Review Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions Sophie Vandermoten a, * , Mark C. Mescher b , Frédéric Francis a , Eric Haubruge a , François J. Verheggen a a University of Liege, Gembloux Agro-Bio Tech, Department of Functional and Evolutionary Entomology, Passage des Déportés 2, B-5030 Gembloux, Belgium b The Pennsylvania State University, Department of Entomology, Center for Chemical Ecology, University Park, PA 16802, USA article info Article history: Received 28 September 2011 Received in revised form 25 November 2011 Accepted 30 November 2011 Keywords: Aphid Alarm pheromone (E)-beta-farnesene Tritrophic interactions Myzus persicae Acyrthosiphon pisum Pheromone biosynthesis abstract Aphids are important agricultural and forest pests that exhibit complex behaviors elicited by pheromonal signals. The aphid alarm pheromone e of which (E)-b-farnesene is the key (or only) component in most species e plays important roles in mediating interactions among individuals as well as multitrophic interactions among plants, aphids, and aphid natural enemies. Though many important questions remain to be answered, a large body of research has addressed various aspects of the biology, physiology, and ecology of aphid alarm pheromones. Here we review recent advances in our understanding of (a) the identity and composition of aphid alarm signals; (b) their biosynthesis and production; (c) their effects on conspecifics; (d) their role as cues for other insect species; and (e) their potential application for the management of pest organisms. Ó 2011 Elsevier Ltd. All rights reserved. 1. Introduction Signaling in insects is dominated by the transmission of semi- ochemicals (from the Greek “semeon” meaning signal). A further distinction is commonly drawn between pheromones, which are directed at conspecifics, and allelochemicals which are directed at members of other species (Nordlund and Lewis, 1976), though, as discussed below, the same semiochemical can serve both functions simultaneously. The first semiochemical to be isolated and identi- fied was the sex pheromone of the silk moth, Bombyx mori L. (Butenandt et al., 1959). In the time since that discovery, extensive research on insect pheromones has resulted in the chemical and/or behavioral elucidation of pheromone components from over 1500 species (Thomson et al., 1999; Tillman et al., 1999). Though insect pheromones are chemically diverse across taxa, they may be broadly classified into six basic functional groups (sex, aggregation, dispersal, alarm, trail and maturation) based on behavioral and physiological reactions induced in conspecifics (Tillman et al., 1999). Alarm pheromones are widespread among insects and other animals (Verheggen et al., 2010) and are the second most commonly produced pheromone used by insects, after sex pheromones (Barbier, 1982). Alarm-pheromone compounds tend to be structurally simple molecules with low molecular weight and high volatility, in keeping with their function as indicators of impending dangers localized in time and space (Payne, 1974). Alarm signaling is thought to be particularly adaptive for species, such as aphids, in which individuals aggregate and exhibit behav- ioral responses to this stimulus (Blum, 1985). Aphids (Homoptera: Aphididae: Aphidinae) are among the most abundant and destructive insect pests of agriculture, particularly in temperate regions, causing direct damage to arable and horticul- tural crops as well as serving as vectors for many important plant diseases (e.g., Basky and Nasser, 1989; Ng and Perry, 2004; Robert et al., 2000; Woodford et al., 1995). Many aphid species engage in cyclical parthenogenesis, which allows rapid population growth and the exploitation of host plant resources. As with most other insect interactions, the signals that mediate conspecific interactions among aphids are primarily chemical; and the most prominent and well-studied interactions among aphids are those mediated by “alarm” pheromones (Verheggen et al., 2010). In addition to warning of the presence of immediate danger, aphid alarm pheromones play a number of additional roles in aphid ecology, including as key foraging cues for many aphid predators (discussed in Section V). Here we review recent literature addressing the identity of aphid alarm pheromones, details of their * Corresponding author. Tel.: þ32 (0)81 622 287; fax: þ32 (0)81 622 312. E-mail addresses: [email protected], entomologie.gembloux@ulg. ac.be (S. Vandermoten). Contents lists available at SciVerse ScienceDirect Insect Biochemistry and Molecular Biology journal homepage: www.elsevier.com/locate/ibmb 0965-1748/$ e see front matter Ó 2011 Elsevier Ltd. All rights reserved. doi:10.1016/j.ibmb.2011.11.008 Insect Biochemistry and Molecular Biology xxx (2012) 1e9 Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016/j.ibmb.2011.11.008

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
at SciVerse ScienceDirect
Insect Biochemistry and Molecular Biology xxx (2012) 1e9
Contents lists available
Insect Biochemistry and Molecular Biology
journal homepage: www.elsevier .com/locate/ ibmb
Review
Aphid alarm pheromone: An overview of current knowledge on biosynthesisand functions
Sophie Vandermoten a,*, Mark C. Mescher b, Frédéric Francis a, Eric Haubruge a, François J. Verheggen a
aUniversity of Liege, Gembloux Agro-Bio Tech, Department of Functional and Evolutionary Entomology, Passage des Déportés 2, B-5030 Gembloux, Belgiumb The Pennsylvania State University, Department of Entomology, Center for Chemical Ecology, University Park, PA 16802, USA
a r t i c l e i n f o
Article history:Received 28 September 2011Received in revised form25 November 2011Accepted 30 November 2011
Keywords:AphidAlarm pheromone(E)-beta-farneseneTritrophic interactionsMyzus persicaeAcyrthosiphon pisumPheromone biosynthesis
* Corresponding author. Tel.: þ32 (0)81 622 287; faE-mail addresses: [email protected],
ac.be (S. Vandermoten).
0965-1748/$ e see front matter � 2011 Elsevier Ltd.doi:10.1016/j.ibmb.2011.11.008
Please cite this article in press as: Vandermfunctions, Insect Biochemistry and Molecula
a b s t r a c t
Aphids are important agricultural and forest pests that exhibit complex behaviors elicited by pheromonalsignals. The aphid alarm pheromone e of which (E)-b-farnesene is the key (or only) component in mostspecies e plays important roles in mediating interactions among individuals as well as multitrophicinteractions among plants, aphids, and aphid natural enemies. Though many important questions remainto be answered, a large body of research has addressed various aspects of the biology, physiology, andecology of aphid alarm pheromones. Here we review recent advances in our understanding of (a) theidentity and composition of aphid alarm signals; (b) their biosynthesis and production; (c) their effectson conspecifics; (d) their role as cues for other insect species; and (e) their potential application for themanagement of pest organisms.
� 2011 Elsevier Ltd. All rights reserved.
1. Introduction
Signaling in insects is dominated by the transmission of semi-ochemicals (from the Greek “semeon” meaning signal). A furtherdistinction is commonly drawn between pheromones, which aredirected at conspecifics, and allelochemicals which are directed atmembers of other species (Nordlund and Lewis, 1976), though, asdiscussed below, the same semiochemical can serve both functionssimultaneously. The first semiochemical to be isolated and identi-fied was the sex pheromone of the silk moth, Bombyx mori L.(Butenandt et al., 1959). In the time since that discovery, extensiveresearch on insect pheromones has resulted in the chemical and/orbehavioral elucidation of pheromone components from over 1500species (Thomson et al., 1999; Tillman et al., 1999).
