JOURNAL OF VIROLOGY, Mar. 1988, p. 1084-1087 Vol. 62, No. 3 0022-538X/88/031084-04$02.00/0 Copyright © 1988, American Society for Microbiology Birnavirus Precursor Polyprotein Is Processed in Escherichia coli by Its Own Virus-Encoded Polypeptide MITTUR N. JAGADISH,* VIKKI J. STATON, PETER J. HUDSON, AND AHMED A. AZAD CSIRO, Division of Protein Chemistry, 343 Royal Parade, Parkville 3052, Australia Received 1 June 1987/Accepted 30 November 1987 The cDNA fragment of the large RNA segment of infectious bursal disease virus 002-73, when expressed in Escherichia coli, produces .precursor polyprotein (N-VP2-VP4-VP3-C), most of which is then processed to generate constituent polypeptides. Using cDNA fragments containing site-specific mutations and two monoclo- nal antibodies that are specific to VP2 and VP3 of mature virus particles, we demonstrated that the VP4 protein is involved in processing of the precursor polyprotein to generate VP2 and VP3 and excluded the possibility of internal initiation for the generation of VP3. Infectious bursal disease virus (IBDV), a member of the birnavirus group, is the causative agent of a highly conta- gious immunodepressive disease of young chickens (15). The virus destroys the precursors of antibody-producing B cells, thereby causing severe immunodeficiency in chickens and making them susceptible to other avian pathogens. In vitro translation (1) and sequencing (10) studies have shown that in IBDV strain 002-73, the smaller RNA segment codes for a single protein of 90 kilodaltons (kDa) (VP1), and the larger RNA segment codes for three proteins with approximate molecular sizes of 52 (VP2), 32 (VP3), and 28 kDa (VP4). VP2 has a calculated molecular size in excess of 50 kDa but separates as two polypeptides of 41 (VP2a) and 37 kDa (VP2b) on Laemmli gels. There appears to be a precursor product relationship between VP2a and VP2b (10). Extensive immunological and recombinant DNA studies have shown that VP2 is a major, conformational-dependent, host-protective immunogen (2a; K. J. Fahey, K. Erny, and J. Crooks, J. Gen. Virol., in press), whereas VP3 contains a minor virus-neutralizing epitope which is immunoreactive in the presence of sodium dodecyl sulfate (SDS) (2, 8, 9). The nucleotide and peptide sequence analyses (10) have shown that the coding region of the large RNA segment is mono- cistronic and encodes a polyprotein in which the viral polypeptides are arranged in the order N-VP2-VP4-VP3-C. However, the precise borders of the three coding regions have not yet been defined. Large recombinant cDNA molecules containing most (3,100 base pairs [bp]) of the coding region (plasmid PO) of the large segment have been constructed by restriction and ligation of smaller overlapping cDNA fragments through common restriction sites (10). When expressed in Esche- richia coli, PO produces correctly processed and unfused VP3 as the primary species along with some higher-molecu- lar-weight species that are all recognized by a monoclonal antibody (MAb) specific to a region of VP3. The fusion polyprotein produced from PO must then be specifically processed in E. coli to produce the constituent polypeptides that include VP3. Alternatively, the generation of VP3 in E. coli could be caused by internal initiation at a procaryote- type ribosome-binding site. Recent work from this labora- tory has shown that large N-terminal deletions of VP4 prevent processing of the precursor protein (2a). However, it * Corresponding author. does not completely exclude the possibility of internal initi- ation. To further determine whether the generation of VP3 is caused by internal initiation or by processing activities originating from either the bacterial or viral genome and then to