
Ind.
20
Crompton, Mapleton, Ill. 20
Crompton, Memphis, Tenn. 30
Dial, Montgomery, Ill. 30
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 14
Dow, Freeport, Tex. 140
Lever, Hammond, Ind. 25
Lonza, Painesville, Ohio 20
Marietta American, Olive Branch,
Miss.
2
Procter & Gamble, Ivorydale, Ohio 150
Starchem, Fostoria, Tex. 20
Uniqema, Chicago, Ill. 35
Total 557
*Millions of pounds per year of refined glycerine.
Dow Chemical is the only producer of synthetic glycerine. Others obtain
glycerine as a byproduct in soap and oleochemicals production using natural fats and
oils as raw materials. Colgate refines glycerine at Jeffersonville using purchased
crude.
Algroup lonza, formerly known as Lonza Inc. expanded glycerin capacity at
Painesville, Ohio, in 1997 to 15 million pounds, and again in 1999 to 20 million
pounds.Archer Daniels Midland Company had planned to build a 50-million-pound-
per-year glycerine plant at Cedar Rapids, Iowa, based on a proprietary fermentation
process. The project was due online in 2000, but in 1999 the project was been put on
hold because of glycerine market conditions.
And last year another ethanol from corn producer, High Plains Corp.,
announced plants to install a 10-million-pound-per-year glycerin recovery unit at its
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 15
ethanol plant in Colwich, Kan. But earlier this year, this project too was put on
indefinite hold.
Unichema International acquired by ICI in May 1997 as part of its
acquisition of Unilever's specialty chemicals businesses. Its name was changed to
Uniqema, on January 1, 1999, and became part of a new ICI business, The Uniqema
Group. Uniqema also includes ICI surfactants and synthetic lubricants, Mona
Industries and Solaveil.The Unilever glycerine and surfactants plant at Hammond,
Ind., operates as part of its Lever Brothers Company subsidiary.
In 1999, Crompton & Knowles and Witco merged to form CK Witco. The
company was renamed Crompton Corp. in 2000. Witco brought on a new still at
Mapleton, Ill., in January, 1999. The still upgrades crude glycerine to refined
material. Crompton Corp. is known to be looking to divest its refined products
operation, which is considered a non-core business.
In October 2001 Procter & Gamble Chemicals announced plans for and
additional 50,000 metric tons of glycerine capacity through a new grassroots
refinery. Although the lead option for the site is Kuantan, Malaysia, additional sites
in North America and Europe are also under consideration. P&G had earlier
increased its glycerine refining capacity by 10,000 metric tons as a result of
debottlenecking in West Thurrock, England and Cincinnati, Ohio.
(2) Strength
The market sector for personal care products is growing at 3.5% annually, as
the increasing of the generation consumes more skin care creams. The strong growth
of sunscreen lotions in this sector also reflects the greater public concern these days
about exposure to sun rays. Good solubility and taste give glycerine an edge on
sorbitol in toothpastes and mouthwashes, and the oral care sector is growing at
approximately 1.5% annually. The best performing sector, is food. In year 2000,
food product consumed about 49.4 million-kilogram glycerine and its derivatives.
Glycerine in the food sector is growing better than 4% annually as a result of the
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 16
continuing trend towards lowering the fat content in foods, particularly baked goods.
These three sectors together represent 64% of glycerine application.
(3) Weakness
However, the glycerine market experienced major swings in market
conditions from the mid-1990s to the present time. In 1995, the industry was supply-
limited, but by 1998 glycerine was abundant and production was declining. By the
end of 1999 and into 2000, the glycerine market was again tight. It is not so much as
a declining demand in 2001, but more of an increase in supply that has put
additional pressure into the marketplace. This is because there is a significant
increase of crude glycerine in biodiesel-related generation in Europe, where crude
glycerine is one of its by products. Some demand has moderated though. Glycerine’s
use in polyether polyols and alkyd resins is shrinking at 1 percent and 1.5 percent
annually. These application areas have both been hurt by the decrease in GDP
growth. Moreover, alkyd resins have been facing increased competition by water-
based acrylic and vinyl formulations.
(4) Prices
The prices for glycerine also increase in these recent years. As new uses are
found and the economies of developing countries are grow, the demand for
glycerine is rising at this time. However the increases in supply have been unable to
match increases in demand. Thus, prices for refined glycerine are now very high.
Currently, refined glycerine sells for US $2.32/kg although in the recent past,
refined glycerine prices have been as low as US $1.25/kg. It may also caused by the
increasing of the raw material prices especially palm oil.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 17
Figure 1.3: World Market Demand for Glycerine
Glycerine is effective 10% or more of fat or oil molecule, so the price of
glycerine is almost depends to the oil or fat itself in the economic of oleochemicals.
Increasing supply will cause price to fall, whilst high prices will cause substitution
by other polyoils and price will fall. In the year 199 and 2000, the world price for
glycerine has varied by as much as $ 1000/ton.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 18
Figure 1.4: U.S. Refined Glycerine Pricing History - 99.5% U.S.P.
1.2.3 Asian Market
The world production of basic oleochemical is expected to increase from 5.0
million tonnes in 1995 to 7.8 million tonnes in 2010. The Asean countries are
expected to contribute 3.5 million tonnes or equal 50% from the world demand.
Malaysia gives the highest percentage, 70% or 1.2 million tonnes from the Asean
output.
In this century, the well development of oleochemical industry in Asian will
also brings to the very high demand for glycerine. It is an advantage if the glycerine
was produced from the vegetables because of the changing in customer demand in
more nature product.
Excluded Malaysia, Indonesia and Philippines are just starting to put
themselves in the world market as glycerine supplier. It may look as competitors to
Malaysia but it will bring a more stable market price for glycerine in the world. It is
because it can cover the demand for the product in industry.
In Philippines, the oleochemicals industry is a strategic sector owing to its
linkage with the Coconut and Palm Oil Industries. These sectors are highly
complementary in their socio-economic and business setting, particularly the high
value-adding impact of downstream processing (oleofoods/oleochemicals) to these
upstream industries (CNO / PO processing). Development of oleochemical ventures
could easily transform the coconut exports and fledgling palm oil industries into at
least $4-5 billion export industries.
As a foreign exchange earner, the industry generated substantial export
earnings of $299.5 million from 1996-2001. The specific leading products stearyl
alcohols, industrial fatty alcohols and refined glycerine. The major destinations
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 19
included Japan, USA, Korea, the Netherlands, Taiwan, China, India, Thailand,
Belgium and France.
As oleochemical manufacturers are either local large enterprises or joint
ventures with foreign companies operating overseas plants, they maintain well-
established distribution channels through which, marketing activities are made for
both export and domestic markets.
Some of the factory in Philippines that involves in producing glycerine are
listed in table 1.7 below:
Table 1.7: Asian Companies Producing Glycerine
COMPANY PRODUCT
United Coconut Chemicals, Inc. - Fatty Acid
- Fatty Alcohol
- Glycerine
Pilipinas Kao, Incorporated - Elem. Resinoid
- Fatty Alcohol Beads
- Hydrogenated Fatty Alcohols
- Methyl Ester
- Refined Glycerine
- Fractionated Alcohol
- Sodium Lauryl Sulfate
- Mono Alkyl Phosphate
- Alkanolamide and Surfactants
- Coco-Tertiary Amines
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 20
- Fractionated Methyl Ester
and/or Fractionated Fatty Alcohol, flaked
Primo Oleochemicals, Inc. - Fatty Alcohol
- Low MW Fatty Acid
- Glycerine
Sakamoto Orient Chemical
Corporation
- Crude Glycerine
- Refined Glycerine
Senbel Fine Chemicals - Fatty Alcohols
- Methyl Esters
- Glycerine
- Alkanolamides
- Others
In Indonesia, there are five producers of basic oleochemicals with an annual
capacity to produce 374,000 tons of fatty acid, 55,900 tons of glycerin, 90,000 tons
of fatty alcohol and 10,000 tons of methyl ester. Three largest producers are located
in Medan namely PT Sinar Oleochemical International (SOCI) of the Sinar Mas
Group, PT Flora Sawita Chemindo and PT Ecogreen Oleochemicals formerly PT
Prima Inti Perkasa of the Salim Oleochemical Group Ecogreen Oleochemicals also
has a factory in Batam formerly named PT Batamas Megah.