Though insect pheromones are chemically diverse across taxa,they may be broadly classified into six basic functional groups (sex,aggregation, dispersal, alarm, trail and maturation) based onbehavioral and physiological reactions induced in conspecifics(Tillman et al., 1999). Alarm pheromones are widespread amonginsects and other animals (Verheggen et al., 2010) and are the
x: þ32 (0)81 622 312.entomologie.gembloux@ulg.
All rights reserved.
oten, S., et al., Aphid alarm pr Biology (2012), doi:10.1016
secondmost commonly produced pheromone used by insects, aftersex pheromones (Barbier, 1982). Alarm-pheromone compoundstend to be structurally simplemolecules with lowmolecular weightand high volatility, in keeping with their function as indicators ofimpending dangers localized in time and space (Payne, 1974).Alarm signaling is thought to be particularly adaptive for species,such as aphids, in which individuals aggregate and exhibit behav-ioral responses to this stimulus (Blum, 1985).
Aphids (Homoptera: Aphididae: Aphidinae) are among themostabundant and destructive insect pests of agriculture, particularly intemperate regions, causing direct damage to arable and horticul-tural crops as well as serving as vectors for many important plantdiseases (e.g., Basky and Nasser, 1989; Ng and Perry, 2004; Robertet al., 2000; Woodford et al., 1995). Many aphid species engage incyclical parthenogenesis, which allows rapid population growthand the exploitation of host plant resources. As with most otherinsect interactions, the signals that mediate conspecific interactionsamong aphids are primarily chemical; and the most prominent andwell-studied interactions among aphids are those mediated by“alarm” pheromones (Verheggen et al., 2010).
In addition to warning of the presence of immediate danger,aphid alarm pheromones play a number of additional roles in aphidecology, including as key foraging cues for many aphid predators(discussed in Section V). Here we review recent literatureaddressing the identity of aphid alarm pheromones, details of their
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e92
production and emission, and their importance in mediatingecological interactions among aphids and between aphids andother organisms. In addition to being of basic scientific interest, thechemical ecology of aphid alarm signaling has significant appliedrelevance because of the status of these insects as important agri-cultural pests, which may be exacerbated by the effects of climatechange and other ecological impacts of human activity (Andersonet al., 2004; Jones, 2009). There is currently significant andincreasing interest in the use of insect semiochemicals in detection,monitoring, and control programs for pests of agricultural crops(Norin, 2007), and the successful implementation of such strategiesrequires a sound understanding of underlying ecological factors.
2. Aphid alarm pheromones
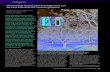
In response to attack by natural enemies, aphids secret dropletsof fluid from specialized structures, named cornicles, located on theupper, posterior surface of the abdomen (Fig. 1) (Büsgen, 1891;Dixon, 1958). The major components of the secretions are triglyc-erides, which appear to function as mechanical defenses by gluingappendages and the cephalic parts of natural enemies (Callow et al.,1973). But, these secretions also contain an alarm pheromone thatinduces behavioral responses in receiving conspecifics (Kislow andEdwards, 1972; Müller, 1983). The nature of the response varieswithin (Montgomery and Nault, 1978) and between species(Montgomery and Nault, 1977) as well as with the context ofpredation and costs of escape (e.g., Arakaki, 1989; Roitberg andMyers, 1978). Typically individuals receiving the alarm signal stopfeeding, move away from the signal, and often drop from the hostplant (Pickett et al., 1992).
Bowers et al. (1972) published the first chemical characteriza-tion of aphid alarm pheromones, identifying the sesquiterpene (E)-b-farnesene (EBF) as the primary component of the alarm phero-mone of several economically important species of aphids. Thiscompound has subsequently been shown to be the primary alarm
Fig. 1. The vetch aphid, Megoura viciae (Hemiptera, Aphididae), with arrows indicatingthe cornicles.
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
pheromone for many additional aphid species, including the greenpeach aphid, Myzus persicae (Sulzer) (Edwards et al., 1973; Franciset al., 2005b; Pickett and Griffith, 1980; Wientjens et al., 1973)and the pea aphid, Acyrthosiphon pisum (Harris) (Du et al., 1998;Francis et al., 2005b; Mostafavi et al., 1996). A second sesquiter-pene, germacrene A, was isolated from alfalfa aphids [Therioaphismaculata (Buckton) and Therioaphis riehmi (Börner)] and shown tofunction as an alarm pheromone (Bowers et al., 1977; Nishino et al.,1977); however, the signalling activity of this compound is limitedto the genus Therioaphis. Later, Pickett and Griffith (1980) showedthat, in addition to EBF, the alarm signal of Megoura viciae Bucktonincluded several monoterpenes (a-pinene, b-pinene and b-limo-nene), with a-pinene having the most important alarm activity.Recently, Francis et al. (2005b) examined volatile emissions ofcrushed aphids from twenty-three species and found that EBF wasthe major, or only, volatile compound released by sixteen of thespecies and aminor component for another five. The remaining twospecies, Euceraphis punctipennis (Zetterstedt) and Drepanosiphumplatanoides (Schrank), did not display any EBF after being crushed,though monoterpenes were isolated. Additional aphid-producedvolatile compounds may also be involved in the process of alarmsignaling, but their biological activity remains to be characterized.
The behavioral response to EBF exhibited by some cruciferousaphids [e.g., Lipaphis erysimi (Kaltenbach) and Brevicoryne brassicae(L.)] is weak compared to that of most other EBF-producing species(Dawson et al., 1986, 1987). These aphids, which feed exclusively onplants in the Brassicaceae, have evolved a particular biochemicalmechanism that accomplishes degradation of plant glucosinolatesinto volatile compounds named isothiocyanates (Francis et al.,2004; Jones et al., 2001). In addition to being directly toxic tosome natural enemies of aphids (Francis et al., 2001; Vanhaelenet al., 2001), isothiocyanates have been shown to act as synergistsof EBF and thereby play an essential key role in the alarm signalingof crucifer-feeding aphids (Dawson et al., 1986, 1987).