understand the mechanisms that may lead to efficient precursor polyprotein processing in E. coli, site-specific mutagenesis of the IBDV cDNA insert in PO was done. Subsequently, unmodified and modified versions of PO were expressed in E. coli, and the expressed proteins were analyzed by immunoblot procedures. As an assay to deter- mine processing of the precursor polyprotein, MAbs specific to VP3 (MAb 17/80) or to the C-terminal end of VP2 (MAb 6; K. J. Fahey et al., manuscript in preparation) were used to locate the processed or unprocessed forms in Western immunoblot analyses of E. coli extracts. E. coli P2136 XPR c1857 (gift from 0. Raibaud), HB2151, and BMH71-18 (5) were used for expression of PO and the mutagenized derivatives. A detailed description of the meth- ods used for expression of IBDV proteins in E. coli P2136, isolation and characterization of proteins by polyacrylamide gel electrophoresis, immunodot assays, and Western blot analysis has been reported earlier (2, 10). Materials and methods for E. coli transformation, plasmid DNA isolation, restriction endonuclease analysis, and ligation are those routinely used and described before (2, 10, 11, 13). Plasmid PO (10) contains most (3,100 bp) of the coding region of the large segment of IBDV 002-73 fused to the C-terminal end of the 115-kDa hybrid protein that encodes the cro-lacZ gene directed by the XPR promoter in the pEX3 vector (19). The expression of PO and its derivatives is thermoinducible in E. coli P2136, which contains the c1857 temperature repressor of A promoter-directed transcription. Plasmid pUC4K Apr Kmr (20), which contains the restriction site mobilizing (RSM) element that codes for the Tn903 Kmr gene, and plasmid pMJ102 Tcr Kmr (this work), in which the ca. 1.3-kilobase EcoRI RSM element was cloned into the EcoRI site of pSUP205 (18), were used as sources of mutagenic DNA in insertional mutagenesis experiments. When expressed in E. coli P2136 under thermoinducible conditions, plasmid PO produced proteins of several sizes that are IBDV specific (see Fig. 2 and 4b, lane PO). In a Western blot analysis with MAb 17/80 that is specific for the denatured VP3 region, clone PO produced three abundant proteins, with sizes of approximately 220, 60, and 32 kDa, and several less abundant species. The generation of 60- and 1084

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

JOURNAL OF VIROLOGY, Mar. 1988, p. 1084-1087 Vol. 62, No. 30022-538X/88/031084-04$02.00/0Copyright © 1988, American Society for Microbiology
Birnavirus Precursor Polyprotein Is Processed in Escherichia coliby Its Own Virus-Encoded Polypeptide
MITTUR N. JAGADISH,* VIKKI J. STATON, PETER J. HUDSON, AND AHMED A. AZADCSIRO, Division of Protein Chemistry, 343 Royal Parade, Parkville 3052, Australia
Received 1 June 1987/Accepted 30 November 1987
The cDNA fragment of the large RNA segment of infectious bursal disease virus 002-73, when expressed inEscherichia coli, produces .precursor polyprotein (N-VP2-VP4-VP3-C), most of which is then processed togenerate constituent polypeptides. Using cDNA fragments containing site-specific mutations and two monoclo-nal antibodies that are specific to VP2 and VP3 of mature virus particles, we demonstrated that the VP4 proteinis involved in processing of the precursor polyprotein to generate VP2 and VP3 and excluded the possibility ofinternal initiation for the generation of VP3.
Infectious bursal disease virus (IBDV), a member of thebirnavirus group, is the causative agent of a highly conta-gious immunodepressive disease of young chickens (15). Thevirus destroys the precursors of antibody-producing B cells,thereby causing severe immunodeficiency in chickens andmaking them susceptible to other avian pathogens.