Two other producers are PT Cisadane Raya Chemicals (CRC) in Tangerang
and PT Sumi Asih Oleochemicals in Bekasi. SOCI, which started operation in 1994,
is a subsidiary of PT Smart Corp. of the Sinar Mas Group (SMG) with co-owners
including Nippon Oil & Fat (NOF) Corp., Shiseido Company Ltd, Hitachi Zosen
Corp. and Marubeni Corp. from Japan. PT Ecogreen Oleochemicals was formerly
owned by the Salim Group, but in late 2000, it was acquired by the Wings Group in
a tender through the Carhart Investment Pte Ltd consortium, which is the investment
vehicle of Bhakti Investama.
Ecogreen Oleochemicals from its two factories has an annual capacity to
produce 90,000 tons of fatty alcohol and 12,500 tons of glycerin. Sumi Asih, which
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 21
started operation in 1984, has an annual capacity to produce 90,000 tons of fatty acid
and 15,000 tons of glycerin and 32,000 tons of stearic acid (stabilizer) and metallic
soap.
Sumi Asih has established cooperation with Goldschmidt AG from Germany
to produce oleochemical derivatives namely betain - a shampoo additive - with an
annual capacity of 20,000 tons. Sumi Asih has also divested its 25% stake in
Goldschmidt Sumi Asih (GSA) to Goldschmidt. Previously GSA was a joint venture
between Sumi Asih and Goldschmidt AG each with a 50% stake.
Sumi Asih also established PT Biolina Trio Sintesa to produce 100% stearin
with an investment of US$ 8 million. It has an annual capacity to produce 75 million
sticks of candle a year.
PT Cisadane Raya Chemical (CRC), a pioneer in oleochemical industry
started operation in 1979. IT has an annual capacity to produce 120,000 tons of
products including 70,000 tons of fatty acid, 15,000 tons of glycerin, 10,00 tons of
distilled fatty acid and 25,000 tons of bar soap. CRC in Tangerang also produce
edible oil with an annual capacity of 180,000 tons. In June 2000, the company
secured the ISO-9002 certificate from the Dutch Council for Accreditation.
Table 1.8: Producers of Oleochemicals and Capacity in Indonesia, 2002
Companies Location Production capacity
(ton/year)
Cisadane Raya Chemicals, PT Tangerang Fatty acid - 120,000
Glycerine - 15,000
Stearic acid - 30,000
Ecogreen Oleochemicals, PT (d/h Salim
Oleochemical)
Medan Fatty alcohol - 30,000
Fatty acid - 5,000
Glycerine - 12,500
Methyl ester - 10,000
Batam Fatty alcohol - 60,000
Sumi Asih Oleochemicals, PT Bekasi Fatty acid - 90,000Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 22
Glycerine - 15,000
Sinar Oleochemical International (SOCI),
PT
Medan Fatty acid - 80,000
Glycerine - 8,000
Flora Sawita Chemindo, PT Deli Serdang Fatty acid - 52,000
Glycerine - 5,400
Total
Fatty acid - 347,000
Glycerin - 55,900
Stearic Acid - 75,600
Fatty alcohol - 90,000
Methyl ester - 10,000
1.2.4 Local Market
The oleochemical industries are growing in Malaysia. Starting in 1979, the
first fatty acid plant was built in Malaysia and producing over 30 thousand metric
tons annually. Two other plants were open after that in 1981 with combined capacity
of about 62 thousand metric tons. In 1990, there have been six oleochemical plants
in Malaysia with total annual capacity of 250 thousand metric tons.
An industrial development program in Malaysia, the Industrial Master Plan
(IMP), calls for n increase to 750 thousand metric tons by 1995. in 1993, the US
Department of Agriculture (USDA) estimated Malaysia’s oleochemical capacity at
600 thousand metric tons. This has been accomplished by several joint venture
projects between Malaysian companies and foreign companies.
As example, Procter and Gamble joined with Felda Mills Corporation, a
subsidiary of the Malaysian Federal Land Development Authority (Felda) to build a
$50 million natural fatty alcohol and glycerine plant production, that which began in
1992 and the capacity is 60 thousand metreic tons annually.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 23
Now days, in Malaysia, glycerine is one of the component exports of major
oleochemical products. In 2002, 12.59% of oleochemical product that has been
export is glycerine, this equal to 160 kMT or RM 344.86 million. The total income
from oleochemical export at that moment is RM 2,739.2 million or 1, 267, 942
tonnes. This brings the bright future for the investment in glycerine production.
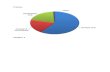
Figure 1.5: Export of Major Oleochemical Products in 2002
Figure 1.6: Major Glycerine Supplier Country
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 24
Malaysia is one of the major countries that supply the glycerol in the world
market. In year 2000, Malaysia supplies 43% of the world export. It is followed by
Netherlands (22%) and other countries are France, USA, Czech Republic and the
others. The main factor for the Malaysia to become the main country that supplies
glycerine is the developing of palm oil industry in this country.
The reason of this phenomenon is the development of oil palm industry in
Malaysia. The increasing of production of palm oil has increase the oleo chemical
industry including the production of glycerine.
Malaysia also becomes the main country that supplies the glycerine for
United States of America. In March 2003, 87.65% of United States imports for
glycerine come from Malaysia. The value of the American import is RM5.21
million. It is prove that the production of glycerine can give a very high profit to the
investor.
Figure 1.7: U.S. Imports for Consumption of Glycerine in March 2003
Glycerine was used in lot of industries in our country. Most of the glycerine
is using as the basic oleo chemical product in producing surfactants, emulsifiers,
lubricants and cosmetic products. The highly demand for the glycerine in the
industrial uses make it as a very potential investment.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 25
Figure 1.8: Usage Of Glycerine In Malaysia
Currently in Malaysia, large multi-national companies dominate the
glycerine market. Five companies that produce glycerine in Malaysia are:
a. Lam Soon (M) Sdn. Bhd.
b. Fatty Chemical (M) Sdn. Bhd.
c. Natural Oleochemical Sdn. Bhd.
d. Pan-Century Oleochemical Sdn. Bhd.
e. Palmco Holdings Berhad.
Besides glycerine, the factories also produce other basic oleochemical
product like fatty acid, fatty alcohol. Most of the glycerine was used in the country
and some of them are exported to the other country. The demand for glycerine in our
country also high and there is not enough suppliers to cover it. This brings to the
bright future in investing in this field.
Gly World (M) Sdn. Bhd
Esterification with Fatty Acid
Transesterification with Fatty Monoester
Transesterification with Monoglycerides
Glycerol
Surfactants
Emulsifiers
Lubricants
Cosmetic Products
Shampoo, Foam, Shower Bath, Gels
Food Industry
Engine, Hydraulic, Gearbox oil
Molding, Chain Saw Oil
Acidic
Basic
Basic

Chapter I– Project Conception - Literature Survey I- 26
1.2.5 The Raw Material
Good quality glycerine can be derived either naturally from vegetable or
animal origin, or synthetically from epichloohydrin but for marketing reason, it is
most suitable to use the vegetable based raw material as the requirement from many
customer. It is because of the religious sensibilities and the purity.
Over 20% of the worldwide production of fats and oils were used in non-
edible products in 1990. The major raw material that is use is tallow, coconut oil,
palm oil and palm kernel oil. The prices for the four major raw materials for the oleo
chemical market as given in the Chemical Marketing Report (November 1993) are
shown below:
Table 1.9: Prices of Major Raw Materials of Oleochemicals in
November 1993
Raw Material Price US$/ MT
Palm Kernel 418
Palm Oil 407
Coconut Oil 522
Tallow (inedible) 319
If we compare, the price for palm oil is the lowest. This will reduce the raw
material cost in producing the oleochemical product.
Some other reasons of the choice are:
a) The market price for CPO is low in Malaysia. So we can reduce the raw
material cost.
b) CPO is one of the major palm oil products in Malaysia. It can be getting
easily in a large quantity.
c) It is a biological substance. Easier to process the waste and reduce the
pollution.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 27
Malaysia, as the main manufacture of palm oil product in the world, has
produce million tonnes of crude palm oil, crude palm kernel oil and it derivatives
every year. In year 2002, Malaysia has produce 1,473 k MT crude palm kernel oil.
Indonesia has produced 1,050 k MT and Thailand and Philippines, each 90 and 8 k
MT. This will give an enough raw material for the production of glycerine.
The average price of CPO in 2004 for local delivered is RM 1,416 per tonne
(The Star, 6 December 2004). The refined glycerine was sold at RM 8.81/kg. The
refined glycerine market may be more complex to penetrate than the crude glycerine
market. This is because, for crude glycerine, a small number of refineries represent
the entire potential customer base. In contrast, refined glycerine can be sold to
hundreds of different companies.
