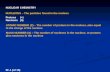
Atomic Number Number of Protons

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Characteristics of Electron
Charge = -1, mass = 1/1836 amu or 0.0005 amu, location =
outside nucleus
2 isotopes of Cl: 75% Cl-35 & 25% Cl-37.Calculate avg. atomic mass.
Avg. atomic mass = .75(35) + .25(37) = 35.5 amu
Rutherford’s Experiment: Results
1) Most of the alpha particles went straight through. Most of the atom is empty space.
2) Some of the alpha particles were deflected back. The nucleus was tiny, but contained most of the mass of the atom.
Schrodinger’s Model
Modern or Quantum Mechanical Model
Source: http://www.dlt.ncssm.edu/TIGER/chem1.htm#atomic
Bohr Configuration of Na = 2-8-1
2 electrons in energy level 18 electrons in energy level 21 electron in energy level 3
Bohr Model
Electrons are restricted to specific orbits or shells or principle energy levels.Each shell holds a specific # of electrons.Each shell has a specific energy & radius.Energy of electron must match energy of shell.
Excited State
Bohr modelAn electron has absorbed heat, light, or electrical energy and moved to a higher energy level.Unstable. Returns to ground state quickly by emitting a photon.
Continuous Spectrum
Spectrum produced by holding a prism in sunlight. Contains light at every wavelength.
Bright Line Spectrum
Visible light produced by electrons in atom returning to ground state: light of only a few wavelengths is present.
Each element has a unique bright line spectrum. Used to identify elements.
Wavelengths of bright lines correspond to difference between energy levels.
Source: http://www.dlt.ncssm.edu/TIGER/chem1.htm#atomic
Related Documents