In vitro translation (1) and sequencing (10) studies haveshown that in IBDV strain 002-73, the smaller RNA segmentcodes for a single protein of 90 kilodaltons (kDa) (VP1), andthe larger RNA segment codes for three proteins withapproximate molecular sizes of 52 (VP2), 32 (VP3), and 28kDa (VP4). VP2 has a calculated molecular size in excess of50 kDa but separates as two polypeptides of 41 (VP2a) and37 kDa (VP2b) on Laemmli gels. There appears to be aprecursor product relationship between VP2a and VP2b (10).Extensive immunological and recombinant DNA studieshave shown that VP2 is a major, conformational-dependent,host-protective immunogen (2a; K. J. Fahey, K. Erny, andJ. Crooks, J. Gen. Virol., in press), whereas VP3 contains aminor virus-neutralizing epitope which is immunoreactive inthe presence of sodium dodecyl sulfate (SDS) (2, 8, 9). Thenucleotide and peptide sequence analyses (10) have shownthat the coding region of the large RNA segment is mono-cistronic and encodes a polyprotein in which the viralpolypeptides are arranged in the order N-VP2-VP4-VP3-C.However, the precise borders of the three coding regionshave not yet been defined.
Large recombinant cDNA molecules containing most(3,100 base pairs [bp]) of the coding region (plasmid PO) ofthe large segment have been constructed by restriction andligation of smaller overlapping cDNA fragments throughcommon restriction sites (10). When expressed in Esche-richia coli, PO produces correctly processed and unfusedVP3 as the primary species along with some higher-molecu-lar-weight species that are all recognized by a monoclonalantibody (MAb) specific to a region of VP3. The fusionpolyprotein produced from PO must then be specificallyprocessed in E. coli to produce the constituent polypeptidesthat include VP3. Alternatively, the generation of VP3 in E.coli could be caused by internal initiation at a procaryote-type ribosome-binding site. Recent work from this labora-tory has shown that large N-terminal deletions of VP4prevent processing of the precursor protein (2a). However, it
* Corresponding author.
does not completely exclude the possibility of internal initi-ation.To further determine whether the generation of VP3 is
caused by internal initiation or by processing activitiesoriginating from either the bacterial or viral genome and thento understand the mechanisms that may lead to efficientprecursor polyprotein processing in E. coli, site-specificmutagenesis of the IBDV cDNA insert in PO was done.Subsequently, unmodified and modified versions of PO wereexpressed in E. coli, and the expressed proteins wereanalyzed by immunoblot procedures. As an assay to deter-mine processing of the precursor polyprotein, MAbs specificto VP3 (MAb 17/80) or to the C-terminal end of VP2 (MAb 6;K. J. Fahey et al., manuscript in preparation) were used tolocate the processed or unprocessed forms in Westernimmunoblot analyses of E. coli extracts.
E. coli P2136 XPR c1857 (gift from 0. Raibaud), HB2151,and BMH71-18 (5) were used for expression of PO and themutagenized derivatives. A detailed description of the meth-ods used for expression of IBDV proteins in E. coli P2136,isolation and characterization of proteins by polyacrylamidegel electrophoresis, immunodot assays, and Western blotanalysis has been reported earlier (2, 10). Materials andmethods for E. coli transformation, plasmid DNA isolation,restriction endonuclease analysis, and ligation are thoseroutinely used and described before (2, 10, 11, 13).