1.3 Several Strategic Plant Locations
The manufacture of refined glycerine can be classified as oleochemical
project. This industries must be therefore be sited in a special zone provided by government.
In Malaysia, the locations of the oleochemical plants are majority located at
Petaling Jaya Selangor, Prai Pulau Pinang and Pasir Gudang Johor. The location
of any purpose plant plays a very important role as it can affect on the success of
the plant and its operation. The choice of the final site should be based on a
complete survey of the advantages and disadvantages of all available location.
For the production of 100 KMT per year of refined glycerine, there is five main
location industrial estates have been shortlist for our new plant. They are:
1. Teluk Panglima Garang Industrial Estate, Selangor
2. Prai Industrial Complex, Prai, Penang.
3. Pasir Gudang Industrial Estate, Johor.
4. Gebeng (Phase IV) Industrial Area in Pahang.
5. Teluk Kalong Industrial Area Terengganu.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 28
1.3.1 Criteria of Plant Site Selection
The assessment of site location for sitting a plant would depend mainly on several factors such as:
(1) Production and Distribution Factors
a) Freight rates
Competitive points
Differentials
Favorable territory
b) Markets
Local area
Favorable area
Competitive area
National area
c) Competitive, feeder and consumer industries
d) Municipal restriction
Nuisance laws relating to fumes
Waste disposal
e) Corporation fees and taxes
f) Fuel
Types of fuel
Thermal efficiency
Reserve and alternate sources
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 29
(2) Specific Factors
a) Transportation facilities
Highways – regularly used for short distance and generally small
quantities.
Availability of various services and project rates.
Water – cheaper but may be slow and irregular.
Railroads – dependable for light and heavy shipping aver all distance.
Pipelines – for gases and liquids, particularly for petroleum product.
Air – for business transportation for personnel.
b) Regulatory Laws
Zoning ordinances.
Highway ordinances.
Building codes.
Waste-disposal codes.
c) Site characteristics
Soil structure.
Costs of site.
Control of site.
Room of expansion.
Access to railroads, highways and water.
Site and facilities available by expansion on present company –
owned property.
d) Flood and fire control
Flood history.
Flood control.
Fire hazards in surrounding area.
e) Community factors
Rural or urban.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 30
Medical facilities – hospitals, doctors, etc.
Housing costs.
Recreation facilities.
Cultural and religion aspects – mosque, surau, libraries, theatres, etc.
School system.
f) Waste disposal
Regulation laws.
Steam carry – off possibilities.
Air – pollution possibilities.
g) Vulnerability to wartime attack
General industry concentration.
Distance from important facilities.
h) Taxes
States and local taxes.
i. Income
ii. Property
iii. Use
iv. Franchise
v. Unemployment insurance
Low assessment or limited term exemption to attract industry.
(3) Major Factors
a) Reasonable land price
The land will influence the working capital. In terms of reasonable, it
means that with the good incentive from local government and low land
price. If the land chosen is not classified as industrial area, the condition
should be change first. State and local tax rates on property income,
unemployment insurance and similar items vary one location to another.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 31
b) Labors
The types and supply of labor available in the vicinity of proposed
plant site must be examined. Some of the factors that should be considered
on labor are supply, kinds, nationality, diversity, intelligence, wage scales,
efficiency and costs. Consideration also should be given to prevailing pay
scales, restrictions on number of hours worked per week, competing
industries that can cause dissatisfaction or high turnover rates among the
workers and variations in the skill and productivity of the workers there for
industrial housing, safety-first movement, welfare institutions, better
sanitation and lunchroom have all contributed to the solution of labor welfare
and problems as well as the radio and automobile, also have helped towards
building up and maintaining a supply satisfied and contented laborers. This
factor can contribute to the saving of the operation cost.
c) Distance From Market
Demand versus distance, inventory storage requirements, growth or
decline, competition-present and future. The location of market or
intermediate distribution centers affects the cost of product distribution and
the time required for shipping. The buyer usually finds that it is more
advantage to purchase from nearby sources. Market of major final product
and by-product should be considered.
d) Waste disposal
Another serious consideration that should be made before choosing a
site is the disposal of waste liquors and waste products from the chemical
plant. If there is a sewer in the street adjoining to the property, the quantity
of waste liquors to be disposed off should be estimated and the size of the
street sewer should be checked to determine whether it could take care of the
liquors. If the waste liquors such as acids or alkaline, contain solids, or has
other objectionable features, it is advisable to learn from the local authorities
whether the disposal of such liquor in the sewerage system is permissible.
Any waste from atomic energy plants cannot just be disposed of by dumping
it into sewers or rivers.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 32
Usually most chemical plants often simply dispose of their waste by
locating on a stream, rivers or at the tidewater. However, this is no longer
satisfactory, for there is a growing list of state, which has instituted
legislation against such pollution by industrial wastes. Seepage through the
ground is also can be used as another method of waste disposal. Before that,
soil tests should be made to determine whether the soil is porous enough to
permit the disposal of considerable quantities of liquor with out
accumulation. It is also advisable to check the topographic factor of the area
to determine where the liquor will sleep in order to avoid trouble from
neighboring plants or the local authorities. Towns lower down the valley mat
draw their water supply from the drainage shed upon which the plant is
situated.
Nevertheless, a systematic toxic and industrial waste will be more
effective as disposal waste will be treated accordingly. This method is
getting popular and encourages using as will product non-polluting, clean
and environmental friendly.
e) Climate
Seasonal range
Precipitation
Humidity and temperature conditions.
Wind
Hurricane, tornado and earthquake history.
Investment required for construction.
f) Raw materials or semi-finished products
These particularly important if large volumes of raw material are
consumed because these permit considerable reduction in transportation and
storage charge. Any way attention should be given to the purchased price of
the raw materials, distance from the source of supply, purity of the raw
materials, reserve stock and storage requirements. In order to save the
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 33
transportation costs, the plant should be located near to the raw material
supply and sources.
g) Transportation Facilities
The relation of railways, ports or road facilities to market is so close
in making a prudent investigation of transportation rates before definitely
deciding upon a plant location. Water, railroads and highways are the
common means of transportation used by major industrial concern. There is
usually need for convenient air and rail transportation facilities. A location
which has several competing railroads, ports and road networks as well as
waterways in order that the competition will help to maintain low rates and
give better service should be chosen.
h) Government Incentives
In order to attract new investors, the government will offer an
incentive to them, the better the incentive offered the feasibility to build the
plant there would be better.
i) Water supply
Sources
Mineral analysis
Bacterial content
Turbidity
Quantity
Temperatures
Costs
The process industries use a lot of water for cooling, washing steam
generation and as raw material. So the plant must be located where supply
water is available. The temperature, mineral content, silt or sand content and
cost for supply must also be considered when choosing a water supply.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 34
j) Energy Supply
Hydroelectric
Public service
Alternate source
Power and steam requirement is high in most industrial plants and
fuel is ordinarily to supply this utilities. Location should near to the
hydroelectric installation if the plant using electrolytic process. If the plant
requires large quantities of oil or coal, location near a source of supply may
be essential for economic operation. The local cost of power can help
determine whether power should be purchased or self-generated. Local
authorities or whoever provided power supply to locations should have a
contingency plan if power failure occurs during plant operations beside
contingency plan from the plant it self.
(4) Port Facilities
i) Prai Industrial port:
Blue Orient Shipping:
- Warehouses provided
- 20 minutes to Penang airport
- 10 minutes distribution to three other locations
- Located in the Free Commercial Zone
ii) Pasir Gudang Port:
Haulers:
- Kontena Nasional Sdn. Bhd.
- MISC Haulage Sdn.Bhd
- Shapadu kontena Berhad
- Multimodal Freight Transportation Sdn. Bhd.
- Konsortium Perkapalan Berhad
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 35
iii) Kuantan Port
Facilities:
- Berth
Berth Container:
- Length = 200m
- Draft = 11.2m
Two units with length of 450m have been completed in 1999.
Multipurpose Berth
- Three units
- Length = 525m each
- Draft = 11.2m each
- Storage:
- Seven godowns
- Total area = 31.75m2
- Open yard = 41.25 m2
- Container freight
- Stations - 9.6m2
- Yard – 72.0 m2
- Port Equipments:
Container crane
- 40 tons capacity
- Able to handle 20’, 40’ and 45’ container.
- Multipurpose crane
- 30.5 tons-container capacity
- 40 tons-sling capacity
- 16 tons-grab capacity
Presently, the port has total area of 605.3 hectares with the following comprises:
- 48.4 hectares used for operation purposes.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 36
- 69.2 hectares accommodate a water basin.
