CASE REPORT SECONDARY GLAUCOMA ET CAUSA TRAUMATIC CATARACT AND ANTERIOR UVEITIS Created by: Sri Nowo Minarti I11110042 SMF MATA RSUD DOKTER SOEDARSO MEDICAL FACULTY TANJUNGPURA UNIVERSITY

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
CASE REPORT
SECONDARY GLAUCOMA ET CAUSA TRAUMATIC CATARACT AND ANTERIOR UVEITIS
Created by:Sri Nowo MinartiI11110042
SMF MATA RSUD DOKTER Soedarso Medical facultyTANJUNGPURA UNIVERSITYPontianak 2014
AGREEMENT SHEET
Shall been agreed a case report with a title
SECONDARY GLAUCOMA ET CAUSA TRAUMATIC CATARACT AND ANTERIOR UVEITIS
This working paper was arranged to fulfill the requirement ofOpthalmology Module Clinical Assistant
Agreed by,Pontianak, September 2014Consulent,
dr. Liesa Zulhidya, Sp.M NIP. 196703121993092001
Arranged by :
Sri Nowo MinartiNIM. I11110042
CHAPTER IINTRODUCTION
Ocular trauma is a common cause of unilateral blindness in children and young adults; persons in these age groups sustain the majority of severe ocular injuries. Young adults, especially men, are the most likely victims of penetrating ocular injuries. Domestic accidents, violent assaults, exploding batteries, sports-related injuries, and motor vehicle accidents are the most common circumstances in which ocular trauma occurs. Severe ocular trauma may cause multiple injuries to the lids, globe, and orbital soft tissues (Eva and Whitcher, 2007). Open-globe injuries or penetrating injuries are the most devastating forms of ocular trauma. They are caused by sharp objects that penetrate the cornea and sclera into deeper structure (Lang, 2006). These damage can induce occurence of some sequelae, such as secondary glaucoma, traumatic cataract, uveitis, and corneal ulcer, which affecting the visual acuity (Eva and Whitcher, 2007). Glaucoma refers to certain eye diseases that affect the optic nerve and cause vision loss. Many factors are associated with an increased risk of developing glaucoma, some of which are elevated IOP, a family history, ethnic background, and older age. Most, but not all, of these diseases typically produce elevated pressure inside the eye, called intraocular pressure (IOP). An elevated IOP is the most important risk factor for the development of glaucoma. Secondary glaucoma refers to an increased IOP that is a result of a structural problem within the eye. Secondary glaucoma is a type of glaucoma which is caused by other ocular diseases or factors such as inflammation, trauma, bleeding, tumors, medication, and physical or chemical influences (Lang, 2006). This form of glaucoma is different because treatment is aimed at treating the underlying cause as well as lowering the increased pressure within the eye.Uveitis is one of the major causes of blindness in the world. Uveitis is composed of a diverse group of disease entities, which in total has been estimated to cause approximately 10% of blindness. Uveitis is broadly classified into anterior, intermediate, posterior and panuveitis based on the anatomical involvement of the eye. (Agrawal et al, 2010).Uveitis has many subtypes and many potential associations with systemic conditions and has always been one of the most challenging diagnoses in ophthalmology (Babu; Rathinam, 2010). Anterior uveitis is the commonest form of intraocular inflammation with a varying incidence in the general population of various countries around the world. The potential severe consequences of recurrent or untreated anterior uveitis are probably underestimated. Anatomically, anterior uveitis involves inflammation of the iris alone (iritis), anterior part of ciliary body (anterior cyclitis) or both structures (iridocyclitis) (Agrawal et al, 2010). Anterior uveitis can caused by trauma, chronic diarrhea, Reiter disease, herpes simplex, Behcet syndrome, Posner Schlosman syndrome, post-operative, adenovirus, parotitis, influenza, chlamydial infection, rheumathoid arthritis and etc. (Ilyas S, 2007)Traumatic cataract can occur by penetrating eye injury or blunt trauma that can be seen in days or years (Ilyas, 2007). The lens becomes white soon after the entry of a foreign body, since interruption of the lens capsule allows aqueous and sometimes vitreous to penetrate into the lens structure. (Vaughan and Asburys, 2007).
CHAPTER IILITERATURE REVIEW
2.1. Penetrating Injuries of the EyePenetrating injuries cover the entire spectrum of clinical syndromes. Symptoms can range from massive penetration of the cornea and sclera with loss of the anterior chamber to tiny, nearly invisible injuries that close spontaneously. The latter may include a fine penetrating wound or the entry wound of a foreign body. Depending on the severity of the injury, the patients visual acuity may be severely compromised or not influenced at all. One of the most common sequelae is atraumatic cataract. The rupture in the lens capsule allows aqueous humor to penetrate, causing the lens to swell. This results in lens opacification of varying severity. Large defects will lead to total opacification of the lens within hours or a few days. Smaller defects that close spontaneously often cause a circumscribed opacity. Typically, penetration results in a rosette-shaped anterior or posterior subcapsular opacity (Lang, 2006). Depending on the severity of the injury, the followingdiagnostic signs will be present in an open-globe injury: a. The anterior chamber will be shallow or absent.b. The pupil will be displaced toward the penetration site.c. Swelling of the lens will be present (traumatic cataract).d. There will be bleeding in the anterior chamber and vitreous body.e. Hypotonia of the globe will be present.The rupture of the lens capsule and vitreous hemorrhage often render examination difficult as they prevent direct inspection. These cases, and any patient whose history suggests an intraocular foreign body, require one or both of the following diagnostic imaging studies:a. Radiographs in two planes to determine whether there is a foreign body in the eye.b. CT studies, which allow precise localization of the foreign body and can also image radiolucent foreign bodies such as Plexiglas.TreatmentFirst aid.Where penetrating trauma is suspected, a sterile bandage should be applied and the patient referred to an eye clinic for treatment. Tetanus immunization or prophylaxis and prophylactic antibiotic treatment are indicated as a matter of course. Surgery.Surgical treatment of penetrating injuries must include suturing the globe and reconstructing the anterior chamber. Any extruded intraocular tissue (such as the iris) must be removed. Intraocular foreign bodies should be removed when the wound is repaired (i.e., by vitrectomy and extraction of the foreign body).Late sequelaea. Improper reconstruction of the anterior chamber may lead to adhesions between the iris and the angle of the anterior chamber, resulting in secondary angle closure glaucoma. b. A retinal injury (for example at the site of the impact of the foreign body) can lead to retinal detachment. c. Failure to remove iron foreign bodies can lead to ocular siderosis, which causes irreparable damage to the receptors and may manifest itself years later. d. Copper foreign bodiescause severe inflammatory reactions in the eye (ocular chalcosis) within a few hours. Symptoms range from uveitis and hypopyon to phthisis bulbi (shrinkage and hypotonia of the eyeball). e. Organic foreign bodies(such as wood) in the eye lead to fulminant endophthalmitis.