Plasmid PO (10) contains most (3,100 bp) of the codingregion of the large segment of IBDV 002-73 fused to theC-terminal end of the 115-kDa hybrid protein that encodesthe cro-lacZ gene directed by the XPR promoter in the pEX3vector (19). The expression of PO and its derivatives isthermoinducible in E. coli P2136, which contains the c1857temperature repressor of A promoter-directed transcription.Plasmid pUC4K Apr Kmr (20), which contains the restrictionsite mobilizing (RSM) element that codes for the Tn903 Kmrgene, and plasmid pMJ102 Tcr Kmr (this work), in which theca. 1.3-kilobase EcoRI RSM element was cloned into theEcoRI site of pSUP205 (18), were used as sources ofmutagenic DNA in insertional mutagenesis experiments.When expressed in E. coli P2136 under thermoinducible
conditions, plasmid PO produced proteins of several sizesthat are IBDV specific (see Fig. 2 and 4b, lane PO). In aWestern blot analysis with MAb 17/80 that is specific for thedenatured VP3 region, clone PO produced three abundantproteins, with sizes of approximately 220, 60, and 32 kDa,and several less abundant species. The generation of 60- and
1084

NOTES 1085
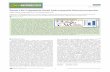
Progressive Dele
lnb PO(3100blPOABgl n-Pst1POBsm-Pst1
VP2 '--VP4 VP3_
EtIoR 9to
N, C
t
Sp,Isl~J. ,-I
,
tlons 5N, Apal.fonp) _
POASph1-Pst1
Internal Deletions
(i) PO&Apo 1PO&Nco 1POoSca 1
Insertions
O9 PO B
PO B1
PO E
PO E 1
PO E B
I -4
EcoRl
I
(
Base Substitutions
PO tKR-ICI
FIG. 1. Mutagenesis of the large segment in PO. (la) Restrictionendonuclease map of the SmaI-Pstl cDNA fragment encodingalmost the entire length of the IBDV large segment. VP2, VP3, andVP4 are the three protein coding regions. *, stop signal; RR and KR,suspected cleavage sites at the VP2-VP4 and VP4-VP3 junctions,respectively; , locations ofAXAASG/E sequence repeats. (lb)3'-Progressive-end deletions. (1c) Internal deletions. --- -, Re-gions deleted. (1d) Site-specific insertions. -, pUC4K RSM ele-ment containing Kmr flanked by termination signals and restrictionsites in a symmetrical order (see text for details); -, interruption or
shift in the reading frame. The insert in PO.B.1 is GTC GAC GGATCC and that in PO.E.1 is CCC CGG ATC CGT CGA CGG ATCCGG GGA ATT. (le) Base substitutions indicating the change ofLys-Arg (KR) to Ile-Cys (IC).
32-kDa proteins strongly suggests that there is proper pre-cursor polyprotein processing in E. coli, even if it is not tocompletion. If there is precise processing of the large poly-protein at the VP2-VP4 and VP4-VP3 junctions, then amongthe abundant species, the 220-kDa species is likely to repre-sent the unprocessed 115-kDa (cro-lacZ)+VP2-VP4-VP3(IBDV) fusion protein, and the 60-kDa species is likely torepresent the VP4-VP3 fusion protein which is the product ofprocessing at the VP2-VP4 junction and the most abundantVP3 which is the product of processing at the VP4-VP3junction or at both junctions of the polyprotein.To determine whether the cro-lacZ-VP2 fusion protein
was produced also, as a result of cleavage at the VP2-VP4junction, expressed proteins from PO in E. coli were sub-jected to Western blotting and reacted with MAb 6. TheMAb 6-binding region is present at the C-terminal end ofVP2 between the first Scal site and the unique XhoI site ofthe IBDV large segment (Fig. la; 2a). PO predominantlyproduced a protein with an approximate size of 160 kDa (seeFig. 4c), which accounts for the 115-kDa cro-lacZ proteinfused to VP2 that indicates the occurrence of cleavage at theVP2-VP4 junction. The less-intense larger bands may repre-sent the remaining unprocessed large polyprotein and theapproximately 195-kDa protein species resulting from pro-
cessing at the VP4-VP3 junction. There is a small amount ofunprocessed 220-kDa protein which is detectable by MAb17/80 (see Fig. 4d) but which reacts weakly to MAb 6 (seeFig. 4c).The appearance of several less-abundant proteins recog-
nized by both MAbs 17/80 and 6, however, indicates thatseveral of the larger unprocessed and processed forms are
probably subjected to nonspecific proteolytic degradation inE. coli. Furthermore, the presence of the large 220- as well
as the 60-kDa protein indicates that processing of the largeprecursor polyprotein in E. coli is incomplete.To further map the regions functionally involved in pro-
cessing of the large polyprotein in E. coli, we engineeredsite-specific deletions, insertions, and base substitutions inPO (Fig. lb through d). Constructs POAApaI, POANcol,and POAScaI represent clones in which specific internaldeletions were made by restriction of PO with the appropri-ate restriction enzyme and ligation in a diluted ligation bufferto favor self-ligation of the vector. According to the DNAsequence data, in all three constructs the original readingframe would be restored after correct ligation. Site-directedinsertional mutagenesis was carried out in the following way.A 1.3-kilobase RSM element carrying the gene for kanamy-cin resistance (Kmr) from pUC4K or pMJ102 was clonedinto the BamHI (PO.B) or EcoRI (PO.E) site in the VP4region. Insertions of 4 codons at the BamHI site (PO.B.1) orof 10 codons at the EcoRI site (PO.E.1), both in phase withthe IBDV translation reading frame, was achieved by excis-ing the RSM element with an enzyme other than the oneused for initial cloning of the RSM element, followed byreligation. In construct PO.E.B, the region between EcoRIand BamHI was deleted and replaced with one codon (CCC)by cleaving PO.E with BamHI, followed by religation of theplasmid. However, the replacement of 182 bp with 3 bpshould result in a frameshift downstream of the EcoRI site inthe IBDV genome, as determined from the sequence data(10).PO and the mutagenized derivatives were expressed in E.