- 205.7 hectares for industrial purposes.
- 11.4 hectares for commercial purposes.
- 151.7 hectares reserved for future expansion.
- 12.5 hectares accommodates recreation and staff quarters.
- 106.4 hectares consist of road, hill and drainage.
- The port is in the midst of RM300 million-expansion program.
iv) Kemaman Port
Dedicated liquid bulking terminal under construction:
- Length of 240 meters.
- Ship capacity up to 40,000 DW.
- Handling capacity 15,000 tons per day.
- Pipeside 8” – 12” (50 in number)
It is an all weather port facing South China Sea. The draft at East Wharf has
17 meters draft (upgraded from initially 13 meters in October 1996). It can handle
ships up to 100,000 DWT. Upgrading exercises would cater 120,000 DWT vessels.
Currently dry general and liquid cargo on average two to three million tons per year.
Current capacity estimated at 6.84 millions tons per year (Tango crane).
It consists of three selections:
- East wharf (684 m)
- Supply base (650 m)
- Petroleum Export Terminal
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 37
Table 1.10: Comparison of short-listed of the potential site location
Selection
Criteria
Teluk Panglima Garang
Industrial Estate,
Selangor
Prai Industrial Complex, Prai, Penang
Pasir Gudang
Industrial Estate,
Johor
Phase IV, Gebeng
Kuantan Pahang
Teluk Kalong,
Terengganu
Developer Perbadanan Kemajuan
Negeri Selangor
(PKNS)
Pulau Pinang
Economic
Development
Corporation
Johor State
Economic
Development
Corporation
(JCORP)
Perbadanan
Tempatan Pasir
Gudang (PBTG)
Land
Price
(RM per
sq ft)
7.00 – 20.00 12.20 – 21.00 8.00 – 22.00 2.00 – 12.00
Total area of 4000
acres
0.46 – 4.18
Total area of 516.18
hectares
Raw
Material
Supplier
Southern Edible Oil
Industries (M) Sdn.
Bhd.
United Plantation Oil
Mill
Sime Darby Edible
Sime Darby Edible
Product
Limited(SDEPL)
Cargill Palm
Products Sdn. Bhd.
Felda Vegetable Oil
Gly World (M) Sdn. Bhd
1 1 331
2
1 1 2

Chapter I– Project Conception - Literature Survey I- 38
Socktek Sdn. Bhd
UNITATA Berhad
Anson Oil Mill Sdn
Bhd.
Lee Oil Mill Sdn. Bhd
Sime Darby Edible
Product
IOI Edible Oils Sdn
Bhd.
Felda Marketing
Services Sdn. Bhd.
KL-Kepong Edible Oil
Sdn. Bhd.
Welli Edible Oil Sdn.
Bhd.
Ngo Chew Hong Oils
& Fats (M) Sdn Bhd
Kempas Edible
Oil. Sdn. Bhd.
Product
Wilma Edible Oil
Sdn. Bhd.
Palmco Oil Mill
KeckSeng(M) Bhd.
Marakot Industries
Public Company
Limited.
Johore Tenggara
Oil Palm Sdn. Bhd.
Mewaholeo
Industries Sdn. Bhd.
Products Sdn. Bhd.
Incentive given
State Government
itself.
Waste
Disposal
Kualiti Alam Sdn.
Bhd.
Kualiti Alam Sdn.
Bhd.
Kualiti Alam Sdn.
Bhd
Kualiti Alam Sdn.
Bhd
Kualiti Alam Sdn.
Bhd
Gly World (M) Sdn. Bhd
32 2

Chapter I– Project Conception - Literature Survey I- 39
Distance
from
Nearest
Town
15 km from Sepang
25 km from Shah
Alam
25 km from Klang
50 km from KL
35 km from Petaling
Jaya
118 km from Alor
Setar
25 km from Kulim
37 km from Sungai
Petani
40 km from
Butterworth
48 km from Johor
Bharu
5 km from Johor
Port
45 km from
Kuantan Town
4 km from Kuantan
Port
9.6 km from
Kemaman District
Types of
Industries
Small, medium and
heavy industry
Oleochemical industry
Chemical
Petro-chemical
Sea related industry
oleochemical
industry
Light, medium and
heavy industry
Sea related industry
Chemical and
plastic
Petrochemical
Medium and heavy
industry
Chemical
Petrochemical
Medium and heavy
industry
Road
Facilities
North-South Highway
(Bukit Kayu Hitam to
Singapore)
Persekutuan Highway
PLUS Highway
North-South
Highway (Bukit
Kayu Hitam to
Singapore)
Pulau Pinang Bridge
Pasir Gudang to
Kim Kim River
Bridge over Kim
Kim River
Bridge to Johor
Main road to KL,
JB, Singapore
340km East-Coast
Highway, linking
Karak and
Coastal highway
Terengganu – KL
and Singapore
Gly World (M) Sdn. Bhd
2 2 2
2 2 32 3
12 2 3 3
2 2

Chapter I– Project Conception - Literature Survey I- 40
ELITE Highway
Sepang International
Airport
South Klang Valley
Expressway
Dedicated Highway
Lebuhraya B15
River
North-South
Highway (Bukit
Kayu Hitam to
Singapore)
Highway from Pasir
Gudang-Tanjung
Kupang-Tuas,
Singapore
Terengganu via
Kuantan
Kuala Lumpur-
Kuantan travel
reduced to at least
two hours.
Airport
Facilities
KLIA Bayan Lepas
International Airport
Sultan Ismail
Airport, Senai
Changi
International
Airport, Singapore
Kuantan Airport Airport – Kuala
Terengganu
Seaport Klang Port Pulau Pinang Port Johor Port (Pasir
Gudang)
Kuantan Port
Transportation
Kemaman Port
Kuantan port
Gly World (M) Sdn. Bhd
23 3
1 1 2 2 1
23

Chapter I– Project Conception - Literature Survey I- 41
Tanjung Pelepas
Port
under Kuantan Port
Consortium (KPC)
Railway PUTRA LRT
Butterworth-Pasir
Gudang-
Singapore(KTM)
(787 km)
Express Rail Link
Butterworth-Pasir
Gudang-
Singapore(KTM)
(787 km)
Butterworth-Pasir
Gudang-Singapore
(KTM)
Kuantan Port-
Gebeng-
KemamanPort-
Kerteh to Tuk Arun
in Terengganu
Electricity IPP Kuala Langat
Banting(Genting
Sanyen Power Sdn.
Bhd)
Sultan Salahudin Abd.
Aziz ( Stesen
Janakuasa Kapar :
2420MW)
IPP Petaling Jaya (Projass
Engineering Sdn. Bhd. ;
IPP Perai
(Voltage:1500MW)
Tariff :
Tariff D (low voltage,
less than 6.6kV supply)
All units : 25.8 cent
Sultan Iskandar
Power Station
(Voltage: 220-240
V; max 132 KVA
taped from 275
KVA line)
IPP YTL Power
Generation Sdn.
Bhd.
Tg. Gelang,
Kuantan
Tenaga Nasional
Berhad (800 MW)
Paka Power Plant
(800 MW)
Tasik Kenyir
Hydroelectric Dam
(600 MW)
IPP YTL Power
Generation Sdn.
