Biology 1111K Lecture 2

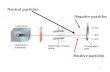
Biology 1111K Lecture 2. Slide 2 - particles Slide 3 – particle charge.
Dec 28, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Slide 4 – Atomic symbols
Element Atomic symbol
Hydrogen H
Carbon C
Potassium K
Magnesium Mg
Calcium Ca
Slide 10 – C 14 half-life sample
Time (Y) Isotope Amount Element Amount
0 C14 20 N14 0
1500 C14 17.5 N14 2.5
3000 C14 15 N14 5
6000 C14 10 N14 10
Slide 16 – chemical formulas
H2O – water - 2 hydrogen and one oxygen
CO2 – carbon dioxide - 1 carbon, 2 oxygen
H2SO4 – sulfuric acid – 2 hydrogen, one sulfur, and four oxygen
HCl – hydrochloric acid – one hydrogen, one chlorine
Slide 25 – acids and bases
Acids – molecules that dissociates in water and releases hydrogen ions (H+). When dissociation is complete, the acid is called a strong acid.
HCl H+ and OH-
H2SO4 H+ and HSO4-
Bases – molecules that either takes up hydrogen ions (H+) or releases hydroxide ions (OH-). When dissociation is complete, the base is known as a strong base.
NaOH Na + and OH –
Pure water is neutral since in its rare ionic form it gives off equal numbers of hydrogen and hydroxide ions.
H2O H+ and OH-
Slide 28 – inorganic vs. organic molecules
Inorganic molecule Organic molecule
Usually contains positive and negative ions Always contains carbon and hydrogen
Usually ionic bonding Covalent bonding
Contains small numbers of atoms Large numbers of atoms
Associates with non-living matters Living matters
Slide 35 – polymers and monomers
Polymer Monomer
Polysaccharide Monosaccharide
Polypeptide Amino acid
Nucleic acid Nucleotide
Slide 53 – purines and pyrimidine
Pyrimidine Purine
DNA Cytosine (C)
Thymine (T)
Adenine (A)
Guanine (G)
RNA Cytosine (C)
Uracil (U)
Adenine (A)
Guanine (G)
Related Documents