3. Recent discoveries relating to the biosynthetic pathway ofaphid alarm pheromone
As described above, three metabolites (all isoprenoids) havebeen shown to have aphid alarm pheromone activity: EBF, ger-macrene A, and a-pinene (Fig. 2). We currently have little directknowledge of the mechanisms by which these compounds areproduced in aphids or their regulation. Byers (2005) observed thatquantities of EBF present in individuals of the cotton aphid, Aphisgossypii Glover, were correlated with body weight and suggestedthat such correlation was consistent with pheromone productionover all life stages. However, there is no strong evidence that aphidsproduce alarm pheromone at all developmental stages, and thehypothesis that alarm pheromone components such as EBF areproduced only by early instars cannot be rejected. As the emissionof aphid alarm pheromone occurs almost instantaneously inresponse to attack or other inducing stimuli, it seems very likelythat the production of pheromone components occurs in antici-pation of an attack rather than at the time of emission, but thelocation and mechanistic details of alarm pheromone storageremain unknown. Studies using radiolabeled intermediates orprecursors could be helpful in confirming the de novo synthesis ofalarm pheromone in aphids. However, this approach ideallyrequires knowledge of the timing of product formation that iscurrently unavailable for aphids. Moreover, the calibration oflabelling studies to ensure that sufficient recovery of labeledmaterial without disruption of normal metabolism or productformation can be challenging (Morgan, 2010).
Despite our limited knowledge of the exact biosynthetic path-ways producing aphid alarm pheromone, the biosynthesis of
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
Fig. 2. Pathways leading to the production of terpene compounds in aphids; white and gray boxes denote known and putative pathways, respectively. The first schematic shows thepathway involving prenyltransferases and leading to the production of mono- and sesquiterpene precursors. The two other schematics show the conversion reactions of mono- andsesquiterpene precursors into a-pinene, germacrene A and (E)-b-farnesene. These reactions are catalyzed by enzymes belonging to the class of terpene synthases. DMAPP,dimethylallyl diphosphate; IPP, isopentenyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate. OPP denotes the diphosphate moiety.
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e9 3
similar mono- and sesquiterpenes is well documented in othersystems, especially plants (Davis and Croteau, 2000; Dewick, 2002;Hick et al., 1999; Yu and Utsumi, 2009). In the remainder of thissection, we describe recent discoveries regarding terpene biosyn-thesis (in aphids, other insects, and plants) that may shed light onthe biosynthesis of the isoprenoid components of aphid alarmpheromones.
3.1. Early steps of isoprenoid biosynthesis catalyzed byprenyltransferases
Most of the approximately 30,000 known terpenes are gener-ated by the mevalonate pathway or, the methyl-erythritol 4-phosphate pathway in plant plastids, through a common biosyn-thetic mechanism in which the C5 dimethylallyl diphosphate(DMAPP, C5) undergoes a 10-4 condensation with its homoallylicisomer, isopentenyl diphosphate (IPP, C5), to form geranyl diphos-phate (GPP, C10). The latter may then undergo additional conden-sations with IPP to yield longer products such as farnesyldiphosphate (FPP, C15) and geranylgeranyl diphosphate (GGPP,C20). These three prenyl diphosphate compounds are the commonprecursors of monoterpenes, sesquiterpenes, and diterpenesrespectively (Poulter and Rilling, 1981).
Generally, each of these diphosphates is generated by a specificshort-chain isoprenyl diphosphate synthase (also referred to as“prenyltransferase”) named according to its final product: geranyldiphosphate synthase, farnesyl diphosphate synthase, and ger-anylgeranyl diphosphate synthase (Fig. 2) [for detailed descriptionsof theses enzymes see Vandermoten et al. (2009a)].
The first biochemical evidence for the de novo synthesis ofa monoterpene in insects came from studies of the production ofthe aggregation pheromone components ipsdienol and E-myrcenolin double spined bark beetles (Ivarsson et al., 1993). The first animalgeranyl diphosphate synthase was subsequently obtained bycloning cDNA from the bark beetle Ips pini (Say) (Gilg et al., 2005),and its implication in the biosynthesis of an aggregation
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
pheromone was demonstrated (Blomquist et al., 2010). Interest-ingly, in addition to its main prenyltransferase activity, the enzymedisplayed terpene synthase activity and was able to produce myr-cene when GPP alone is provided as a substrate (Gilg et al., 2009).Similar bifunctional activity was observed with the recombinantMalus domestica Borkh (apple) a-farnesene synthase, whichproduced little FPP under certain conditions (Green et al., 2007).
In insects, farnesyl diphosphate synthases have been mostintensively studied because of their implication in the biosyntheticpathway of the sesquiterpenoid juvenile hormone (Castillo-Graciaand Couillaud, 1999; Cusson et al., 2006; Kikuchi et al., 2001;Koyama et al., 1985; Sen and Sperry, 2002; Sen et al., 2007).Recently, a geranylgeranyl diphosphate gene involved in termitedefense secretions was also cloned (Hojo et al., 2007).
In aphids, Lewis et al. (2008) and Vandermoten et al. (2008)identified the first animal short-chain prenyltransferase that cangenerate both geranyl diphosphate (C10) and farnesyl diphosphate(C15), by using an in vitro assay with isopentenyl diphosphate (C5)and dimethylallyl diphosphate (C5) as substrates. Although therecombinant enzyme yielded more geranyl diphosphate than far-nesyl diphosphate e a tendency that became more pronouncedwith increasing concentrations of dimethylallyl diphosphate e itgenerated only farnesyl diphosphate when supplied with geranyldiphosphate as the sole allylic co-substrate. In addition, therecombinant aphid enzyme could generate bothmonoterpenes andsesquiterpenes in linked assays where appropriate terpene syn-thases were added to the assay buffer (Lewis et al., 2008).
In an extension of this work, Vandermoten et al. (2009b) usedhomology modelling in combination with site-directed mutagen-esis and molecular dynamics to show that both Gln107 and Leu110residues are implicated in the mechanism by which aphid geranyldiphosphate/farnesyl diphosphate synthase regulates its productratio. Compared to other prenyltransferases, for which regulation ofproduct chain-length is mainly defined by the steric hindranceprovided by amino acid residues neighbouring the first aspartate-rich motif (one of the seven conserved domains present in all
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e94
prenyltransferases), the mechanism in aphids seems morecomplex, depending also on the nature of the interactions amongkey residues of the binding pocket and on substrate concentrations.
3.2. Final steps of isoprenoid biosynthesis catalyzed by terpenesynthases
To date, no terpene synthases have been reported from aphids.However several studies provide information regarding thebiosynthesis of mono- and sesquiterpenes in plants. Based on thiswork, the cloning of a geranyl diphosphate/farnesyl diphosphatesynthase in several aphid species (Lewis et al., 2008;Ma et al., 2010;Vandermoten et al., 2008) and evidence that the isoprenoidcomponents of the aphid alarm pheromone including EBF areproduced by a pathway linked to juvenile hormone (Gut et al., 1987;van Oosten et al., 1990), a putative schematic of the terpenoidpathway in aphids can be proposed (Fig. 2).
As described by Bohlmann et al. (1997), monoterpene synthasescatalyze the divalent metal ion-dependent ionization and isomer-ization of GPP to enzyme-bound linalyl diphosphate, which,following rotation about C2eC3, undergoes a second ionizationfollowed by cyclization to the a-terpinyl cation, the cyclic inter-mediate for both monocyclic and bicyclic products. Cyclic mono-terpenes, such as pinenes, may arise by a subsequent deprotonationstep from the C3-methyl (to b-pinene) or from the methylene (C4)to a-pinene (Schwab et al., 2001).