Bhd. (808MW)
Gly World (M) Sdn. Bhd
2 2 3 3 3
2 2 32

Chapter I– Project Conception - Literature Survey I- 42
17 MW)
Water
Supply
Sungai Selangor water
supply
Sungai Semenyih
water supply
Tariff :
0 – 35m3 : RM1.80
More than 35m3 : RM 1.92
Penang Water Supply
Corporation
Empangan Bersia
Tariff:
0 -20m3 : RM0.52
21 - 40m3 : RM0.70
41 – 200m3 : RM0.90
More than 200m3 :
RM1.00
Syarikat Air Johor
Loji Air Sungai
Layang
Sungai Buloh
(Capacity: 24
mg/day; Flowrate:
0.04-30.0 liter per
second)
Tariff:
0 - 20m3 : RM1.68
More than 20m3 :
RM2.24
Loji Air Semambu
Tarif :
1st 700 m3 : RM 0.95
More than 700 m3 :
RM 1.15
Surrounding
district especially
from Dungun
Kenyir Dam
Tarif :
0 – 227 m3 : RM 0.92
More than 227 m3 :
RM 0.84
Resident
Area
Petaling Jaya
Shah Alam
Kuala Lumpur
Seberang Perai
Georgtown
Butterworth
Pasir Gudang
Kempas
Johor Bahru
Gly World (M) Sdn. Bhd
3 2 32 3
3 331
1

Chapter I– Project Conception - Literature Survey I- 43
Klang
Sepang
Bukit Mertajam
Human
Resources
Training facilities such as:
Politeknik Shah Alam
Universiti Teknologi
MARA
Malaysia Agriculture
Research and
Development Institute
(MARDI)
Universiti Kebangsaan
Malaysia
Universiti Putra
Malaysia
Universiti Islam
Antarabangsa
Training facilities such
as:
Universiti Teknologi
MARA
Universiti Sains
Malaysia
Universiti Utara
Malaysia
Training facilities such
as:
Universiti
Teknologi
Malaysia, Skudai
Universiti
Teknologi MARA,
Segamat
Politeknik Pasir
Gudang
Institut Latihan
Perindustrian Pasir
Gudang
Johor Skills
Development
Center (JSDEC)
Johor
Technovation Park
Polytech
nic Kuantan
Institut
Kemajuan Ikhtisas
Pahang (IKIP)
Gly World (M) Sdn. Bhd
2 2 2
2 2

Chapter I– Project Conception - Literature Survey I- 44
Total Site
value
Gly World (M) Sdn. Bhd
27
25
28
32
23
2 2

Chapter I– Project Conception - Literature Survey I- 45
1.3.2 Selected Site
After considering the available site locations that depend upon several
factors including primary and specific factors, Gebeng (Phase IV), Kuantan Pahang
is chosen as the site for this proposed refined glycerine plant selected as a totally
satisfactory solution for future benefits. Generally the main reasons why Gebeng
(Phase IV), Kuantan Pahang have been chosen as the glycerine refinary industrial
area are:
i) Gebeng Industrial Estate had been specialized for heavy and medium
industries where waste treatment is easy to carry out.
ii) Close to raw material sources. We can get our raw materials (RBDPO)
from Cargill Palm Products Sdn. Bhd and Felda Vegetable oil Products
Sdn. Bhd. In Gebeng Industrial Estate. So, the price of raw materials is
cheaper.
iii) Reasonable land price (RM 2.00 – RM 12.00 per square feet) compared
to the other sites. Since our plant is quite small, the land price won’t
affect the economic analysis much.
iv) Since the industrial is new, so there is much space for us to construct our
plan and for our future expansion. The available area is 4000 acres
(1,618.76 hectares).
v) Common pipe rack link Gebeng and Kuantan Port.
vi) This location is quite near to Kuantab Port (4 km), so that any trade
involving import and export product to the other countries can be
accomplished easily.
vii) Far enough from residents. This site is located 45 km from Kuantan.
viii) Good transportation in terms of road facilities, railway, airport and
seaport to get raw materials and market our product.
ix) Attractive incentives from Pahang State Government. The state
government is giving Pioneer Status, Investment Tax Allowance (ITA),
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 46
Infrastructure Allowance, Incentives for Strategic Projects, Exemption on
Import Duties, and Discount on Electricity Bills etc.
x) Adequate supply of electricity and water. There are Paka Power Plant,
IPPYTL Power Generation Sdn. Bhd. And Tasik Kenyir Hydroelectric to
supply electricity. On the other hand, Semambu Water Treatment can
supply water.
xi) Training facilities are given by Universiti Teknologi Malaysia, Indera
Mahkota, Politeknik Sultan Ahmad Shah (POLISAS), Universiti
Teknologi MARA, Bandar Jengka, Universiti Islam Antarabangsa,
Kuantan and Institut Kemajuan Ikhtisas Pahang (IKIP).
xii) Suitable climate (Annual rainfall: 2500 mm, average temperature: 25-27 0C)
xiii) Other facilities: Eastern Corridor Incentives, establishment of Multi
National Company (MNC’s) etc.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 47
1.3.3 Proposed Plant Layout
1.4 Physical and Chemical Property Data
Tabulated below are the physical and chemical properties of the components involved in this design (Perry, 1984).
Gly World (M) Sdn. Bhd
Par
kin
g L
ot
Par
king
L
ot Par
kin
g L
ot
Par
kin
g L
ot
Secu
rity
Office &
A
dministration
Departm
ent
Sec
uri
ty
Pla
nt A
rea
Pla
nt
Uti
lity
Ch
emic
al
War
eho
use
Fire Station
Future
ExpansionTank Farm
Canteen
Toil
et
Sur
au

Chapter I– Project Conception - Literature Survey I- 48
1.4.1 Glycerine
Chemical Name: Glycerine (Glycerol)
Physical Properties ValueFormula C3H8O3
Physical State LiquidColor ClearOdor Faint odorSolubility in Water Miscible in water; insoluble in
CloroformMolecular Weight 92.095Critical Temperature (F) 1070.33Critical Pressure (psia) 1087.788Critical Volume (ft3/lbmol) 4.228874Melting Point (F) 64.72398Normal Boiling Point (F) 550.13IG Heat of formation (Btu/lbmol) -248452.3Specific gravity 60 F 1.265331 Heat of vaporization (Btu/lbmol)Min value at T (K) = 291.33Max value at T (K) = 850Coefficients:ABCDE
9.035704E+070
1.1067E+084.8319E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 291.33Max value at T (K) = 850Coefficients:ABCDE
13.708433.788321
9.2382E-012.4386E-018.5000E+022.2114E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 291.33Max value at T (K) = 850Coefficients:C1C2C3C4C5
0.0095222277497165
9.9986E+01-1.3808E+04-1.0088E+013.5712E-196.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 298.15Max value at T (K) = 1200.15
115314.2249812.9
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 49
Coefficients:ABCDE
9.6490E+041.5187E+058.2120E+021.8280E+053.2720E+03
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 187.4Max value at T (K) = 561Coefficients:ABCDE
168553348146.3
7.8468E+044.8071E+02
000
1.4.2 Lauric Acid
Chemical Name: Lauric Acid (Dodecanoic Acid)
Physical Properties ValueFormula C12H24O2
Physical State LiquidColor WhiteOdor Slight odor of bay oilSolubility in Water InsolubleMolecular Weight 200.32Critical Temperature (F) 877.73Critical Pressure (psia) 281.3731Critical Volume (ft3/lbmol) 11.29302Melting Point (F) 110.894Normal Boiling Point (F) 569.66IG Heat of formation (Btu/lbmol) -275149.4Specific gravity 60 F 0.89273631 Heat of vaporization (Btu/lbmol)Min value at T (K) = 291.33Max value at T (K) = 850Coefficients:ABCDE
9.807806E+070
1.3164E+085.2913E+01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 291.33Max value at T (K) = 850Coefficients:A
4.3510921.418513
3.7897E-01Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 50
BCDE
2.6716E-017.4300E+022.9396E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 291.33Max value at T (K) = 850Coefficients:C1C2C3C4C5
0.041180881946081
2.0156E+02-2.0484E+04-2.4334E+018.0558E-186.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 298.15Max value at T (K) = 1200.15Coefficients:ABCDE
300015.2739509.3
2.1052E+056.3439E+05-1.4923E+034.0290E+05-6.8190E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 187.4Max value at T (K) = 561Coefficients:ABCDE
300015.2739509.3
2.1685E+056.7377E+02
000
1.4.3 Myristic Acid
Chemical Name: Myristic Acid (Tetradecanoic Acid)
Physical Properties ValueFormula C14H28O2
Physical State SolidColor WhiteOdor None reportedSolubility in Water Insoluble Molecular Weight 228.38Critical Temperature (F) 917.33Critical Pressure (psia) 246.5641Critical Volume (ft3/lbmol) 12.99097Melting Point (F) 129.596Normal Boiling Point (F) 619.16
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 51
IG Heat of formation (Btu/lbmol) -293636Specific gravity 60 F 0.88954641 Heat of vaporization (Btu/lbmol)Min value at T (K) = 327.37Max value at T (K) = 765Coefficients:ABCDE
1.063899E+080
1.4572E+085.6325E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 327.37Max value at T (K) = 765Coefficients:ABCDE
3.7763351.233171
3.2938E-012.6710E-017.6500E+022.9570E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 327.37Max value at T (K) = 765Coefficients:C1C2C3C4C5
0.025596441687843
2.0948E+02-2.177E+04
-2.53218E+017.2474E-186.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 300Max value at T (K) = 1500Coefficients:ABCDE
335012.3864542.7
2.4692E+057.5533E+05-1.6468E+035.2798E+05-7.5355E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 327.37Max value at T (K) = 599.32Coefficients:ABCDE
505872682873.4
2.9280E+056.5086E+02
000
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 52
1.4.4 Palmitic Acid
Chemical Name: Palmitic Acid (Hexadecanoic Acid)
Physical Properties ValueFormula C16H32O2
Physical State ChipsColor WhiteOdor n/aSolubility in Water Insoluble Molecular Weight 256.43Critical Temperature (F) 937.13Critical Pressure (psia) 219.0136Critical Volume (ft3/lbmol) 14.68893Melting Point (F) 145.04Normal Boiling Point (F) 663.8IG Heat of formation (Btu/lbmol) -311692.7Specific gravity 60 F 0.88385921 Heat of vaporization (Btu/lbmol)Min value at T (K) = 327.37Max value at T (K) = 765Coefficients:ABCDE