2.2. Glaucomaa. DefinitionGlaucoma is a disorder in which increased intraocular pressure damages the optic nerve. This eventually leads to blindness in the affected eye. Primary glaucoma refers to glaucoma that is not caused by other ocular disorders. Secondary glaucoma may occur as the result of another ocular disorder or an undesired side effect of medication or other therapy.Two major forms of glaucoma exist: open-angle glaucoma, in which aqueous humor has free access to the trabecular meshwork, and angle-closure glaucoma, in which access of the aqueous humor to the trabecular meshwork is obstructed. Both forms of glaucoma demonstrate a progressive optic neuropathy with visual field loss and characteristic structural changes, including thinning of the retinal nerve fiber layer and excavation of the optic nerve head. Intraocular pressure (IOP) does not define glaucoma, and many with glaucoma have IOP measurements that are also found in individuals without glaucoma.b. EpidemiologyGlaucoma is the second leading cause of blindness worldwide. The frequency of bilateral blindness amongst persons with glaucoma varies across populations, with substantial bilateral blindness from glaucoma observed in developing countries with poor access to eye care, and in populations where angle-closure glaucoma predominates. Combining demographic projections with prevalence models for open-angle and angle-closure glaucoma, it has been estimated that 61 million people worldwide will have glaucoma by 2010, and 8.4 million will be bilaterally blind from the disease . Growth and aging of the worlds population are expected to result in significant increases in these numbers. While PACG remains less common than POAG, the numbers of individuals expected to be blind from both types of glaucoma is nearly equal given the higher morbidity of PACG.Population-based studies with low rates of pseudoexfoliation report rates of secondary glaucoma (resulting from pseudoexfoliation, vein occlusions, diabetes, uveitis, trauma and other causes) between 0.15% and 0.7%, with secondary glaucoma comprising 418% of all glaucoma cases. Whether or not pseudoexfoliation glaucoma is a primary or secondary form of glaucoma depends on definition, and researchers vary in how they present their data. Secondary glaucomas other than pseudoexfoliation occur infrequently. In populations where pseudoexfoliation is found, its prevalence varies substantially, with the highest rates found in Scandinavian populations. People with pseudoexfoliation have substantially higher mean intraocular pressures, and have a significantly higher risk of glaucoma.
c. PathophysiologyThe average normal intraocular pressure of 15mm Hg in adults is significantly higher than the average tissue pressure in almost every other organ in the body. Such a high pressure is important for the optical imaging and helps to ensure several things:1) Uniformly smooth curvature of the surface of the cornea.2) Constant distance between the cornea, lens, and retina.3) Uniform alignment of the photoreceptors of the retina and the pigmented epithelium on Bruchs membrane, which is normally taut and smooth.The aqueous humor is formed by the ciliary processes and secreted into the posterior chamber of the eye. At a rate of about 26 l per minute and a total anterior and posterior chamber volume of about 0.20.4ml, about 12% of the aqueous humor is replaced each minute. The aqueous humor passes through the pupil into the anterior chamber. As the iris lies flat along the anterior surface of the lens, the aqueous humor cannot overcome this pupillary resistance until sufficient pressure has built up to lift the iris off the surface of the lens. Therefore, the flow of the aqueous humor from the posterior chamber into the anterior chamber is not continuous but pulsatile.Any increase in the resistance to pupillary outflow (pupillary block) leads to an increase in the pressure in the posterior chamber; the iris inflates anteriorly on its root like a sail and presses against the trabecular meshwork. This is the pathogenesis of angle closure glaucoma.Various factors can increase the resistance to pupillary outflow.
The aqueous humor flows out of the angle of the anterior chamber through two channels:1) The trabecular meshwork (Fig. 10.1 [C]) receives about 85% of the outflow, which then drains into the canal of Schlemm. From here it is conducted by 2030 radial collecting channels into the episcleral venous plexus (D).2) A uveoscleral vascular system receives about 15% of the outflow, which joins the venous blood (E).3) The trabecular meshwork (C) is the second source of physiologic resistance.4) The trabecular meshwork is a body of loose sponge-like avascular tissue between the scleral spur and Schwalbes line. Increased resistance in present in open angle glaucoma.
d. Classification
e. Glaucoma Associated With Ocular Trauma1) Definition Glaucomatous damage to the optic nerve related to elevated intraocular pressure associated with acute or prior ocular trauma.2) EpidemiologyGlaucoma is a common complication following ocular trauma. It can present immediately following injury or even years or decades later. There exists a plethora of potential causes of glaucoma following ocular trauma. It is important to be familiar with the various types of glaucoma in this setting as well as their pathogenesis. Recent cohort studies examining the relationship of glaucoma following ocular injury to several baseline structural and functional ocular characteristics are now available. The risk of developing glaucoma in 3627 patients in the United States Eye Injury Registry with penetrating ocular injury was 2.67%. The development of glaucoma in these patients was independently associated with advancing age, lens injury, poor baseline acuity, and inflammation.In a similar study, 6021 patients in the registry who experienced ocular contusion injury were found to have a 3.4% risk of developing glaucoma at 6 months after their injury. The development of glaucoma was independently associated with: advancing age, visual acuity worse than 20/200, iris injury, lens injury, or angle recession.A smaller prospective review of 100 consecutive patients with traumatic glaucoma in India were found to have a greater risk of postconcussional glaucoma associated with traumatic cataracts, angle recession of more than 180, significant iris injury, and displacement of the lens. Penetrating injuries were more likely to result in glaucoma if there was evidence of an adherent leucoma and/or evidence of lenticular damage or displacement..
3) PathophysiologyIn the initial period following an ocular injury, the intraocular pressure may be normal, high, or low. Several mechanisms exist to explain a low pressure. These mechanisms include aqueous hyposecretion based on ciliary contusion and inflammation, increased egress of aqueous through a cyclodialysis cleft, or loss of integrity of the wall of the globe. The presence of ocular hypotension or normal intraocular pressure does not preclude the development of glaucoma at a later date. Whether glaucoma is present initially or at a later date, it is generally a reflection of reduced facility of outflow of aqueous humor. One may categorize the existence of traumatic glaucoma relative to the time of onset of the glaucoma (immediate or delayed) and the type of trauma that caused the injury. The type of trauma may be divided into blunt force trauma or penetrating trauma. A broader classification would include chemicals, electromagnetic radiation, and surgery as additional causes of trauma that might induce glaucoma. We must also remind ourselves that glaucoma may also occur as a result of the therapeutic modalities.