The first step in reactions involving the sesquiterpene synthasesis the divalent cation-dependent ionization of the prenyl diphos-phate precursor to create an enzyme bound allylic carbocation(Davis and Croteau, 2000). The allylic carbocation generated in theionization step may then undergo a deprotonation from the C-3methyl (Crock et al., 1997) or a cyclization (de Kraker et al., 1998) toproduce EBF or germacrene A, respectively.
Most of the mono- and sesquiterpene synthases that have beencloned display a highly conserved aspartate-rich motif (DDxxD),involved in the binding of the diphosphate moiety of the allylicsubstrate and a conserved RxR motif (located 35 amino acidsupstream the DDxxD motif) presumed to help direct the diphos-phate anion away from the reactive carbocation after ionization(Keeling and Bohlmann, 2006).
Future efforts to identify the aphid terpene synthases may makeuse of cDNA library screening or similarity-based PCR cloningapproaches. But, previous attempts to use plant sequences forhomology cloning were unsuccessful (Lewis et al., 2008). Andalthough, the genome of the pea aphid, A. pisum, has recentlybecome available, the search for sequences displaying character-istic motifs of the terpene-synthase family (described above) hasyet to yield results (Gerardo et al., 2010). This is not necessarilysurprising, as comparison of the gene sequences of various plantterpene synthases shows that, amongmono-, sesqui- and diterpenesynthases, genes cloned from closely related plants were moresimilar to each other, irrespective of their functions, than to thosecloned from distant species but having the same function. Thus, wemay expect aphid terpene synthases to display a low sequencesimilarity to those used by plants, making the challenge of theiridentification more challenging.
4. Function of aphid alarm pheromone in signaling amongconspecifics
4.1. Benefits and costs related to emission of aphid alarmpheromone
In addition to disseminating the alarm pheromone (Kislow andEdwards, 1972), the release of sticky cornicle secretions e
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
containing mainly triglycerides (Callow et al., 1973) e may providedirect fitness benefits to the emitter, by gluing appendages (e.g.,mouthparts, antennae, ovipositor, etc.) of natural enemies (Strong,1967) permanently or temporarily incapacitating the attacker(Butler and O’Neil, 2006). However, aphids typically emit cornicledroplets not when initially encountering a predator but only afterbeing physically attacked (Nault and Phelan, 1984), resulting in theemitter’s escape in only w10% of attacks (Dixon, 1958; Edwards,1966). Thus, the function of cornicle secretions in colony defensemay be more significant than their role as a direct defensivemechanism for individual aphids. Indeed, given that nearby indi-viduals in the context of an aphid colony are often clone-mates,both alarm signals and physical defenses deployed during preda-tion events can presumably have substantial inclusive fitnessbenefits for the aphid producing them, as the nearby individualswho benefit share exactly the same genotype (Mondor andRoitberg, 2004).
Consistent with this observation, Robertson et al. (1995) foundthat A. pisum individuals were more likely to emit alarm phero-mone in response to simulated predation when living in proximityto conspecifics as opposed to heteroclonal conspecifics or hetero-specifics. Additional evidence for inclusive fitness benefits inaphids derives from the apparent role of cornicle secretions inscent-marking predators (Mondor and Roitberg, 2004). The emis-sion of cornicle droplets not only interferes mechanically with thepredation event, but can also deposit pheromone droplets directlyonto the predator. As a result, the tagged predator carries the alarmsignal as it continues to search for other individuals to prey on,thereby increasing the likelihood that clone-mates escape preda-tion. In a recent study, Wu et al. (2010) provided the evidence ofkin-directed altruistic defense by showing that grain-aphid corniclesecretions provide no direct fitness benefits to the signaler but dobenefit relatives.
Despite the apparent benefits of alarm signaling, considerableevidence indicates that the production and emission of corniclesecretions has both physiological and ecological costs for aphids.Because cornicle secretions primarily comprise triglycerides(Callow et al., 1973), they are likely costly to produce for aphids,which lack access to lipids in their diets. In addition, as EBF isproduced de novo (Gut and van Oosten,1985) in a pathway linked tojuvenile hormone (Gut et al., 1987; van Oosten et al., 1990),precursors leading to the formation of EBF could presumably beotherwise employed in other biosynthetic pathways thatcontribute to development and offspring production. Mondor andRoitberg (2003) found that the release of even a single cornicledroplet by third or fourth-instar pre-reproductive aphids hadmeasurable fitness consequences by delaying or decreasingreproduction. Moreover, alarm pheromone emissions can imposesignificant ecological costs, as the volatiles contained in them mayattract natural enemies to the emitting individual or to the colony(see Section V).
Aphids thus face trade-offs, in the production of alarm signals, inpart because signals that can enhance colony defense againstimmediate threats also serve as attractants for other naturalenemies. As a consequence, there should be a selective advantagefor aphids to minimize EBF emission to situations where thebenefits of alarm communication are higher than the costs ofreleasing EBF. For example, it has been demonstrated that A. pisumindividuals regulate their alarm pheromone emission according totheir social environment, with smaller colonies releasing less EBFthan larger ones (Verheggen et al., 2009). There is also evidencethat members of an aphid colony are not all equally able or inclinedto release alarm signals. In A. pisum, the amount of EBF in cornicledroplets was reported to increase significantly from the first to thesecond instars, remain high in third and fourth instars, and then
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e9 5
decrease significantly from fourth instars to 4-day-old adults(reproductives) (Mondor et al., 2000). The emission of higher levelof EBF by pre-reproductive adults might be correlated to the highprobability of having related clones nearby as pre-reproductiveaphids are most likely to occur in dense colonies (Mondor et al.,2000). In addition, adults may have a higher capability to escape(by walking away or dropping from the plant) compared to pre-reproductive aphids, which are less mobile (Mondor et al., 2000).In the same aphid species, Schwartzberg et al. (2008b) reported nosignificant difference in peak EBF emission among juvenile instars,but found that the peak emission of adults was significantly lower.Schwartzberg et al. (2008b) also examined the timing of alarmpheromone release under predation, observing that (i) the initialburst of alarm signal emission is likely to be the most important,because nearby aphids react within a few seconds of perceiving thesignal, but (ii) while most of the EBF that pea aphids producedduring attacks by lacewing larvae volatilized during the first15 min, measurable quantities continued to be emitted for 60 min.
Considering that aphid colonies are mostly clonal, aphids mightalso be expected to adjust their propensity to signal in response tothe genetic composition of the colony, but studies addressing thispossibility are currently lacking.