9.982221E+07115266.7
1.2647E+084.1760E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 327.37Max value at T (K) = 765Coefficients:ABCDE
3.3237651.1012
2.9241E-012.6805E-017.7600E+022.9470E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 327.37Max value at T (K) = 765Coefficients:C1C2C3C4C5
0.0012604151507850
6.3503E+02-4.5621E+04-8.7646E+013.5199E-052.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 300Max value at T (K) = 1500Coefficients:
381011.7985759.6
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 53
ABCDE
2.8475E+058.6494E+051.6878E+036.1107E+057.6735E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 327.37Max value at T (K) = 599.32Coefficients:ABCDE
577842.6816698.4
-4.8415E+05-3.4568E+00
000
1.4.5 Stearic Acid
Chemical Name: Stearic Acid (Octadecanoic Acid)
Physical Properties ValueFormula C18H36O2
Physical State SolidColor WhiteOdor Slight tallow-like odorSolubility in Water Slightly soluble in water Molecular Weight 284.48Critical Temperature (F) 987.53Critical Pressure (psia) 197.2513Critical Volume (ft3/lbmol) 16.33883Melting Point (F) 157.28Normal Boiling Point (F) 707.36IG Heat of formation (Btu/lbmol) -328459.6Specific gravity 60 F 0.8471 Heat of vaporization (Btu/lbmol)Min value at T (K) = 342.75Max value at T (K) = 804Coefficients:ABCDE
1.223813E+083169.206
1.7410E+086.3436E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 342.75Max value at T (K) = 804Coefficients:AB
2.9717310.9867467
2.6257E-012.6778E-01
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 54
CDE
8.0400E+023.1050E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 342.75Max value at T (K) = 804Coefficients:C1C2C3C4C5
0.0058632971339989
2.3303E+02-2.4932E+04-2.8343E+016.2621E-186.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 300Max value at T (K) = 1500Coefficients:ABCDE
437912.11100061
3.2620E+059.4730E+051.6260E+036.4154E+057.4310E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 342.75Max value at T (K) = 648.3499Coefficients:ABCDE
653938.8852080.7
4.3171E+056.4837E+02
000
1.4.6 Oleic Acid
Chemical Name: Oleic Acid (9-Octadecanoic Acid)
Physical Properties ValueFormula C18H34O2
Physical State LiquidColor Colorless to pale redOdor LardlikeSolubility in Water Insoluble Molecular Weight 282.46Critical Temperature (F) 946.13Critical Pressure (psia) 201.5842Critical Volume (ft3/lbmol) 16.01846Melting Point (F) 56.08403Normal Boiling Point (F) 679.73IG Heat of formation (Btu/lbmol) -288820.9
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 55
Specific gravity 60 F 0.89341 Heat of vaporization (Btu/lbmol)Min value at T (K) = 286.53Max value at T (K) = 781Coefficients:ABCDE
1.12485E+08180974.2
1.3470E+083.9430E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 286.53Max value at T (K) = 633.15Coefficients:ABCDE
3.1676452.253934
2.6810E-012.6812E-017.8100E+022.8970E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 286.53Max value at T (K) = 633.15Coefficients:C1C2C3C4C5
1.074907E-0599453.47
1.8610E+02-2.0.15E+04-2.2472E+016.1199E-182.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 298.15Max value at T (K) = 1500.1Coefficients:ABCDE
417795.11069776
3.2000E+059.3620E+05-1.7431E+036.7540E+057.8250E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 286.53Max value at T (K) = 550Coefficients:ABCDE
517916.91114050
4.5900E+05-8.6600E+023.7400E+00
00
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 56
1.4.7 Linoleic Acid
Chemical Name: Linoleic Acid (9,2 Octadecadienoic Acid)
Physical Properties ValueFormula C18H32O2
Physical State LiquidColor Clear, very slight yellowOdor n/aSolubility in Water Insoluble Molecular Weight 280.45Critical Temperature (F) 935.33Critical Pressure (psia) 204.494Critical Volume (ft3/lbmol) 15.85828Melting Point (F) 23.00001Normal Boiling Point (F) 670.73IG Heat of formation (Btu/lbmol) -23215.3Specific gravity 60 F 0.908781 Heat of vaporization (Btu/lbmol)Min value at T (K) = 268.15Max value at T (K) = 775Coefficients:ABCDE
1.130197E+080
1.3387E+083.9870E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 268.15Max value at T (K) = 628Coefficients:ABCDE
3.2893612.250581
2.1880E-012.3756E-017.7500E+022.8600E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 268.15Max value at T (K) = 628Coefficients:C1C2C3C4C5
6.448264E-07100576.3
1.4363E+02-1.8347E+04-1.6000E+013.0041E-186.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 298.15Max value at T (K) = 1500Coefficients:
394299.81049282
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 57
ABCDE
3.3200E+051.0420E+062.2884E+037.9840E+059.2350E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 268.15Max value at T (K) = 628Coefficients:ABCDE
527508.3844896
2.9100E+058.8200E+02
000
1.4.8 Linolenic Acid
Chemical Name: Linolenic Acid (9,12,15-Octadecatrienoic Acid)
Physical Properties ValueFormula C18H30O2
Physical State LiquidColor n/aOdor n/aSolubility in Water Insoluble Molecular Weight 278.435Critical Temperature (F) 944.33Critical Pressure (psia) 208.8543Critical Volume (ft3/lbmol) 17.13976Melting Point (F) 12.02Normal Boiling Point (F) 677.93IG Heat of formation (Btu/lbmol) -174118Specific gravity 60 F 0.92120871 Heat of vaporization (Btu/lbmol)Min value at T (K) = 262.05Max value at T (K) = 780Coefficients:ABCDE
1.158786E+080
1.3680E+084.0540E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 262.05Max value at T (K) = 780Coefficients:AB
3.3638880.9356537
2.2204E-012.3731E-01
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 58
CDE
7.8000E+022.8570E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 262.05Max value at T (K) = 780Coefficients:C1C2C3C4C5
1.014866E-071444430
1.4949E+02-1.8892E+04-1.6791E+013.2413E-186.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 300Max value at T (K) = 1200Coefficients:ABCDE
378008925310.7
2.6915E+058.7906E+051.6363E+036.2440E+057.4228E+02
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 390Max value at T (K) = 585Coefficients:ABCDE
586543795914.5
1.6780E+051.0737E+03
000
1.4.9 Arachidic Acid
Chemical Name: Eicosanoic (Arachidic Acid)
Physical Properties ValueFormula C20H40O2
Physical State CrystalsColor WhiteOdor None reportedSolubility in Water Practically insoluble in water Molecular Weight 312.536Critical Temperature (F) 1018.13Critical Pressure (psia) 179.8467Critical Volume (ft3/lbmol) 18.10086Melting Point (F) 167.54Normal Boiling Point (F) 746.6IG Heat of formation (Btu/lbmol) -349267.8
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 59
Specific gravity 60 F 0.88415841 Heat of vaporization (Btu/lbmol)Min value at T (K) = 348.23Max value at T (K) = 821Coefficients:ABCDE
1.078658E+08118190.2
1.3593E+084.1900E-01
000
2 Liquid Density (kmol/m3)Min value at T (K) = 348.23Max value at T (K) = 821Coefficients:ABCDE
2.6923750.8883711
2.2977E-012.5964E-018.2100E+023.4829E-01
3 Vapor Pressure (Pascal)Min value at T (K) = 348.23Max value at T (K) = 821Coefficients:C1C2C3C4C5
0.0025786241211789
2.4459E+02-2.6449E+04-2.9833E+015.9700E-186.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 300Max value at T (K) = 1500Coefficients:ABCDE
484021.91176239
3.5814E+056.7278E+057.2784E+024.4834E+051.9194E+03
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 348.23Max value at T (K) = 610.5Coefficients:ABCDE
715714.4967964.7
8.3000E+05-1.0640E+032.1130E+00
00
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 60
1.4.10 Water
Chemical Name: Water
Physical Properties ValueFormula H2OPhysical State LiquidColor Colorless-clear-water-whiteOdor OdorlessSolubility in Water n/a Molecular Weight 18.015Critical Temperature (F) 705.56Critical Pressure (psia) 3207.977Critical Volume (ft3/lbmol) 1.017076Melting Point (F) 32.00001Normal Boiling Point (F) 212IG Heat of formation (Btu/lbmol) -103963.5Specific gravity 60 F 11 Heat of vaporization (Btu/lbmol)Min value at T (K) = 273.16Max value at T (K) = 647.35Coefficients:ABCDE
4.473567E+070
5.2053E+073.1990E-01-2.1200E-012.5800E-01
02 Liquid Density (kmol/m3)Min value at T (K) = 273.16Max value at T (K) = 333.15Coefficients:ABCDE
55.5826154.70285
5.4590E+003.0542E-016.4713E+028.1000E-02
3 Vapor Pressure (Pascal)Min value at T (K) = 273.16Max value at T (K) = 647.13Coefficients:C1C2C3C4C5
15.1066193718E+07
7.2550E+01-7.2067E+03-7.1385E+004.0460E-062.0000E+00
4 Ideal gas Heat Capacity (J/kmol-K)Min value at T (K) = 100Max value at T (K) = 1500Coefficients:
3335947105.12
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 61
ABCDE
3.3359E+042.6798E+042.6093E+038.8880E+031.1676E+03
5 Liquid Heat Capacity (J/kmol-K)Min value at T (K) = 273.15Max value at T (K) = 533.15Coefficients:ABCDE
76150.5789393.9
2.7637E+05-2.0901E+038.1250E+00-1.4116E-029.3701E-06
General Equations
1 H =
2 =
3 P = exp
4 Y =
5 Y =
1.5 Review and Screening of Alternative Process
1.5.1 Process Description
Glycerol is produced from natural triglycerides found in fats and oils
including a variety of vegetable oil such as coconut oil, palm kernel oil, palm oil,
soybean oil, canola oil and sunflower oil as well as tallow using three processes such
as saponification, hydrolysis, and transesterification (methanolysis).
