C H A P T E R 3 biochemistry

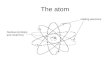
C H A P T E R 3 biochemistry. Atomic Structure: Protons = Electrons = Neutrons = Mass = Valence Electrons = Currently unstable Needs to obtain, give,
Dec 25, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Atomic Structure:Protons = Electrons = Neutrons = Mass = Valence Electrons =Currently unstable Needs to obtain, give, or share 4 electrons
to become stable.
VERSATILITY OF CARBON
Can form single bonds,
double bonds, triple bonds, or a
combination those bonds.
Both of the above pictures demonstrate aromatic hydrocarbonssince they contain a benzene ring in their structure and contain
only hydrogen and carbon
Alkanes – aliphatic hydrocarbon with all single bonds
Alkenes – aliphatic hydrocarbon with at least one double bond
Alkynes – aliphatic hydrocarbon with at least one triple bond
INTRODUCTION AND REVIEW
What are organic compounds?
By what process do monomers become polymers?What has to be removed?
By what process do polymers become monomers?What has to be added?
There are 4 main groups of organic molecules essential to life: CARBOHYDRATES, PROTEINS, LIPIDS, and NUCLEIC ACIDS
CARBOHYDRATES
Elements =
Monomers =
Monosaccharides General Formula =
Have a carbonyl group (C double bonded to O) Straight chain or ring structure
DISACCHARIDES
Disaccharide means… 2 sugars are joined by what process? What had been removed to allow the
disaccharide to form? Sucrose (ordinary table sugar) = one glucose
and one fructose Lactose (milk sugar) = one glucose, one
galactose Maltose (malt sugar) = 2 glucose
POLYSACCHARIDESMany sugars
Cellulose StarchMade by plants Made by plantsMakes up 50% of woodCell wall of plants GlycogenThousands of glucose Hundreds of
glucose branchedin a long chain Stored in animal cells
LIPIDS
Elements = Groups of Lipids =
triglyceride, phospholipid, wax, steroid
Nonpolar (do not dissolve in water)
Store energy effectively in C-H bonds
Saturated fats are solid at room temperature
Unsaturated fats are liquid at room temperature (such as plant oils).
4 TYPES OF LIPIDSTriglycerides, Phospholipids, Waxes,
Steroids
Phospholipids = 2 fatty acids joined to glycerol
2 layers of phospholipids make up the cell membrane = lipid bilayer
Phospholipid bilayer = barrier between the inside and outside of the cell.
Most of the body is water, the cell membrane is lipid…..lipids do not dissolve in water, so barrier forms.
4 TYPES OF LIPIDSTriglycerides, Phospholipids, Waxes,
Steroids
Wax = carboxylic acid chain (fatty acid) joined to alcohol chain (-OH)
Wax = water-proof, protective covering
Related Documents