4.2. Responses mediated by aphid alarm pheromone
In most species of Aphidinae, EBF triggers behavioral responsesthat range from feeding cessation and withdrawal of the stylet todropping off the host plant (Pickett et al., 1992). In addition to theseimmediate behavioral responses, the perception of aphid alarmpheromone can also have longer-term consequences for aphidcolony composition and dispersal. For example, it has been shownthat aphid alarm pheromone mediates A. pisumwing polyphenismunder both laboratory (Kunert et al., 2005; Podjasek et al., 2005)and field conditions (Hatano et al., 2010) e aphid wing polyphen-isms have previously been reported in response to the presence ofboth natural enemies (Kunert and Weisser, 2003; Sloggett andWeisser, 2002; Weisser et al., 1999) and their search cues (Dixonand Agarwala, 1999; Mondor et al., 2005, 2004). The release ofthe alarm pheromone by attacked aphids causes increased move-ment of individual aphids within the colony, in turn increasing thefrequency of physical contact. Increased frequency of such contactsamong individuals e which presumably also occurs due tocrowding in dense populations e has been shown to result ina higher proportion of winged dispersing morphs among offspring(Kunert et al., 2005). However, Kunert andWeisser (2005) providedevidence that antennae are crucial for the elicitation of wing pol-yphenism in the presence of natural enemies, suggesting thatchemical cues play a direct role, perhaps in combination withtactile and/or visual cues, in the elicitation of such responses.
After exposing maturing A. pisum aphids to increasing concen-tration of EBF vapor, Podjasek et al. (2005) observed that winginduction in offspring was correlated in a relatively linear mannerto the amount of pheromone presented. However, Kunert et al.(2005) reported that A. pisum react more strongly to thefrequency of pheromone releases than to the amount of pheromonereleased during each signaling event. This observation is consistentwith the results of two later studies, which found that aphids do notpropagate or amplify the alarm signal by emitting additional EBF inresponse to perception of the alarm pheromone (Hatano et al.,2008a; Verheggen et al., 2008b). Thus, the frequency, rather thanthe intensity of alarm pheromone emission events, may determinethe scale of the response induced.
Interestingly, wing induction in response to EBF exposure wasnot observed when M. persicae colonies were reared on transgenicEBF-emitting plants (de Vos et al., 2010; Kunert et al., 2010), on
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
which aphids exhibit habituation to EBF (after three generations,aphids continued to emit EBF normally but did not exhibit a strongalarm response). Moreover de Vos et al. (2010) documentedsignificant gene expression changes 30 min after exposure to EBF(15% of the genes displayed an altered expression response).
Several other reports have shown variation in escape behavior,wing induction, or alarm pheromone emission among aphidspecies and lineages (Müller, 1983; Schwartzberg et al., 2008a;Weisser and Braendle, 2001), suggesting a genotypic basis forshort- and long-term responses to alarm pheromone exposure.
5. Function of aphid alarm pheromone in a multitrophiccontext
Although semiochemicals provide a powerful way for organismsto communicate and coordinate their behaviors, they also provideopportunities for other organisms to intercept and exploit signalsintended for conspecifics e as reviewed by Hatano et al. (2008b).Predators and parasitoids frequently exhibit innate responses tochemical cues reliably associated with hosts and prey, and there isalso abundant evidence that learning of profitable chemical cuesfrequently occurs (Du et al., 1997; Mölck et al., 2000). Consequently,there are numerous examples of natural enemies having learned orevolved to use the pheromones of their prey as foraging cues (Vetand Dicke, 1992), and this has led to exploration of the potentialuses of aphid semiochemicals within integrated managementstrategies to manipulate the behavior of aphid natural enemies (seeSection VI).
Among aphid natural enemies, primary parasitoids of aphids arefound in only two taxa, the sub-family Aphidiinae (Hymenoptera:Braconidae) and the genus Aphelinus (Hymenoptera: Aphelinidae).These two Hymenopteran groups, both specialized on aphids, laytheir eggs in larvae and adult instars of their host e as reviewed byLe Ralec et al. (2010). Although many of the long-range olfactorycues used by aphid parasitoids for host location may originate fromthe host plant, several studies indicate that aphid parasitoids mayuse honeydew or aphid pheromones as short-range cues e asreviewed by Rehman and Powell (2010). Micha and Wyss (1996)reported that EBF attracted Aphidius uzbekistanicus Luzhetskifemales in a Y-tube olfactometer bioassay, suggesting that EBF mayact as a host finding kairomone in this species. However, theseauthors suggested that its effects over longer distances might belimited due to the relatively high concentrations required to inducea response. Similar results were observed for the parasitoid Aphi-dius ervi (Haliday), which displayed an electrophysiologicalresponse to EBF but at levels higher than those found in aphidcornicle droplets (Du et al., 1998). More recently, Foster et al. (2005)observed that EBF attracted Diaeretiella rapae (McIntosh).
Many aphid predators have also been found to utilize aphidalarm pheromones as foraging cues. For example, electroantenno-gram recordings from a variety of predatory species showedconsistently higher responses to EBF than to other structurallysimilar molecules, indicating olfactory adaptation for the percep-tion of aphid alarm pheromone (Verheggen et al., 2008a; Zhu et al.,1999). Moreover, behavioral studies have demonstrated significanteffects of aphid alarm pheromone on the foraging behavior ofhoverflies (e.g., Francis et al., 2005a; Verheggen et al., 2008a);ground beetles (e.g., Kielty et al., 1996); and lady beetles (e.g., Acaret al., 2001; Al Abassi et al., 2000; Francis et al., 2005a; Verheggenet al., 2007) e though different groups of investigators have re-ported conflicting results regarding the attractiveness of EBF insome lady beetle species (Mondor and Roitberg, 2000; Nakamuta,1991). For most of these aphid natural enemies, variation in thenature and intensity of responses to aphid alarm pheromone isobserved among species and between larval and adult stages.
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e96
In addition to effects on aphid natural enemies, aphid alarmpheromone has been shown to mediate mutualistic interactionsbetween ants and aphids, in which ants protect “myrmecophilic”aphids while collecting aphid honeydew (a rich source of carbo-hydrates). Nault et al. (1976) observed that ants became veryaggressive in the presence of alarm pheromone and increased theirrate of attack on aphid predators, but did not attack aphids. Incontrast, when alarm pheromone was applied to colonies of aphidspecies that are not ant-tended (non-myrmecophilic), ants becameaggressive towards the aphids themselves (Nault et al., 1976). Morerecently, ants’ ability to perceive EBFwas confirmed byMondor andAddicott (2007).
It should be noted that many of the experiments, detailed aboveand demonstrating evidences of the attractiveness of aphid alarmpheromone to aphid natural enemies, were performed undervariable EBF concentrations: sometimes using concentrationshigher than those typically emitted by aphids under enemy attack.Thus, while there is strong evidence that aphid alarm pheromoneacts as a foraging cue for many aphid predators and parasitoids,additional studies conducted under realistic field conditions wouldhelp to clarify the exact role of such cues (e.g., in short- and long-range host location) and their ecological significance.