4) Immediate or Early-Onset Glaucoma After TraumaContusionIntraocular pressure elevation in the setting of blunt trauma with a notable absence of clinical evidence of tissue damage may be noted on occasion. Gonioscopy is entirely normal with no evidence of angle recession and there is no evidence of blood or abnormal pigment in the angle. Flare and cells may be evident at the slit lamp. The presumed mechanism of this type of glaucoma is reduced outflow facility as a result of trabecular inflammation. The course of this glaucoma is usually brief and self limited although a trial of topical anti-inflammatory drops in addition to any intraocular pressure-lowering agents may hasten improvement and shorten the clinical course.
Trabecular DisruptionEvidence of trauma-related changes to the trabecular meshwork has been previously documented in a study utilizing gonioscopy within the first 48 h following injury. Documented abnormalities ranged from sharply demarcated hemorrhage into Schlemms canal and possibly the outer trabecular sheets to full-thickness rupture of the trabecular meshwork for part of its circumference. A trabecular flap may be created with a point of rupture at or just below the insertion of the trabecular sheets at Schwalbes line. This flap is typically hinged in the region of the scleral spur. Lesions such as these at the trabecular meshwork may or may not be associated with elevated intraocular pressure at the time of injury. Trabecular lesions may scar with time and become increasingly difficult to recognize over time. Although angle recession is associated with the late development of glaucoma, the occurrence of late glaucoma may correlate better with the amount of trabecular disruption observed acutely.
HyphemaThe presence of hyphema following ocular trauma is an indicator of significant intraocular injury. Cho et al. compared the clinical characteristics of 18 patients with very poor visual outcome after nonperforating hyphema to 166 patients with better visual outcome after nonperforating hyphema. The presence of posterior segment injuries, anterior segment injuries, poor initial visual acuity, glaucoma, vitreous hemorrhage, and eyelid laceration were all associated with long-term poor visual outcome. Hyphema may produce glaucoma via several mechanisms including contusion/inflammation of the trabecular meshwork, physical disruption of the meshwork, and plugging of the meshwork with red blood cells. In addition, a large clot in the anterior chamber may even produce pupillary block by entirely occluding the pupillary aperture.Pressure elevation in association with hyphema may threaten vision as a result of optic nerve damage, compromised blood flow to the posterior segment, or corneal blood staining. Intraocular pressure elevation occurs in up to 27% of patients acutely; however, this pressure elevation is often mild and self-limited.The duration and level of intraocular pressure required to damage the optic nerve for a given individual is difficult to determine. Read and Goldberg studied 137 hyphema patients prospectively and determined that optic atrophy tended to occur with intraocular pressures at or greater than 35 mmHg with durations varying from 5 to 14 days. Optic atrophy as a direct result of the trauma itself may be a confounding factor in such studies.Corneal blood staining occurs more readily in the presence of an intraocular pressure greater than 25 mmHg that has persisted for at least 6 days. If the corneal endothelial cells are already compromised as a result of the trauma itself or pre-existing disease, the risk of staining with only marginal pressure elevation is even greater.Rebleeding into the anterior chamber can be a devastating complication that typically occurs between days 2 and 6 following the initial injury. The reported incidence of rebleeding is somewhere between 6% and 33% based on several studies. Markedly elevated intraocular pressure and its attendant complications are a particular concern with rebleeding. Aminocaproic acid decreases the rate of rebleeding in some patients. Systemic corticosteroids have been recommended by some to reduce the incidence of rebleeding. However, Spoor and associates showed no benefit from oral corticosteroids in a prospective study. Although aminocaproic acid may reduce the incidence of rebleeding in some patients, exaggerated clot lysis in patients with larger hyphemas may develop 1-2 days following therapy discontinuation with associated acute pressure elevation as a result of lysed cells and debris obstructing outflow. This and the fact that aminocaproic acid may have systemic side effects including nausea and vomiting as well as the relatively low incidence of rebleeds overall may account for why some clinicians choose not to employ this drug.Acute pressure elevation in the setting of hyphema may be treated with conventional pharmacologic agents, with the exception of miotic agents and prostaglandin agents. Both of these agents may excacerbate any inflammation that is already present and so they are not generally used as first-line agents. Cycloplegic agents and topical corticosteroids are often employed in the treatment of any associated inflammation following hyphema. The potential for either topical or systemic steroids to produce intraocular pressure elevation with more chronic use must be kept in mind.If the intraocular pressure remains elevated at a level that threatens the optic nerve or the cornea in spite of medical therapy, then surgical intervention may be necessary. Many surgical procedures have been reported in the literature including anterior chamber washout, mechanical clot expression, delivery of the clot with a cryoprobe, automated hyphemectomy, and ultrasonic emulsification and aspiration of the clot. Adjunctive procedures may include peripheral iridectomy to relieve clot-induced papillary block. Trabeculectomy has been used to achieve pressure normalization. Cyclodiathermy to control recurrent bleeding has also been described.Paracentesis and anterior chamber washout is the simplest and safest procedure for clot evacuation. This can be performed by simple irrigation or by manual coaxial irrigation and aspiration. Removal of the entire clot may not be necessary. This technique also spares the conjunctiva for future filtration surgery if it becomes required.
2.3. Traumatic CataractThe incidence of these lens opacities is higher in men than in women due to occupational and sports injuries (Lang, 2006). Traumatic cataract can occur by penetrating eye injury or blunt trauma that can be seen in days or years (Ilyas S, 2007). The lens becomes white soon after the entry of a foreign body, since interruption of the lens capsule allows aqueous and sometimes vitreous to penetrate into the lens structure (Eva and Whitcher, 2007).Blunt trauma does not result in rupture of the capsule, may cause an anterior and/or posterior subcapsular cataract or both. Initially, fluid influx causes swelling and thickening of the lens fibers. Later the fibers become less swollen; the anterior subcapsular region whitens and may develop a characteristic flower-shaped pattern, or an amorphous or punctate opacity (Yanoff, 2009). Small perforation caused by penetrating injury will close immediately because of ephitelial proliferation so the opacity only in the small area (Ilyas, 2007). A small capsular penetrating injury may result in a localized lens opacity. A larger rupture results in rapid hydration and complete opacification. Penetrating injuries can be caused by accidental or surgical trauma such as a peripheral iridectomy or during a vitrectomy. (Yanoff, 2009)
Traumatic Cataract
Traumatic cataract and iridodialysis Concussion cataract occurs mainly due to imbibition of aqueous and partly due to direct mechanical effects of the injury on lens fibres. It may assume any of the following shapes (Khurana, 2007):a. Discrete subepithelial opacities are of most common occurrence.b. Early rosette cataract (punctate). It is the most typical form of concussion cataract. It appears as feathery lines of opacities along the star-shaped suture lines; usually in the posterior cortex.c. Late rosette cataract. It develops in the posterior cortex 1 to 2 years after the injury. Its sutural extensions are shorter and more compact than the early rosette cataract.d. Traumatic zonular cataract. It may also occur in some cases, though rarely.e. Diffuse (total) concussion cataract. It is of frequent occurrence.f. Early maturation of senile cataract may follow blunt truma.