coli. The proteins expressed were analyzed on an SDS-polyacrylamide gel, transferred to nitrocellulose blots, andreacted with MAb 17/80. Site-specific insertion of 10 codonsin phase into the EcoRI site (Fig. 2, lane PO.E.1) near the 5'end of VP4 did not drastically affect the production ofcorrectly processed VP3 protein. However, insertion of fourcodons in phase into the BamHI site (Fig. 2, lane PO.B.1) inthe middle of the VP4 coding region inhibited correct pro-cessing of the precursor polyprotein and instead mainlyproduced the large precursor molecule of approximately 220kDa. The mutation at the BamHI site had affected process-ing either by inactivating or modifying the gene productencoded by the VP4 region or by altering the conformational
r" 1 ,.
FIG. 2. MAb 17/80-probed Westem blot analysis of extracts ofE. coli transformed with plasmid PO and PO derivatives on a 12.5%polyacrylamide gel run under SDS denaturing conditions. The bandswere visualized by horseradish peroxidase reaction. The lanes aredescribed in the text.
VOL. 62, 1988
.-,v

1086 NOTES
structure of the polyprotein in such a way that it was nolonger subjected to proper proteolytic cleavage.
Site-specific deletion of the 330-bp (12.2-kDa) NcoI frag-ment in the POANcoI clone resulted in two major bands, oneof less than 220 kDa and one of about 48 kDa (Fig. 2). Bothbands account for the loss of ca. 12.2 kDa from the corre-sponding large 220- and 60-kDa precursors expressed fromPO. A band of 20 kDa (32 to 12.2 kDa) was not seen whichmay be due to the absence of cleavage at the VP4-VP3junction or absence of the cleavage site itself in POANcol.
Site-specific deletion of 390 bp (14.4 kDa) in the POAApaIclone (Fig. 2) resulted in three major and several minorprotein bands. One predominant band is slightly less than the208-kDa band in POANcol and thus may account for the lossof 14.4 kDa from the precursor polyprotein. The major band,which is about 45 kDa (60 to 14.4 kDa), is perhaps thetruncated equivalent of the VP4-VP3 fusion protein. Thereasons for the generation of the approximately 60-kDa bandin POAApaI is not clear. Expressed proteins from thePOAApaI clone seem to be subjected to more nonspecificdegradation than those from PO or POANcol (Fig. 2). InPOAScaI (Fig. 2), proteins specific to MAb 17/80 were notdetected because of the deletion of the MAb 17/80-bindingregion. It has been observed that the epitope that reacts withMAb 17/80 spans the second ScaI site (unpublished results).The above data from deletion and site-specific insertion
analyses show that the VP4 polypeptide-coding region isinvolved in precursor polyprotein processing at the VP4-VP3junction to generate VP3.