6. Potential uses of alarm pheromone for management ofaphids
In addition to obviously intriguing questions regarding basicbiology, physiology and ecology of aphid alarm pheromone, thestudy of these semiochemicals also has applied implications for themanagement of pest species in agriculture. Insect pheromoneshave already been successfully deployed for pest management. Themost common approaches to date have used sex pheromones e
especially lepidopteran sex pheromones (Copping, 2001) e formass trapping or mating disruption via air permeation (Witzgallet al., 2010). Efforts have also been made to incorporate alarmpheromones as repellents in push-pull strategies, in order to makethe protected resource unattractive to the pest (Cook et al., 2007).
Following identification of EBF as the key component of theaphid alarm pheromone of most species of Aphidinae, researchersbegan discussing the possibility of using this semiochemical torepel aphids (Bowers et al., 1972). The potential use of aphid alarmpheromone as a direct control mechanism has been explored ina number of studies. For example, a significant reduction in thenumber of A. pisum adults on plants treated with EBF was reported(Wohlers, 1981). It has also been suggested that the wild potatomay directly repel M. persicae aphids by releasing EBF in pulses,thereby mimicking the EBF emission by aphids (Gibson and Pickett,1983). And EBF appears to reduce settling of the pea aphid and bluealfalfa aphid on alfalfa (Mostafavi et al., 1996). However, thisbehavioral response appears to be strongly affected by othercommon plant volatiles, including (E)-b-caryophyllene, which actsas an inhibitor of alarm pheromone activity (Dawson et al., 1984;Mostafavi et al., 1996). Considering the instability of synthetic EBFwhen exposed to air, Bruce et al. (2005) investigated the potentialuse of essential oils with high EBF content and observed that slow-release formulations of essential oil fromHemizygia petiolata Ashby(Lamiaceae) reduced pea aphid populations on plants (Bruce et al.,2005). Finally, laboratory and field experiment conducted on peaaphids (Hatano et al., 2010; Kunert et al., 2005) demonstrated thatEBF treatments mediate wing induction, and thus might reduceaphid population density.
Less promising results were obtained by Kunert et al. (2010)when investigating whether EBF emission provided benefits fortransgenic EBF-emitting plants against the aphid M. persicae.Although no metabolic cost of EBF synthesis was demonstrated,
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
there was no evidence of direct benefits for the plants: EBF emis-sion did not appear to repelM. persicae, reduce its reproduction, norincrease the proportion of winged offspring. These authors sug-gested two possible explanations: (i) habituation to EBF of aphidsreared on these constitutively EBF-producing plants and (ii) thataphids might only react to EBF emitted in pulses (mimicking EBFemission by aphids as noted above).
However, even if the (continuous) production of EBF by trans-genic plants is not effective as a direct defense against aphids, thistechnique might have promising applications in integrated pestmanagement via the kairomone function of EBF as a cue for aphidnatural enemies. Indeed, Beale et al. (2006) first reported thetransformation of Arabidopsis thaliana (L.) Heynh. plants to producethe aphid alarm pheromone EBF and demonstrated that EBFemission enhanced the level of M. persicae control achieved byaphid parasitoids D. rapae (McIntosh). More recently, de Vos et al.(2010), using the same aphid-plant system, observed that habitu-ated M. persicae aphids failed to respond to aphid-emitted EBF andsuffered of higher levels of predation by the coccinelid predatorHippodamia convergens Guérin-Méneville. Further studies areneeded to determine whether such effects can be effectivelyexploited to enhance biological control of aphid pests in agricul-tural settings.
7. Conclusions
As described above, recent work on diverse topics relating to theproduction and ecological functions of aphid alarm pheromoneshas greatly enhanced our understanding of the biology and ecologyof these signals. The alarm pheromones of several aphid specieshave been isolated and identified, and numerous studies haveaddressed the behavioral responses induced in receiving individ-uals and the ecological roles of aphid alarm pheromone in intra-and interspecific interactions. Moreover, these studies suggestpotentially promising avenues for the deployment of aphid alarmpheromones for the management of these pest species. Despitethese advances, many fundamental questions about aphid alarmsignaling remain unanswered. Priorities for future work in the areaof the biosynthesis of aphid alarm compounds should includeidentifying the enzymes involved into the last step of mono- andsesquiterpene biosynthesis, determining the location of theirproduction and storage, and elucidating their regulation.
Furthermore, while there is some evidence that the size andcomposition of aphid colonies are modulating factors of EBFemission, we still lack a detailed understanding of how aphidsregulate alarm pheromone production and emission in response toecological and social context and of the individual and the inclusivefitness benefits that balance the apparent physiological andecological costs. Moreover, while laboratory experiments areinvaluable tools for revealing mechanisms, additional field studiesare needed to test ecological relevance of the observed effects. Forexample, it remains to be determined whether aphid alarm pher-omone (applied in pulse treatments or produced by transgenic EBF-emitting plants) might be attractive to natural enemies under fieldconditions. Future work should also more fully explore the broaderecological context in which signaling occurs, paying particularattention to the volatiles emitted by plants that may have syner-gistic or antagonistic effects on foraging behavior of aphid naturalenemies.
The information gained from a deeper understanding of thebiosynthesis and regulation of aphid alarm pheromone as well asthe chemical ecology of aphid-natural enemy interaction willenhance our understanding of the chemical biology and ecology ofaphids, an important group of insect pests, and may facilitate thedesign of novel control strategies.
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e9 7
Acknowledgments
This work was supported by post-doctoral fellowship to S.Vandermoten awarded by the National Fund for Scientific Research(Belgium).
References
Acar, E.B., Medina, J.C., Lee, M.L., Booth, G.M., 2001. Olfactory behavior of convergentlady beetles (Coleoptera: Coccinellidae) to alarm pheromone of green peachaphid (Hemiptera: Aphididae). Can. Entomol. 133, 389e397.
Al Abassi, S., Birkett, M.A., Pettersson, J., Pickett, J.A., Wadhams, L.J., Woodcock, C.M.,2000. Response of the seven-spot ladybird to an aphid alarm pheromone andan alarm pheromone inhibitor is mediated by paired olfactory cells. J. Chem.Ecol. 26, 1765e1771.
Anderson, P.K., Cunningham, A.A., Patel, N.G., Morales, F.J., Epstein, P.R., Daszak, P.,2004. Emerging infectious diseases of plants: pathogen pollution, climatechange and agrotechnology drivers. Trends Ecol. Evol. 19, 535e544.
Arakaki, N., 1989. Alarm pheromone eliciting attack and escape responses in thesugar-cane wooly aphid, Ceratovacuna-lanigera (Homoptera, Pemphigidae).J. Ethol. 7, 83e90.
Barbier, M., 1982. Les phe
romones. Aspects biochimiques et biologiques. Masson,Paris, p. 140.