2.4. Uveitisa. DefinitionThe term "uveitis" denotes inflammation of the iris (iritis, iridocyclitis), ciliary body (intermediate uveitis, cyclitis, peripheral uveitis, or pars planitis), or choroid (choroiditis). Common usage, however, includes inflammation of the retina (retinitis), retinal vasculature (retinal vasculitis), and intraocular optic nerve (papillitis). Uveitis may also occur secondary to inflammation of the cornea (keratitis), sclera (scleritis), or both (Riordan-Eva; Whitcher, 2007)b. EpidemiologyUveitis usually affects people 2050 years of age and accounts for 1020% of cases of legal blindness in developed countries. Uveitis is more common in the developing world than in the developed countries, due in large part to the greater prevalence of infections that can affect the eye, such as toxoplasmosis and tuberculosis. (Riordan-Eva; Whitcher, 2007).Large series of uveitis patients show variation in terms of the relative prevalence of different forms of uveitis. In surveys of patients referred to tertiary centers, anterior uveitis has been shown to account for 28-66% of cases, intermediate uveitis for 5-15%, posterior uveitis for 19-51%, and panuveitis for 7-18%. (Yanoff, 2009)c. EtiologyUveitis can caused by trauma, chronic diarrhea, Reiter disease, herpes simplex, Behcet syndrome, Posner Schlosman syndrome, post-operative, adenovirus, parotitis, influenza, chlamydial infection, rheumathoid arthritis and etc. (Ilyas S, 2007). Traumatic uveitis is often seen in accidental or operative injuries to the uveal tissue. Different mechanisms which may produce uveitis following trauma include (Khurana, 2007):1) Direct mechanical effects of trauma.2) Irritative effects of blood products after intraocular haemorrhage 3) Microbial invasion.4) Chemical effects of retained intraocular foreign bodies5) Sympathetic ophthalmia in the other eye.d. ClassificationDepend on the anatomical location, uveitis can be classified as:Anatomical Classification of Uveitis TypePrimary site of inflammationInclude
Anterior uveitisAnterior chamberIritis, Iridocyclitis, Anterior cyclitis
Intermediate uveitisVitreousPars planitis, Posterior cyclitis, Hyalitis
Posterior uveitisRetina or choroidFocal, multifocal, or diffuse choroiditis, Chorioretinitis, Retinochoroiditis, Retinitis, Neuroretinitis
PanuveitisAnterior chamber, vitreous, and retina or choroid
Anatomical Classification of UveitisAcute uveitis describes the course of a specific uveitis syndrome characterized by sudden onset and limited duration. Chronic uveitis describes persistent inflammation characterized by prompt relapse (in less than 3 months) after discontinuation of therapy. Recurrent uveitis is characterized by repeated episodes of uveitis separated by periods of inactivity without treatment lasting at least 3 months. (Kanski, 2011)e. Occular ManifestationThe clinical manifestations of uveitis vary depending on several factors - the primary site of involvement in the eye, the course of the inflammatory process (e.g., acute or chronic), and the presence of secondary complications arising from the uveitis itself. (Yanoff, 2009)The symptoms of acute anterior uveitis (e.g., human leukocyte antigen HLA-B27-related entities, such as ankylosing spondylitis) generally include pain, redness, photophobia, and blurred vision, which typically develop over a period of hours or days. On the other hand, patients who have chronic anterior uveitis, such as that seen with JIA or Fuchs heterochromic iridocyclitis, may present merely with blurring of vision or mild redness, with little pain or photophobia. Patients who have intermediate or posterior uveitis typically present with floaters or impaired vision secondary to cystoid macular edema or chorioretinal involvement. Patients who have panuveitis may present with any or all of these symptoms. (Yanoff, 2009)f. Examination MethodThe slit lamp is used to examine the surface of the iris under a focused beam of light. Normally no vessels will be visible. Iris vessels are only visible in atrophy of the iris, inflammation, or as neovascularization in rubeosis iridis.Where vessels are present, they can be visualized by iris angiography after intravenous injection of fluorescein sodium dye. Defects in the pigmented layer of the iris appear red under retroillumination with a slit lamp. Slit lamp biomicroscopy visualizes individualcells such as melanin cells at 40-power magnification. The anterior chamber is normally transparent. Inflammation can increase the permeability of the vessels of the iris and compromise the barrier between blood and aqueous humor. The pigmented epithelium of the retina permits only limited evaluation ofthe choroid by ophthalmoscopy and fluorescein angiography or indocyaninegreen angiography. Changes in the choroid such as tumors or hemangiomascan be visualized by ultrasound examination. (Lang, 2006)g. Pathology of UveitisInflammation of the uvea fundamentally has the same characteristics as any other tissue of the body, i.e, a vascular and a cellular response. However, due to extreme vascularity and looseness of the uveal tissue, the inflammatory responses are exaggerated and thus produce special results. (Khurana, 2007)Pathologically, inflammations of the uveal tract may be divided into suppurative (purulent) and nonsuppurative (non-purulent) varieties. Wood has further classified non-suppurative uveitis into a nongranulomatous and granulomatous types. (Khurana, 2007)Purulent inflammation of the uvea is usually a part of endophthalmitis or panophthalmitis occurring as a result, of exogenous infection by pyogenic organisms which include staphylococcus, streptococcus, psuedomonas, pneumococcus and gonococcus. The pathological reaction is characterised by an outpouring of purulent exudate and infiltration by polymorphonuclear cells of uveal tissue, anterior chamber, posterior chamber and vitreous cavity. As a result, the whole uveal tissue is thickened and necrotic and the cavities of eye become filled with pus. (Khurana, 2007) The pathological alterations of the nongranulomatous reaction consists of marked dilatation and increased permeability of vessels, breakdown of blood aqueous barrier with an outpouring of fibrinous exudate and infiltration by lymphocytes, plasma cells and large macrophages of the uveal tissue, anterior chamber, posterior chamber and vitreous cavity. The inflammation is usually diffuse. As a result of these pathological reactions iris becomes waterlogged, oedematous, muddy with blurring of crypts and furrows. As a consequence its mobility is reduced, pupil becomes small in size due to sphincter irritation and engorgement of radial vessels of iris. Exudates and lymphocytes poured into the anterior chamber result in aqueous flare and deposition of fine KPs at the back of cornea. Due to exudates in the posterior chamber, the posterior surface of iris adheres to the anterior capsule of lens leading to posterior synechiae formation. In severe inflammation, due to pouring of exudate from ciliary processes, behind the lens, an exudative membrane called cyclitic membrane may be formed. (Khurana, 2007)The pathological reaction in granulomatous uveitis is characterised by infiltration with lymphocytes, plasma cells, with mobilization and proliferation of large mononuclear cells which eventually become epithelioid and giant cells and aggregate into nodules. Iris nodules are usually formed near pupillary border (Koeppes nodules). Similar nodular collection of the cells is deposited at the back of cornea in the form of mutton fat keratic precipitates and aqueous flare is minimal. Necrosis in the adjacent structures leads to a repairative process resulting in fibrosis and gliosis of the involved area. (Khurana, 2007)h. Anterior UveitisThough anterior uveitis, almost always presents as a combined inflammation of iris and ciliary body (iridocyclitis), the reaction may be more marked in iris (iritis) or ciliary body (cyclitis). Clinically it may present as acute or chronic anterior uveitis. Main symptoms of acute anterior uveitis are pain, photophobia, redness, lacrimation and decreased vision. In chronic uveitis, however the eye may be white with minimal symptoms even in the presence of signs of severe inflammation. (Khurana, 2007). External examination shows ciliary (circumcorneal) injection which has a violaceous hue. (Kanski, 2011)
Ciliary Injection Miosis due to sphincter spasm may predispose to the formation of posterior synechiae unless the pupil is pharmacologically dilated. Endothelial dusting by a myriad of cells is present early and gives rise to a dirty appearance True keratic precipitates (KP) usually appear only after a few days and are usually non-granulomatous. (Kanski, 2011).