Site-specific insertions of the 1.3-kilobase RSM element atboth the EcoRI and BamHI sites prevented the generation ofVP3 protein (Fig. 2). This is likely because of the transcrip-tional and translational termination signals present in theregions flanking the Kmr gene in the RSM element, asdetermined from the published DNA sequence data (16). Thepremature termination signals have abolished the synthesisof the VP3 or larger-molecular-weight proteins specific toMAb 17/80. Similarly, when the region between the EcoRIand BamHI sites within the IBDV fragment in PO wasdeleted (clone PO.E.B), neither a VP3 protein nor any of thelarger species specific to MAb 17/80 was generated. This wasmost likely caused by the alteration of the reading frame 3' tothe EcoRI-CCC-BamHI site of the large segment in PO.E.B.The results from clone PO.E, PO.B, and PO.E.B indicatethat the VP3 protein is not expressed from an independenttranslation initiation site in E. coli but indeed is a processedproduct of the polyprotein.The expressed proteins from PO and the mutagenized
derivatives were analyzed on an SDS-polyacrylamide gel,transferred to nitrocellulose, and reacted with MAb 6 toinvestigate the effect of insertion mutations on proteolyticcleavage at the VP2-VP4 junction. In-phase insertion of fourcodons in the BamHI site and in-phase deletion of 390 bp 3'to the first ApaI site (353 bp downstream of the BamHI site)prevented processing at the VP2-VP4 junction (data notshown).
In POANcol on the other hand, the deletion of 330 bp inthe VP3 region did not have drastic effects on processing atthe VP2-VP4 junction, because a band pattern similar to thatof PO was observed. Similarly, 3'-end deletions in the VP3regions in clones POABglII-PstI, POABsmI-PstI, andPOASphI-PstI did not have any effects on processing at theVP2-VP4 junction (Fig. 3). Thus it seems that the essentialcoding sequences for VP4 processing functions map betweenthe EcoRI site and the first NcoI site of the large segment,because site-specific in-phase insertion of 30 bp (10 codons)
ICL- E
C. CL
d<KC) CDL
v-w
rt
0-C-L
CL-
4% .~~~~160
FIG. 3. MAb 6-probed Western blot analysis of IBDV andextracts of E. coli transformed with plasmid PO and PO derivativeson a 7.5% polyacrylamide gel run under SDS denaturing conditions.The bands were visualized by horseradish peroxidase reaction. Thelanes are described in the text.
at the EcoRI site and in-phase deletion of 330 bp 3' to thefirst NcoI site did not alter processing at the VP4-VP3 andVP2-VP4 junctions, respectively.
Proteolytic cleavage has been shown to occur commonlyat pairs of dibasic residues during processing of somemammalian peptide hormone precursors and plasma pro-teins (6, 17), as well as Saccharomyces cerevisiae ao-factorand killer toxin peptides (3, 12). The sequence of the largesegment of IBDV contains pairs of dibasic residues atpositions 451 and 452 (Arg-Arg; RR) and 721 and 722(Lys-Arg; KR) corresponding to approximate junctions ofVP2-VP4 and VP4-VP3, respectively. Proteolytic cleavageat these dibasic residues would generate proteins with ex-pected sizes of 28.2 (VP4) and 32.5 kDa (VP3). To test thispossibility, the residues KR at 721 and 722 were changed tononbasic Ile-Cys (IC) residues by oligonucleotide-directedmutagenesis, using standard techniques (5). Mutagenized POwas expressed in E. coli, and the proteins were analyzed byusing MAbs 6 and 17/80 (Fig. 4). A MAb 6-specific band thatis larger than the predominant 160-kDa PO band is seen (Fig.4c), suggesting that cleavage at the first junction (VP2-VP4)was also affected. The alteration of KR residues appears tohave inactivated VP4 processing functions perhaps by dras-tically changing the conformation of VP4. It is possible thatthe KR pair of dibasic residues is not the target site forcleavage but is an integral part of VP4 processing functions.It remains to be seen if the residues RR at positions 451 and452 play any role in processing. Suprisingly, the expressedproteins from the PO.KR-IC construct did not react withMAb 17/80 specific to VP3 (Fig. 4b and d), for which thereasons are not clear.The 28-kDa (VP4) protein has been found in very minute
and variable amounts in mature IBDV particles (4). Thisfinding, along with our results from site-specific mutagenesisof the VP4 coding region, suggests that VP4 may be aproteaselike product that is functional when expressed in E.coli. However, no significant homology between VP4 andother known viral or other proteases has been found in oursearch of nucleic acid (GenBank) and protein (NBRF) databases. So, VP4 could be a new type of protease character-istic of the Birnaviridae family, except that in infectious