Basky, Z., Nasser, M.A., 1989. The activity of virus vector aphids on cucumbers. Agr.Ecosyst. Environ. 25, 337e342.
Beale, M.H., Birkett, M.A., Bruce, T.J.A., Chamberlain, K., Field, L.M., Huttly, A.K.,Martin, J.L., Parker, R., Phillips, A.L., Pickett, J.A., Prosser, I.M., Shewry, P.R.,Smart, L.E., Wadhams, L.J., Woodcock, C.M., Zhang, Y.H., 2006. Aphid alarmpheromone produced by transgenic plants affects aphid and parasitoidbehavior. Proc. Natl. Acad. Sci. USA 103, 10509e10513.
Blomquist, G.J., Teran, R.F., Aw, M., Song, M., Gorzalski, A., Abbot, N., Chang, E.,Tittiger, C., 2010. Pheromone production in bark beetles. Insect Biochem. Mol.Biol. 40, 699e712.
Blum, M.S., 1985. Alarm pheromones. In: Kerkut, G.A., Gilbert, L.I. (Eds.), Compre-hensive Insect Physiology, Biochemistry and Pharmacology. Pergamon Press,Oxford, pp. 193e224.
Bohlmann, J., Steele, C.L., Croteau, R., 1997. Monoterpene synthases from Grand fir(Abies grandis) e cDNA isolation, characterization, and functional expression ofmyrcene synthase, (-)(4S)-limonene synthase, and (-)-(1S,5S)-pinene synthase.J. Biol. Chem. 272, 21784e21792.
Bowers, W.S., Nault, L.R., Webb, R.E., Dutky, S.R., 1972. Aphid alarm pheromone:isolation, identification, synthesis. Science 177, 1121e1122.
Bowers, W.S., Nishino, C., Montgomery, M.E., Nault, L.R., Nielson, M.W., 1977.Sesquiterpene progenitor, Germancrene A: an alarm pheromone in aphids.Science 196, 680e681.
Bruce, T.J.A., Birkett, M.A., Blande, J., Hooper, A.M., Martin, J.L., Khambay, B.,Prosser, I., Smart, L.E., Wadhams, L.J., 2005. Response of economically importantaphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 61,1115e1121.
Büsgen, M., 1891. Der honigtau biologische studien an pflanzen und pflanzenlaüsen.Jena. Zeits. Naturwiss. 25, 339e428.
Butenandt, A., Beckmann, R., Stamm, D., Hecker, E., 1959. Über den sexuallockstoffden seidenspinners Bombyx mori. Reindarstellung und konstitution.Z. Naturforschung 14b, 283e284.
Butler, C.D., O’Neil, R.J., 2006. Defensive response of soybean aphid (Hemiptera:Aphididae) to predation by insidious flower bug (Hemiptera: Anthocoridae).Ann. Entomol. Soc. Am. 99, 317e320.
Byers, J.A., 2005. A cost of alarm pheromone production in cotton aphids, Aphisgossypii. Naturwissenschaften 92, 69e72.
Callow, R.K., Greenway, A.R., Griffiths, D.C., 1973. Chemistry of the secretion fromthe cornicles of various species of aphids. J. Insect Physiol. 19, 737e748.
Castillo-Gracia, M., Couillaud, F., 1999. Molecular cloning and tissue expression of aninsect farnesyl diphosphate synthase. Eur. J. Biochem. 262, 365e370.
Cook, S.M., Khan, Z.R., Pickett, J.A., 2007. The use of Push-Pull strategies in inte-grated pest managment. Annu. Rev. Entomol. 52, 375e400.
Copping, L.G., 2001. The Biopesticide Manual: a World Compendium of NaturallyOccuring Biopesticides. The British Crop Protection Council, Farnham, UK, p.450.
Crock, J., Wildung, M., Croteau, R., 1997. Isolation and bacterial expression ofa sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.)that produces the aphid alarm pheromone (E)-â-farnesene. Proc. Natl. Acad. Sci.USA 94, 12833e12838.
Cusson, M., Béliveau, C., Sen, S.E., Vandermoten, S., Rutledge, R.G., Stewart, D.,Francis, F., Haubruge, E., Rehse, P., Huggins, D.J., Dowling, A.P.G., Grant, G.H.,2006. Characterization and tissue-specific expression of two lepidopteran far-nesyl diphosphate synthase homologs: implications for the biosynthesis ofethyl-substituted juvenile hormones. Proteins 65, 742e758.
Davis, E.M., Croteau, R., 2000. Cyclization enzymes in the biosynthesis of mono-terpenes, sesquiterpenes, and diterpenes. Top. Curr. Chem. 209, 54e95.
Dawson, G.W., Griffiths, D.C., Pickett, J.A., Smith, M.C., Woodcock, C.M., 1984.Natural inhibition of the aphid alarm pheromone. Entomol. Exp. Appl. 36,197e199.
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
Dawson, G.W., Griffiths, D.C., Pickett, J.A., Wadhams, L.J., Woodcock, C.M., 1986.Plant compounds that synergize activity of the aphid alarm pheromone. in:British Crop Protection Conference-Pests and Diseases Proc, pp. 829e834.
Dawson, G.W., Griffiths, D.C., Pickett, J.A., Wadhams, L.J., Woodcock, C.M., 1987.Plant-derived synergists of alarm pheromone from turnip aphid, Lipaphis(Hyadaphis) erysimi (Homoptera, Aphididae). J. Chem. Ecol. 13, 1663e1671.
de Kraker, J.W., Franssen, M.C.R., de Groot, A., Konig, W.A., Bouwmeester, H.J., 1998.(þ)-Germacrene A biosynthesis e The committed step in the biosynthesis ofbitter sesquiterpene lactones in chicory. Plant Physiol. 117, 1381e1392.
de Vos, M., Cheng, W.Y., Summers, H.E., Raguso, R.A., Jander, G., 2010. Alarmpheromone habituation in Myzus persicae has fitness consequences and causesextensive gene expression changes. Proc. Natl. Acad. Sci. USA 107, 14673e14678.
Dewick, P.M., 2002. The biosynthesis of C5eC25 terpenoid compounds. Nat. Prod.Rep. 19, 181e222.
Dixon, A.F.G., 1958. The escape responses shown by certain aphids to the presence ofthe coccinellid Adalia decempunctata (L.). Trans. R Ent Soc. London 110, 319e334.
Dixon, A.F.G., Agarwala, B.K., 1999. Ladybird-induced life-history changes in aphids.Proc. R. Soc. Lond. B. 266, 1549e1553.
Du, Y., Poppy, G.M., Powell, W., Wadhams, L.J., 1997. Chemically mediated associa-tive learning in the host foraging behavior of the aphid parasitoid Aphidius ervi(Hymenoptera: Braconidae). J. Insect Behav. 10, 509e522.