Endothelial dusting by cellsAqueous cells indicate disease activity and their number reflects disease severity. Grading of cells is performed with a 2 mm long and 1 mm wide slit beam with maximal light intensity and magnification. This must be performed before mydriasis because in normal eyes cells and pigment clumps may develop after pupillary dilatation. Worsening is defined as either a two-step increase in the level of activity or an increase to the maximum grade. Anterior vitreous cells indicate iridocyclitis. (Kanski, 2011).Hypopyon is a feature of intense inflammation in which cells settle in the inferior part of the anterior chamber (AC) and form a horizontal level. (Kanski, 2011)
Hypopion Posterior synechiae may develop quickly and must be broken down before they become permanent . Low intraocular pressure (IOP) may occur as a result of reduced secretion of aqueous by the ciliary epithelium. (Kanski, 2011)
Extensive posterior synechiaeFundus examination is usually normal, but should always be performed to exclude spillover anterior uveitis associated with a posterior focus, notably toxoplasmosis and acute retinal necrosis (Kanski, 2011). With appropriate therapy the inflammation tends to completely resolve within 56 weeks.The prognosis is usually very good. Complications and poor visual prognosis are related to delayed or inadequate management. Steroid-induced hypertension may occur but glaucomatous damage is uncommon (Kanski, 2011).Chronic anterior uveitis (CAU) is less common than the acute type and is characterized by persistent inflammation that promptly relapses, in less than 3 months, after discontinuation of treatment. The inflammation may be granulomatous or non-granulomatous. Bilateral involvement is more common than in AAU. Presentation is often insidious and many patients are asymptomatic until the development of complications such as cataract or band keratopathy. Because of the lack of symptoms patients at risk of developing CAU should be routinely screened; this applies particularly in patients with juvenile idiopathic arthritis. (Kanski, 2011).External examination usually shows a white eye. Occasionally the eye may be pink during periods of severe exacerbation of inflammatory activity.Aqueous cells vary in number according to disease activity but even patients with numerous cells may have no symptoms. Aqueous flare may be more marked than cells in eyes with prolonged activity and its severity may act as an indicator of disease activity. (Kanski, 2011). It is due to leakage of protein particles into the aqueous humour from damaged blood vessels. It is demonstrated on the slit lamp examination by a point beam of light passed obliquely to the plane of iris. In the beam of light, protein particles are seen as suspended and moving dust particles. This is based on the Brownian movements or Tyndal phenomenon. (Khurana, 2007).
Aqueous Flare and CellsKeratic precipitate are clusters of cellular deposits on the corneal endothelium composed of epithelioid cells, lymphocytes and polymorphs. (Kanski, 2011).
Keratic PrecipitateThe duration is prolonged and in some cases the inflammation may last for many months or even years. Remissions and exacerbations of inflammatory activity are common and it is difficult to determine when the natural course of the disease has come to an end.The prognosis is guarded because of complications such as cataract, glaucoma and hypotony. (Kanski, 2011).i. Intermediate UveitisIntermediate uveitis affects mainly the intermediate zone of the eye -ciliary body, principally the pars plana, peripheral retina, and vitreous. The cause is unknown in most cases, although syphilis, tuberculosis, Lyme disease, and sarcoidosis should be ruled out with appropriate laboratory and ancillary testing. Multiple sclerosis should also be considered. Intermediate uveitis is seen mainly among young adults, affects men and women equally, and is bilateral in up to 80% of cases. (Riordan-Eva; Whitcher, 2007)Common complaints include painless floaters and decreased vision. Minimal photophobia or external inflammation. Usually age 15 to 40 years and bilateral. Vitreous cells, white exudative material over the inferior ora serrata and pars plana (snowbank), cellular aggregates floating in the inferior vitreous (snowballs). Younger patients may present with vitreous hemorrhage. (Elhers, 2008)
j. Pars planitis/intermediate uveitis with snowballsPosterior subcapsular cataract and cystoid macular edema are the most common causes of decreased vision. In severe cases, cyclitic membranes and retinal detachments may occur. Secondary glaucoma is rare. Corticosteroids are used mainly to treat cystoid macular edema or retinal neovascularization. Topical corticosteroids should be tried for 34 weeks to identify patients predisposed to development of corticosteroid-induced ocular hypertension. If no improvement is noted and ocular hypertension does not develop, a posterior sub-Tenon or intraocular injection of triamcinolone acetonide, 40 mg/mL, may be effective. Patients with intermediate uveitis usually do well with cataract surgery. (Riordan-Eva; Whitcher, 2007)k. Posterior UveitisPosterior uveitis refers to inflammation of the choroid (choroiditis). Since the outer layers of retina are in close contact with the choroid and also depend on it for the nourishment, the choroidal inflammation almost always involves the adjoining retina, and the resultant lesion is called chorioretinitis (Khurana, 2007). Presentation varies according to the location of the inflammatory focus and the presence of vitritis. For example a patient with a peripheral lesion may complain of floaters whereas a patient with a lesion involving the macula will predominantly complain of impaired central vision (Kanski, 2011). Blurred vision, floaters, pain, redness, and photophobia typically absent unless anterior chamber inflammation is present (Elsher, 2008). Various visual symptoms experienced by a patient of choroiditis are summarised below (Khurana, 2007):1) Defective vision. It is usually mild due to vitreous haze, but may be severe as in central choroiditis.2) Photopsia. It is a subjective sensation of flashes of light resulting due to irritation of rods and cones.3) Black spots floating in front of the eyes. It is a very common complaint of such patients. They occur due to large exudative clumps in the vitreous.4) Metamorphopsia. Herein, patients perceive distorted images of the object. This results due to alteration in the retinal contour caused by a raised patch of choroiditis.5) Micropsia which results due to separation of visual cells is a common complaint. In this the objects appear smaller than they are.6) Macropsia, i.e., perception of the objects larger than they are, may occur due to crowding together of rods and cones.7) Positive scotoma, i.e., perception of a fixed large spot in the field of vision, corresponding to the lesion may be noted by many patients.Lesions of the posterior segment of the eye can be focal, multifocal, geographic, or diffuse. Those that tend to cause clouding of the overlying vitreous should be differentiated from those that give rise to little or no vitreous cells. The type and distribution of vitreous opacities should be described. Inflammatory lesions of the posterior segment are generally insidious in onset, but some may be accompanied by abrupt and profound visual loss. (Riordan-Eva; Whitcher, 2007)
Retinitis Vitreous opacities due to choroiditis are usually present in its middle or posterior part. These may be fine, coarse, stringy or snowball opacities (Khurana, 2007). Features of a patch of choroiditis in active stage it looks as a pale-yellow or dirty white raised area with ill-defined edges. This results due to exudation and cellular infiltration of the choroid which hide the choroidal vessels. The lesion is typically deeper to the retinal vessels. The overlying retina is often cloudy and oedematous. In atrophic stage or healed stage, when active inflammation subsides, the affected area becomes more sharply defined and delineated from the rest of the normal area. The involved area shows white sclera below the atrophic choroid and black pigmented clumps at the periphery of the lesion (Khurana, 2007).