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 62
Figure 9: Summary of glycerine production routes
Figure 1.9: Summary of glycerine production routes
1.5.1.1 Transesterification process to produce glycerine
The Transesterification process is the reaction of a triglyceride (fat/oil) with
an alcohol to form esters and glycerol. A common product of the transesterification
process is methyl ester produced from oil reacted with methanol. Triglycerides can
readily be transesterification batch wise at atmospheric pressure and slightly
elevated temperature of approximately 60-70 ºC with excess methanol and in
presence of an alkaline catalyst. In most production methanol or ethanol is the
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 63
alcohol used (methanol produces methyl esters, ethanol produces ethyl esters) and is
base catalysed by either potassium or sodium hydroxide.
The figure below shows the chemical process for produced glycerine by
transesterification. The reaction between the fat or oil and the alcohol is a reversible
reaction and so the alcohol must be added in excess to drive the reaction towards the
right and ensure complete conversion. Actually transesterification of fat and oils is
the most commonly used process for the manufacture of methyl ester.
Figure 1.10: Chemical process for produced glycerine by
transesterification
1.5.1.2 Saponification process to produce glycerine
Saponification process is from soap manufacture which triglycerides are
boiled with caustic soda solution and salt. The fatty acid constituents of the fat
combined with soda to form soap, which obtained as an upper layer. The lower
aqueous layer, referred to as spent lye, contains glycerine, water, salt and unchanged
caustic. The spent lye resulting from current soap making processes generally
contain from 10-15% glycerine.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 64
Figure 1.11: Saponification process is from soap manufacture which
triglycerides is boiled with caustic soda solution and salt.
1.5.1.3 Fat splitting process to produce glycerine
In fat splitting or hydrolysis, vegetable or animal fats are split into fatty acid
and glycerine, merely by addition of water without added alkali of fat and oil. Two
early methods were the Twitchell and batch autoclave processes both require
catalyst. The most recent and the most common process are continuous with high
pressure. The splitting takes place at a high (55 Bar) pressure and temperature
(260C) in presence of water.
The fat splitting process employs highly innovative and advanced technology
for continuous fat splitting without a catalyst. The fat and water flow in opposite
directions, resulting in glycerine-water referred to as sweet water. The fatty acids are
withdrawn from the top of the column and the sweet water falls and is withdrawn
from the bottom. Sweet water from hydrolysis of fat contain up to 20% glycerine.
The sweet water is glycerine should be processed promptly after splitting to avoid
degradation and loss of glycerine by fermentation.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 65
Figure 1.12: Fat splitting process to produce glycerine
In order to produce glycerine either as main product or by-product, the three
processes explained above can be selected as alternative. Other than different
substances used in the processes, the properties of all three processes are also
different as shown in table 1 below.
Table 1.11: Properties of the processes
Transesterification Saponification Fat splitting
Type of operation - batch & continuous batch &
continuous
mean temperature
(°C)
approx. 213 - 223.3 78.5 - 95.5 248.9 - 254.4
mean pressure
(psi)
approx. 19.9 - 36.6 16.5 - 18.7 683.9 - 721.7
mean residence
times (min)
220 1556.8 - 1912.3 220 - 240
Chemicals
employed
Alcohol, sodium
hydroxide
NaOH, KOH,
NaCl, Na2O,
Bleach
Optional
1.5.2 Economic Potential
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 66
Table 1.12: Prices of materials
Raw material / product Price (RM/kmole)
Water, H2O 0.02
Methanol 80.23
Natrium hydroxide 28.025
CPO 641.91
Glycerine 1473.52
Economic potential of the processes are calculated as below:
EP = Cost of Products – Cost of Raw Materials
Transesterification process:
Cost (glycerine) – Cost (methanol + CPO )
= RM 1473.52 – RM (3 x 80.23 + 641.91)
=RM 590.65
Saponification process:
Cost (glycerine) – Cost ( NaOH + CPO)
=RM 1473.52 – RM(28.025 + 641.91)
=RM 803.585
Fat splitting process:
Cost (glycerine) – Cost ( CPO + H2O)
=RM (1473.52)-RM(641.91 + 3 x 0.02)
=RM 831.55
1.5.3 Advantages and disadvantages of the alternatives
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 67
The three alternative processes which are transesterification, saponification,
and fat splitting are compared as shown in table 3 below.
Table 1.13: Advantages and disadvantages of alternative process
Process Advantages Disadvantages EP
Transesterification Low operating
temperature and
pressure
Low operating
cost
Usually glycerine
are not remove
from main
product: methyl
ester
Catalyst needed
The free fatty
acid in vegetable
oil can damage
the catalyst easily
Low conversion
and purity of
glycerine
RM 590.65
Saponification Widely used in
manufacture
soap
Lower purity than
fat splitting
process, 10-15%
Lower quality of
crude glycerine-
more purification
process needed
RM
803.585
Fat splitting High purity of
gycerine up to
20%
Better quality of
crude glycerine:
little or no salt
content (less
purification
High operating
temperature and
pressure
RM 831.55
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 68
process)
Less chemical
used
Low cost
Continuous
process catalyst
is not necessary
1.5.4 Advantages of continuous process over batch and Twitchell process
In order to make a good decision in choosing the right route, there are
several criteria that must be considered in selecting the correct production process.
Each potential route is studied and reviewed thoroughly. Some of the essential
considerations are stated as below:-
i. Maximum economic potential:
Choosing a process with high economic potential will determine the
profitable of the plant.
ii. Type of reaction and its condition:
Always consider the simplest reaction. The reaction that can be carried out
around ambient condition is more preferable.
iii. Process safety:
A safety process is more attractive because it can make sure the plant safety
always under control.
iv. Availability and cost of raw material:
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 69
A process with cheaper and long-term available raw material can maintain
the profit and the production of the plant. There is an advantage where the
raw materials are locally available.
v. Yields of a process:
A process with high yields of desired product is more attractive to ensure the
high production of the plant.
vi. Environmental friendly:
All the chemicals involve in the process should be environmental friendly.
Only the chemicals that free of toxic being choose in the process.