J. VIROL.

NOTES 1087
Cl) C)
200.
b0 -
bO6-0
- -
.4
32--
:.
FIG. 4. Western blot analysis of extracts of E. ccwith PO and PO.KR-IC on 12.5% (a and b) or 7polyacrylamide gel run under SDS denaturing condiicleotide-directed base substitutions were performecontaining the XcyI (SmaI)-PstI fragment from I
BMH71-18 and HB2151, essentially as described by CTwo primers, (i) 5' GTGGGGGAAACATATGATGtype negative strand, 5' GTGGGGGAAACGTTTCand (ii) 5' CTTTCGAGCTCGGGGGT 3' (wild-type i5' CTTTCGAGTTCGGGGGT 3'), were used to alterresidues at positions 721 and 722 by substitution wilresidues and to remove the EcoK site-encoding seqaltering the amino acid residues at position 763, respeii allowed us to exploit the EcoK selection properHB2151 and BMH71-18. The mutagenized fragmeniinto pEX3 to generate mutagenized clone PO.KR-panels a and c were probed with MAb 6, and blots id were probed with MAb 17/80. The bands werereaction with [1251]protein A and autoradiography.bands are approximate and are deduced from molecdards (not shown).
pancreatic necrosis virus, the only other well-(member of the group (7, 14), VP4 has surphomology. This perhaps suggests diversity in tand functional aspects of VP4 products and prprotein processing among the Birnaviridae me
ever, further characterization of the VP4 oinfectious pancreatic necrosis virus as well asmembers of the family is required to verify thiof the nonhomologous sequences of VP4.
Site-directed mutational changes are being ithe large segment of IBDV to identify the protage sites. Identification of the cleavage sitiflanking sequences would greatly facilitate the uof the mechanism of IBDV polyprotein prnimprovement in processing efficiency will lehigher yield of VP2 and VP3 in E. coli. Purifiedcan then be used as subunit vaccines in chicke
We thank K. J. Fahey and colleagues for the monoies, I. Macreadie and P. Vaughan for critical readinscript and discussions, and Melissa Brown and I
technical assistance.This research was supported in part by the Aust
Biotechnology Program research grants scheme.
LITERATURE CITED
1. Azad, A. A., S. A. Barrett, and K. J. Fahey. 1985. Thecharacterization and molecular cloning of the double-strandedRNA genome of an Australian strain of infectious bursal diseasevirus. Virology 143:35-44.
2. Azad, A. A., K. J. Fahey, S. A. Barrett, K. M. Erny, and P. J.Hudson. 1986. Expression in Escherichia coli of cDNA frag-
220 ments encoding the gene for the host protective antigen of60 infectious bursal disease virus. Virology 149:190-198.
2a.Azad, A. A., M. N. Jagadish, M. A. Brown, and P. J. Hudson.1987. Deletion mapping and expression in Escherichia coli ofthe large genomic segment of a birnavirus. Virology 161:145-152.
6 3. Bathurst, I. C., S. 0. Brennan, R. W. Carrell, L. S. Conseus,A. J. Brake, and P. J. Barr. 1987. Yeast KEX2 protease has theproperties of a human proalbumin converting enzyme. Science235:348-350.