l. TreatmentThe treatment of uveitis has three main goals: to prevent vision-threatening complications, to relieve the patient's complaints and, when feasible, to treat the underlying disease. (Babu; Rathinam, 2010)1. Mydriatic and Cycloplegic AgentsThese topical medications are used to treat the ciliary spasm that frequently occurs with acute anterior uveitis and to break recently formed posterior synechiae and/or prevent the development of new synechiae. Longer acting agents, such as homatropine, scopolamine, or atropine, are utilized to relieve ciliary spasm, whereas the shorter acting agents (tropicamide or cyclopentolate) may play a role in preventing new posterior synechiae formation in patients who have chronic iridocyclitis (e.g., secondary to JIA) and minimal photophobia in whom the pupil should be kept relatively mobile. (Yanoff, 2009)2. CorticosteroidsCorticosteroids are the drugs of choice in most types of uveitis. They inhibit the inflammatory process by suppressing the arachidonic acid metabolism and activation of complement (Babu; Rathinam, 2010). When administered systemically they have a definite role in non-granulomatous iridocyclitis, where inflammation, most of the times, is due to antigen antibody reaction. Even in other types of uveitis, the systemic steroids are helpful due to their potent non-specific anti-inflammatory and antifibrotic effects. Systemic corticosteroids are usually indicated in intractable anterior uveitis resistant to topical therapy (Khurana, 2007).In panuveitis, both topical and systemic corticosteroids are needed. Depending upon the severity of the disease, oral prednisolone is started in a loading dose of 1 mg/kg/day. As the inflammation subsides, tapering of corticosteroids by 5-10 mg per week is begun within two to four weeks of initiating therapy. Once the eye is completely quiescent, the patient is followed on a maintenance dose ranging from 2.5-10 mg daily of prednisolone. A reasonably long period of low-dose corticosteroids is required as maintenance therapy in VKH(Vogt Koyanagi-Harada) syndrome and SO.(Babu; Rathinam, 2010)The normal response to the corticosteroid therapy may be interrupted by recurrence of uveitis in which case the frequency of instillation of topical drops is increased besides raising the oral corticosteroid to the initial high-dose levels. Unilateral cases may be given a trial with periocular injection of depot corticosteroids into the posterior subtenon space. The side-effects and complications of topical or systemic corticosteroids must be looked for at every follow-up visit of the patient. These include secondary glaucoma, posterior subcapsular cataract, increased susceptibility to infection (ocular or systemic), hypertension, gastric ulcer, diabetes, obesity, growth retardation, osteoporosis and psychosis. (Babu; Rathinam, 2010)3. AntimetabolitesIndicate for sight-threatening uveitis, which is usually bilateral, non-infectious, reversible and has failed to respond to adequate steroid therapy. Steroid-sparing therapy in patients with intolerable side-effects from systemic steroids or those with chronic relapsing disease requiring a daily dose of prednisolone of more than 10 mg. Once a patient has been started on an immunosuppressive drug and the appropriate dose ascertained, treatment should continue for 624 months, after which gradual tapering and discontinuation of medication should be attempted over the next 312 months. However, some patients may require long-term therapy for control of disease activity. (Kanski, 2011)4. Vitrectomy in panuveitisVitrectomy for uveitis began in the late 1970s for diagnostic purposes and for treating infections. Diagnostic vitrectomy combined with PCR can significantly improve diagnostic yield in otherwise idiopathic uveitis, and can frequently make a diagnosis in cases complicated by media opacity or other features that make traditional exam-based diagnosis difficult or impossible. Vitrectomy may be considered as a therapeutic option when uveitis persists despite maximum tolerable medical treatment with corticosteroids and/or other immunosuppressants. It may also be indicated when visual loss occurs due to complications of longstanding inflammations, such as a densely opacified vitreous, scar tissue pulling on the ciliary body causing hypotony, cystoid macular edema, an epiretinal membrane, a dense posterior lens capsule opacification or a tractional retinal detachment.Vitrectomy removes the lodged lymphocytes in the vitreous, inflammatory debris, immune complexes and autoantigens. It also increases the uveal penetration of anti-inflammatory cells. Besides providing a better access for complete removal of the cataractous lens material along with posterior capsule, the combined approach of pars plana lensectomy and vitrectomy allows easy performance of intraocular maneuvers and prevents formation of cyclitic membrane. (Babu; Rathinam, 2010)
CHAPTER IIICASE REPORT
1. Patient identityName: Mr. YSex : Male Age: 31 years oldAddress: Sekayam, Balai Karangan Job : Wiraswasta Religion : CatholicPatient was examined on September 22nd , 2014
2. Anamnesisa. Chief complaint : Pain in left eye since one day agob. History of disease : Patient came to ophthalmologist with pain in left eye as major complaint. Pain was felt by patient after his left eye, accidently, get punctured by a small branch of tree at 10 AM yesterday. Patient was complained about his blurred vision and watery discharge of his left eye. Patient felt of glare when he saw in light condition.There were no headache, nausea, and vomiting. Patient had consulted to general practitioner and had been given some medication, such as, Cendo Xytrol, Mefenamic Acid, and Amoxicillin. But, there was no improvement with those medication.c. Past clinical history :Diabetes mellitus and hypertension history was denied by patient. There was no ocular disease before. Wearing glasses (-).d. Family history Diabetes mellitus and hypertension history in family was denied by patient.3. General Physical assessmentGeneral condition: Good, with mild painAwareness: Compos mentisVital sign :a. Blood Pressure: 110/80 mmHgb. HR: 80 x/minutec. RR: 24 x/minuted. Temperature: 36,2C4. Ophthalmological statusVisual acuity :a. OD : 6/10b. OS: 1/300, with good light projection
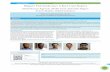
Opacification of lensODOS
Corneal erosioninjection
HypopionFibrin in COA
ODOS
OrthophoriaEye ball positionOrthophoria
Eye Movement