Based on the criteria mentioned above, the advantages and disadvantages of
all potential processes are studied. Comparison is made in between batch and
continuous processes for fat splitting process. The advantages of each type of
processing method are summarized in table 1.14.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 70
Table 1.14 : Summary Of Fat Splitting Process
TYPE OF FAT SPLITTING PROCESS
Characteristics Twitchell Batch autoclave Continuous countercurrent
Temperature (°F) 212 – 220 300 – 350 450 485 approx
Pressure (psig) - 75 – 150 425 – 450 600 – 700
Catalyst Alkyl-aryl sulphonic acids or
cycloaliphatic sulphonic acids, both
used with sulphuric acid 0.75-1.25%
of the charge.
Zinc, calcium,
barium or
magnesium
oxides 1-2%
No catalyst Optional.
Time (hr) 24-48 5-10 2-3 Less than 2
Operation Batch Batch Continuous
Equipment Lead-lined, copper-lined, Monel-line,
or wooden tank.
Copper or stainless-steel autoclave Type 316 stainless tower
Hydrolysed 85-98% hydrolysed.
5-15% glycerol solution obtained,
depending on number of stages and
type of fat.
85-98% hydrolysed.
10-15% glycerol, depending on
number of stages and type of fat.
97-99% hydrolysed
10-25% glycerol depending on
type of fat.
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 71
Screening
criteriaTwitchell Batch autoclave Continuous countercurrent
Economic
potential
RM 266.96 RM 259.61 RM 323.09
Reaction
condition
Reaction occurs at low temperature
(212-220°F) and atmospheric
pressure.
Reaction occurs at higher
temperature (300-450°F) and high
pressure (75-450 psig).
Reaction occurs at highest
temperature (485°F) and highest
pressure (600-700psig).
Process
duration
24-48 hours 2-10 hours Less than 2 hours
Conversion
85-98% hydrolysed 85-98% hydrolysed 97-99% hydrolysed
Purity yield
5-10% glycerol obtained 10-15% glycerol obtained 10-25% glycerol obtained
Gly World (M) Sdn. Bhd
1
23 1
1 2 3
1 21
1 2 3
32

Chapter I– Project Conception - Literature Survey I- 72
Catalyst
Alkyl-aryl sulphonic acids,
cycloaliphatic sulphonic acids and
sulphuric acid 0.75-1.25%.
Zinc, calcium, barium and
magnesium oxides 1-2% or optional.
No catalyst needed.
Handling of
catalyst
Required treatment and removal of
catalyst used.
Required treatment and removal of
catalyst used
Not required as no catalyst used.
Equipment
Require acid and corrosive resistant
equipment.
Require corrosive resistant
equipment.
Not necessary.
Effects on
environment
Emission of noxious and acidic fumes. Emission of noxious and acidic
fumes.
No noxious and acidic fumes are
emitted
Initial cost
Lowest first cost because of relatively
simple and inexpensive equipment.
Low first cost for small scale
compare to continuous process.
High first cost for large scale
production.
Gly World (M) Sdn. Bhd
21 1
1 2 3
1 21
11 2
23 1

Chapter I– Project Conception - Literature Survey I- 73
Product quality
Good quality CPO provides fatty acids
of good colour.
Produces lighter colour fatty acids. Produces best and uniform
product quality with lighter
colour fatty acids.
Raw material
Additional cost needed for catalyst
used.
Additional cost needed for catalyst
used.
Less raw material cost as no
catalyst used.
Others Adaptable to small scale
production as it is batch basis
Fat stocks of poor quality must
often be acid refined to avoid
catalyst poisoning
High steam consumption
Tendency to form dark colour
fatty acids
Need more than one stages for
good yield and high
concentration
Not adaptable to automatic
control
Adaptable to small scale
production as it is batch basis
Rapid cycle time which
minimized polymerization
Can be conducted with or
without catalyst
Not adaptable to automatic
control as continuous process
High labour cost
Need more than one stages
for good yield and high
concentration
Greater operating skill
Gly World (M) Sdn. Bhd
21 3
1 1 2

Chapter I– Project Conception - Literature Survey I- 74
A lot of boiling cycles that
consume energy and human
power
Glycerine produced is more
difficult to purify due to the
presence of acid and catalyst in
sweet water
Total points 17 18 27
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 75
1.5.5 Economic Potential
Table 1.15: Local prices of materials
Material Molecular weight Price (RM/tonne)
Palmitic Acids 256.43 1315.40
Stearic Acids 284.48 1399.24
Oleic Acids 282.46 3018.25
Glycerine 92.09 2918.84
Fats (RBD Palm Oil) 846.363 1680.00
Sulphuric Acid 98.08 2073.40
Zinc oxide 81.38 7049.70
Water 18.016 1.20
Assumption: The utility used such as steam is not considered in economic potential
calculation.
C3H5(OOCR)3 + 3H2O 3RCO2H + C3H5(OH)3
C3H5(OOCR)3 x (846.363 kg/kmol) + 3H2O x (18.016 kg/kmol)
3RCO2H x (269.454 kg/kmol) + C3H5(OH)3 x (92.09 kg/kmol)
846.363 kg C3H5(OOCR)3 + 54.048 kg H2O
808.362 kg RCO2H + 92.09 kg C3H5(OH)3
In 808.362 kg Fatty Acids = 808.362 (0.441 PA + 0.045 SA + 0.392 OA)
= 356.4876 kg PA + 36.3763 kg SA + 316.8779 kg
OA
846.363 kg C3H5(OOCR)3 + 54.048 kg H2O
356.4876 kg PA + 36.3763 kg SA + 316.8779 kg OA + 92.09 kg C3H5(OH)3
0.846363 tonne C3H5(OOCR)3 + 0.054048 tonne H2O
0.3564876 tonne PA + 0.0363763 tonne SA + 0.3168779 tonne OA +
0.09209 tonne C3H5(OH)3
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 76
Price of Fatty Acids = RM 468.92 PA + RM 50.90 SA + RM 956.42 OA
= RM 1476.24
Price of Glycerine = RM 268.80
Price of Palm Oil = RM 1421.89
Price of water = RM 0.06
1.5.5.1 Economic Potential for Twitchell Process
H2SO4
C3H5(OOCR)3 + 3H2O 3RCO2H + C3H5(OH)3
Raw material : Palm Oil, water and 1% Sulphuric Acids ( 3 cycles of water and
sulphuric acids)
Products : Fatty Acids and Glycerine
Price of Sulphuric Acids = 0.01 x 0.900411 tonne x RM 2073.40
= RM 18.67
EP = Products – Raw Materials
= (RM 1476.24 + RM 268.80) – [RM 1421.89 + 3 (RM 0.06 + RM
18.67)]
= RM 266.96
1.5.5.2 Economic Potential for Batch Autoclave Splitting
ZnOC3H5(OOCR)3 + 3H2O 3RCO2H + C3H5(OH)3
Raw material : Palm Oil, water and 1% Zinc Oxide
Products : Fatty Acids and Glycerine
Price of Zinc Oxide = 0.01 x 0.900411 x RM 7049.70
= RM 63.48
EP = Products – Raw Materials
= (RM 1476.24 + RM 268.80) – (RM 1421.89 + RM 0.06 + RM 63.48)
= RM 259.61
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 77
1.5.5.3 Economic Potential for Continuous Counter-current Splitting
C3H5(OOCR)3 + 3H2O 3RCO2H + C3H5(OH)3
Raw material : Palm Oil and water
Products : Fatty Acids and Glycerine
EP = Products – Raw Materials
= (RM 1476.24 + RM 268.80) – (RM 1421.89 + RM 0.06)
= RM 323.09
1.5.6 Process Screening Conclusion
Detail screening has been carried out on the processes mainly base on
economic potential, process safety, quality of the products, effects on environment,
time and energy consumption. Decision has been achieved to choose fat splitting
process as our alternative process to produce glycerine from crude palm oil. This is
base on the higher economic potential, higher products quality and less chemical
usage compare to the other two processes which are transesterification process and
saponification process.
From the fat splitting process, further screening has been done on the three
types of hydrolysis methods which are Twitchell Process, batch autoclave process
and continuous countercurrent process. After detail research, our group has decided
to use continuous countercurrent process as our alternative to produce glycerine
because the process excels in most part of our screening criteria. Below are the
advantages of continuous countercurrent process compare to the other two
processes:
Higher economic potential
Short reaction time
More quality products
Higher conversion and purity yield
Uniform product quality and lighter colour fatty acids
No noxious and acidic fumes are emitted
Gly World (M) Sdn. Bhd

Chapter I– Project Conception - Literature Survey I- 78
No need catalyst
No acid or corrosive resistant equipments are needed
No acid wash or catalyst treatment are required
Higher yield of products
Suitable for large scale production
Gly World (M) Sdn. Bhd
Related Documents