4. Becht, H. 1980. Infectious bursal disease virus. Curr. Top.Microbiol. Immunol. 90:107-121.
5. Carter, P., H. Bedouelle, and G. Winter. 1985. Improved oligo-nucleotide site-directed mutagenesis using M13 vectors. Nu-cleic Acids Res. 13:4431 4443.
6. Douglas, J., 0. Civelli, and E. Herbert. 1984. Polyprotein geneexpression: generation of diversity of neuroendocrine peptides.
sli transformed Annu. Rev. Biochem. 53:665-715.7.5% (c and d) 7. Duncan, R., and P. Dobos. 1986. The nucleotide sequence oftions. Oligonu- infectious pancreatic necrosis virus (IPNV) dsRNA segment A-d in M13mp8 reveals one large ORF encoding a precursor polyprotein. Nu-PO in E. coli cleic Acids Res. 14:5934.-arter et al. (5). 8. Fahey, K. J., I. J. O'Donnell, and A. A. Azad. 1985. Character-'AAC 3' (wild- ization by Western blotting of immunogens of infectious bursaliATGAAC 3') disease virus. J. Gen. Virol. 66:1479-1488.negtive strand, 9. Fahey, K. J., I. J. O'Donnell, and T. J. Bagust. 1985. Antibodythe dibasic KR to the 32 kDa structural protein of infectious bursal disease virusth nonbasic IC neutralizes viral infectivity in vitro and confers protection oniuence without young chickens. J. Gen. Virol. 66:2693-2702.ctively. Primer 10. Hudson, P. J., N. M. McKern, B. E. Power, and A. A. Azad.rties of E. coli 1986. Genomic structure of the large RNA segment of infectioust was replaced bursal disease virus. Nucleic Acids Res. 14:5001-5012.-IC. Blots in 11. Jagadish, M. N., and A. A. Szalay. 1984. Directed transposonIn panels b and TnS mutagenesis and complementation in slow-growing broadvisualized by host range cowpea Rhizobium. Mol. Gen. Genet. 196:290-
. The sizes of 300.:ular size stan- 12. Julius, D., A. Brake, L. Blair, R. Kunisawa, and J. Thorner.
1984. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of
characterized 13. yeast prepro-a-factor. Cell 37:1075-1089.arisinglylittele 13. Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular
xrisingly little cloning: a laboratory manual. Cold Spring Harbor Laboratory,the structural Cold Spring Harbor, N.Y.ecursor poly- 14. Mertens, P. P. C., and P. Dobos. 1982. Messenger RNA ofmbers. How- infectious pancreatic necrosis virus is polycistronic. Natureof IBDV and (London) 297:243-246.,all the other 15. Nick, H., D. Cursiefen, and H. Becht. 1976. Structural ande significance growth characteristics of infectious bursal disease virus. J.
Virol. 18:227-234.
introduced in 16. Oka, A., Sugisaki, H., and M. Takanami. 1981. Nucleotideeolytic cleav- sequence of the kanamycin resistance transposon Tn 903. J.esoandytic eav- Mol. Biol. 147:217-226.:es and their 17. Schwartz, T. W. 1986. The processing of peptide precursors.Inderstanding FEBS Lett. 200:1-9.ocessing. An 18. Simon, R., R. Priefer, and A. Puhler. 1983. A broad host rangead toward a mobilization system for in vivo genetic engineering: transposonVP2 and VP3 mutagenesis in gram negative bacteria. Biotechnology 1:784-ins. 791.
19. Stanley, K. K., and J. P. Luzio. 1984. Construction of a newclonal antibod- family of high-efficiency bacterial expression vectors: identifi-ig of the manu- cation of cDNA clones coding for human liver proteins. EMBOPaul Failla for J. 3:1429-1434.
20. Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7ralian National derived system for insertion mutagenesis and sequencing with
synthetic universal primers. Gene 19:259-268.
VOL. 62, 1988
Related Documents