Movement(+), ptosis (-), lagoftalmos (-), exoftalmos (-), edema (-), blepharospasm (-)PalpebraMovement (+), ptosis (-), lagoftalmos (-), exoftalmos (-), edema (-), blepharospasm (+)
Redness (-), discharge (-), injection (-)ConjungtivaRedness (+), watery discharge (+), injection (+)
Clear, edema (-), infiltrate (-), Ulcer (-)CorneaHazy, edema (+), infiltrate (+), ulcer (-), KP (-)
Clear, deepAnterior chamberShallow, fibrin (+), hypopion (+)
Iris: brown, sinechia (-)Circular pupil, regular, 3 mm in diameter. Direct light pupillary reflex (+), consensual reflex (+)Iris/pupilIris : brown, iridodialysis (+)Pupil is not circular, with tears in iris. Direct light pupillary reflex diminished, consensual reflex diminished
ClearLensWhite opacification
Not performedPosterior segmentFunduscopy : not performedUSG : normal (no retinal detachment, no intraocular foreign body, no fibrin)
5. Shadow test : negative / negative6. Visual field test: normal / normal7. Palpation tonometry : OS > OD
8. ResumePatient came to ophthalmologist with pain in left eye as major complaint. Pain was felt by patient after his left eye, accidently, get punctured by a small branch of wood at 10 AM yesterday. Patient was complained about his blurred vision and watery discharge of his left eye. There were no headache, nausea, and vomiting. Patient had consulted to general practitioner and had been given some medication, such as, Cendo Xytrol, Mefenamic Acid, and Amoxicillin. But, patient felt that there was no eye improvement with those medication.Pain (+), watery (+), photophobia (+), blurred vision (+)Visual acuity was 6/10 OD, 1/300 OS. Left palpebra seems having blepharospasm. Cornea had a lesion cause by puncture of branch of tree and there was edema in cornea.
9. DiagnosisDiagnose : a. OD: -b. OS: Secondary glaucoma et causa traumatic cataract Anterior uveitisDDx : OD: - OS: Primary close angle glaucoma and keratitis
10. Plan for examinationa. Flourescein testb. Tonometryc. Gonioscopy
11. Treatmenta. Atropine eye drops (Cendo tropin), 3 times a dayb. Prostaglandine analog eye drops (latanoprost), 2 times a dayc. Antibiotic (Flouroquinolone) eye drops, every hoursd. Systemic corticosteroid (methyl prednisolone), once a day e. Asetazolamide per oral, 3 times a dayf. KCl 600 mg (Electrolyte) per oral, 3 times a day12. Prognosis a. OD:i. Ad vitam: dubia ad bonamii. Ad functionam : dubia ad bonamiii. Ad sanactionam: dubia ad bonam
b. OS :i. Ad vitam: dubia ad bonamii. Ad functionam : dubia ad bonamiii. Ad sanactionam: dubia ad bonam
CHAPTER IVDISCUSSION
A man, 31 years old, works as a laborer. Patient complains pain in his left eye since one day ago after his eye was get punctured by branch of tree. The patient felt blurred vision in his left eye, there is redness in konjungtiva, photophobia, and watery discharge of his left eye. Fever history was denied by patient. From the ophthalmic examination, the visual acuity of right eye is 6/10, and left eye is 1/300 with good light projection. Eyelids of both eyes are normal, but the left eyes lid is tend to blink and close. Conjunctiva of the left eye has subconjunctival injection with no bleeding. Cornea of the left eye has infiltrate, defect, and edema. The anterior chamber of the left eye is shallow and containing some fibrins, meanwhile the right eyes anterior chamber is shallow and clear. Iris is brown in color, but there is a tears due to the injury in the left eye. Pupil is anisochore, the right pupil has regular circular shaped with 3 mm in diameters. The left eyes pupil is irregular due to the tears in iris. Direct light reflex and consensual reflex for right eye are positive, but diminished in the left eye. The lens is opaque in the left eye and clear in the right eye. Funduscopy is not performed in left eye due to hazy cornea and white opacification of the lens. In USG examination, there is no fibrin or mass founded (clear), and no retinal detachment in vitreous of left eye. In palpation of both eyes, it is found that the left eyes intraocular pressure (IOP) is slightly higher than the right eye.Painfull sensation, which felt by patient, is caused by stimulation of the trigeminal nerve. Cornal edema, increasing intraocular pressure, and inflammation or infection in uvea will affect the trigeminal nerve and produce pain sensation. The patient also complaint about photophobia. Photophobia is intimately, likely inextricably, linked to pain sensation. The trigeminal nerve and its nuclei are the primary mediators of pain sensation to the head. The conjunctiva, cornea, sclera, and uvea (iris, ciliary body, and choroid) are densely innervated with trigeminal fibers, and exquisitely sensitive to pain. Any painful stimulus to these areas (e.g. iritis, uveitis) invariably causes photophobia.Blurry vision can be caused by the refractive media defect such as cornea or the lens, from uvea tract like iris, cilliar corpus, choroid, or the retina. In this case, patient has some problems with the visual refractive media. It is hazy cornea (shows that there is edema in cornea), and white opacification of lens (traumatic cataract). These conditions can cause disrupting of the refraction media and finally, it can hampered the light to retinal.The patients left eye is red because of subconjunctival and cilliary injection. Subconjunctival injection is dilatation of posterior conjunctiva artery. Ciliar injection is dilatation of pericorneal blood vessel (a. cilliar anterior) that can be caused by inflammation in the cornea, corneal ulcer, corpus alienum, inflammation in the uveal tract, glaucoma, endophtalmitis or panophtalmitis.From the ophthalmologic examination, the visual acuity is 6/10 for OD and 1/300 for OS. Visual acuity can decrease because of defect in the cornea, opacity in the lens, inflammation in the uveal tract, retinal impairment, etc. The visual acuity in the patients left eye decreasing suddenly can be caused by defect in the cornea like keratitis or corneal ulcer, or from the uveal tract. Since the patient didnt complain about any discharge from his eye, it can caused by inflammation in uveal tract such as anterior uveitis. Uveitis is inflammation of the iris (iritis, iridocyclitis), ciliary body (intermediate uveitis, cyclitis, peripheral uveitis, or pars planitis), or choroid (choroiditis). Anterior uveitis is iritis and iridocyclitis. Uveitis can cause by corneal infection, systemic infection like syphilis, tuberculosis, systemic immunological disease like sarcoidosis, Vogt-Koyanagi-Harada, arthritis rheumatoid, and trauma, etc.Cornea of the left eye has infiltrate, defect and edema. It can be caused by there is inflammation in the cornea. In anterior uveitis, the infiltrate can be caused by chronic inflammation resulting debris in the anterior chamber that stick to the inner surface of the corneal endothelium and make keratic precipitate. The defect in the cornea is possibly the port of entry of the branch of tree that can cause inflammation to the iris (anterior uveitis) . The anterior chamber is opaque and shallow in the left eye can caused by accumulation of inflammatory cell, debris or fibrin. Iris of both eyes is brown, but there is a tearing of the left eyes iris. The iris is adherent to the lens in several places as a result of inflammation, causing an irregular, fixed pupil. The adhesion may alter the sphincter pupils muscle movement. So the pupil will be anisochore.Cataract can cause blurred vision but the patient didnt complaint about blurred vision in the left eye or white eye before so the most possible cause for the opacity of the lens in the patient is trauma. The lens becomes white soon after the entry of a foreign body or penetrating trauma, since interruption of the lens capsule allows aqueous and sometimes vitreous to penetrate into the lens structure. Trauma does not result in rupture of the capsule, may cause an anterior and/or posterior subcapsular cataract or both. Initially, fluid influx causes swelling and thickening of the lens fibers. Later the fibers become less swollen; the anterior subcapsular region whitens and may develop a characteristic flower-shaped pattern, or an amorphous or punctate opacity.Secondary glaucomas are caused by other ocular diseases or factors such as inflammation, trauma, bleeding, tumors, medication, and physical or chemical influences. The differential diagnosis of secondary glaucoma is primary close angle glaucoma. The symptoms which appear in this patient are general symptoms of glaucoma. But primary close angle glaucoma can be diagnose when there is no another underlying ocular disease or factor which induce the glaucoma.The differential diagnose for OS is keratitis. Keratitis can caused by viral, bacterial, or fungal infection with or without trauma before. The symptom is the patient feel usually blurred, there is watery or purulent discharge, change in corneal surface related to cause, there is cilliar injection and conjunctival injection, the pupil is normal or myosis but the pupil still reactive to the light. In the patient, there is no discharge, and the pupil is irregular and doesnt reactive to the light so keratitis can be disregard. And for the traumatic cataract the differential diagnose is senile cataract and traumatic lens dislocation. Since the opacity of the lens happened after the patient stabbed by wire, the senile cataract can be disregard. Small perforation caused by penetrating injury will close immediately because of ephitelial proliferation so the opacity only in the small area like found in the patient. Lens dislocation can occur after a trauma and can be no symptom if the dislocation is partial but if the lens floating in the vitreous the patient will have blurred vision and red eye. Iridodonesis, a quivering of the iris when the patient moves the eye, is a common sign of lens dislocation and is due to the lack of lens support. This is present both in partially and in completely dislocated lenses. But in the patient, iridodonesis cant be find.The non medicamentous treatment in this patient is close the eye with bandage when the patient work to avoid exposure to dust, wood, foreign body, and ultraviolet light. Medical treatment which given to this patient are atropine (cendo tropin) eye drops, prostaglandin analog (latanoprost) eye drops, antibiotic (levofloxacin) eye drops, oral steroid, oral asetazolamide (glaukon), and oral KSR. Mydriatic agent (atropine) is given to promote comfort by relieving spasm of the ciliary muscle and pupillary sphincter, and to break down recently formed posterior synechiae. Atropine can decrease the inflammation of the eye too. Prostglandin analog (latanoprost) is used to decreased the intraocular pressure by facilitating the aqueous humor outflow. Antibiotic (levofloxacin) is given to heal the bacterial infection (uveitis) of the eye. Systemic steroid is used to decrease the inflammation. Corticosteroid has the function to reduce the inflammation of the eye by inhibit cyclo-oxygenase and lipoxygenase pathways, decrease complement level, migration of lymphocytes, production of vasoactive amines and interleukin, circulating monocytes and macrophage activity. Oral steroid is chosen than steroid eyedrop because there is defect in cornea. If topical steroid is given to corneal defect, it can disturb the healing process and fungi can easily grow, which can destroy the corneal structure. Oral asetazolamide is used to decrease the aqueous humor production. Asetazolamide can block anhidrase carbonic enzyme which make diuresis, decrease secretion of aquoeus humor up to 60% and decrease the intraocular pressure. Because asetazolamide is a diuretic drug, it could disturb the electrolyte balance in the body, so KSR is given to avoid this effect.
CHAPTER VCONCLUSION
A 31 years old man complain pain in his left eye since one day ago after his eyes got punctured by small branch of tree. Ophthalmologic examination show subconjunctival and cilliar injection, irregular pupil, not reactive to light, tears in iris, opacity of the lens, fibrin and hypopion in anterior chamber. Anterior chamber looks shallow, and with palpation tonometry, the left eye has slighlty higher pressure than right eye. The therapy include non-medicamentous (use bandage to avoid exposure to foreign body, dust, and UV) and medicamentous (mydriatic agent [atropine], prostaglandin analog [latanoprost], antibiotic [levofloxacin], systemic corticosteroid [methyl prednisolone], asetazolamide, and KSR for the left eye).
BIBLIOGRAPHY
Ilyas S. 2007. Ilmu Penyakit Mata edisi ketiga. Jakarta: Balai Penerbit FKUI.Kanski, Jack J; Bowling B. 2011. Clinical Ophthalmology: A Systematic Approach, 7th edition. UK: Elveiser.Khurana A. 2007. Comprehensive Ophtalmology 4th Edition. India: New Age International Limited Publisher.Lang, GK., 2000. Ophthalmology: A Short Textbook. New York: Thieme.Eva, P.R., and Whitcher, J.P. 2007. Vaughan and Asburys General Ophthalmology 17th Edition. USA: McGrawHillYanoff, M. and Duker, JS., 2009. Yanoff and Dukers Ophthalmology. 3rd Edition. UK: Mosby Elsevier.
Related Documents