Du, Y., Poppy, G.M., Powell, W., Pickett, J.A., Wadhams, L.J., Woodcock, C.M., 1998.Identification of semiochemicals released during aphid feeding that attractparasitoid Aphidius ervi. J. Chem. Ecol. 24, 1355e1368.
Edwards, J.S., 1966. Defence by Smear: supercooling in the cornicle Wax of aphids.Nature 211, 73e74.
Edwards, L.J., Siddball, J.B., Dunham, L.L., Uden, P., Kislow, C.J., 1973. Trans-beta-farnesene, alarm pheromone of the green peach aphid, Myzus persicae (Sulzer).Nature 241, 126e127.
Foster, S.P., Denholm, I., Thompson, R., Poppy, G.M., Powell, W., 2005. Reducedresponse of insecticide-resistant aphids and attraction of parasitoids to aphidalarm pheromone; a potential fitness trade-off. Bull. Entomol. Res. 95, 37e46.
Francis, F., Lognay, G., Wathelet, J.P., Haubruge, E., 2001. Effects of allelochemicalsfrom first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae)trophic levels on Adalia bipunctata. J. Chem. Ecol. 27, 243e256.
Francis, F., Lognay, G., Haubruge, E., 2004. Olfactory responses to aphid and hostplant volatile releases: E-B-Farnesene an effective kairomone for the predatorAdalia bipunctata. J. Chem. Ecol. 30, 741e755.
Francis, F., Martin, T., Lognay, G., Haubruge, E., 2005a. Role of (E)-ß-farnesene insystematic aphid prey location by Episyrphus balteatus larvae (Diptera: Syr-phidae). Eur. J. Entomol. 102, 431e436.
Francis, F., Vandermoten, S., Verheggen, F., Lognay, G., Haubruge, E., 2005b. Is the(E)-ß-farnesene only volatile terpenoid in aphids? J. App. Entomol. 129, 6e11.
Gerardo, N.M., Altincicek, B., Anselme, C., Atamian, H., Barribeau, S.M., de Vos, M.,Duncan, E.J., Evans, J.D., Gabaldon, T., Ghanim, M., Heddi, A., Kaloshian, I.,Latorre, A., Moya, A., Nakabachi, A., Parker, B.J., Perez-Brocal, V., Pignatelli, M.,Rahbe, Y., Ramsey, J.S., Spragg, C.J., Tamames, J., Tamarit, D., Tamborindeguy, C.,Vincent-Monegat, C., Vilcinskas, A., 2010. Immunity and other defenses in peaaphids, Acyrthosiphon pisum. Genome Biol. 11, R21.
Gibson, R.W., Pickett, J.A., 1983. Wild potato repels aphids by release of aphid alarmpheromone. Nature 302, 608e609.
Gilg, A.B., Bearfield, J.C., Titigger, C., Welch, W.H., Blomquist, G.J., 2005. Isolation andfunctional expression of an animal geranyl diphosphate synthase, and its role inbark beetle pheromone biosynthesis. Proc. Natl. Acad. Sci. USA 102, 9760e9765.
Gilg, A.B., Tittiger, C., Blomquist, G.J., 2009. Unique animal prenyltransferase withmonoterpene synthase activity. Naturwissenschaften 96, 731e735.
Green, S., Friel, E.N., Matich, A., Beuning, L.L., Cooney, J.M., Rowan, D.D., MacRae, E.,2007. Unusual features of a recombinant apple alpha-farnesene synthase.Phytochem 68, 176e188.
Gut, J., van Oosten, A.M., 1985. Functional-significance of the alarm pheromonecomposition in various morphs of the green peach aphid, Myzus persicae.Entomol. Exp. Appl. 37, 199e204.
Gut, J., Harrewijn, P., van Oosten, A.M., van Rheenen, B., 1987. Additional functions ofalarm pheromones in development processes of aphids. Meded. Fac. Land-bouwwet. Rijksuniv. Gent. 52, 371e378.
Hatano, E., Kunert, G., Bartram, S., Boland, W., Gershenzon, J., Weisser, W.W., 2008a.Do aphid colonies amplify their emission of alarm pheromone? J. Chem. Ecol.34, 1149e1152.
Hatano, E., Kunert, G., Michaud, J.P., Weisser, W.W., 2008b. Chemical cues mediatingaphid location by natural enemies. Eur. J. Entomol. 105, 797e806.
Hatano, E., Kunert, G., Weisser, W.W., 2010. Aphid wing induction and ecologicalcosts of alarm pheromone emission under field conditions. Plos One 5 (6),e11188.
Hick, A.J., Luszniak, M.C., Pickett, J.A., 1999. Volatile isoprenoids that control insectbehaviour and development. Nat. Prod. Rep. 16, 39e54.
Hojo, M., Matsumoto, T., Miura, T., 2007. Cloning and expression of a geranylgeranyldiphosphate synthase gene: insights into the synthesis of termite defencesecretion. Insect Mol. Biol. 16, 121e131.
Ivarsson, P., Schlyter, F., Birgersson, G., 1993. Demonstration of de Novo pheromonebiosynthesis in Ips duplicatus (Coleoptera: Scolytidae): inhibition of Ipsdienoland E-Myrcenol production by compactin. Insect Biochem. Mol. Biol. 23,655e662.
Jones, R.A.C., 2009. Plant virus emergence and evolution: Origins, new encounterscenarios, factors driving emergence, effects of changing world conditions, andprospects for control. Virus Res. 141, 113e130.
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e98
Jones, A.M., Bridges, M., Bones, A.M., Cole, R., Rossiter, J.T., 2001. Purification andcharacterization of a non-plant myrosinase from the cabbage aphid Brevicorynebrassicae (L.). Insect Biochem. Mol. Biol. 31, 1e5.
Keeling, C.I., Bohlmann, J., 2006. Genes, enzymes and chemicals of terpenoiddiversity in the constitutive and induced defence of conifers against insects andpathogens. New Phytol. 170, 657e675.
Kielty, J.P., Allen-Williams, L.J., Underwood, N., Eastwood, E.A., 1996. Behavioralresponses of three species of ground beetle (Coleoptera: Carabidae) to olfactorycues associated with prey and habitat. J. Insect Behav. 9, 237e250.
Kikuchi, K., Hirai, M., Shiotsuki, T., 2001. Molecular cloning and tissue distribution offarnesyl pyrophosphate synthase from the silkworm. J. Insect Biotech. Sericol.70, 167e172.
Kislow, C., Edwards, L.J., 1972. Repellent odour in aphids. Nature 235, 108e109.Koyama, T., Matsubara, M., Ogura, K., 1985. Isoprenoid enzyme systems of silkworm.
II. Formation of the juvenile hormone skeletons by farnesyl pyrophosphatesynthase II. J. Biochem. 98, 457e463.
Kunert, G., Weisser, W.W., 2003. The interplay between density- and trait-mediatedeffects in predator-prey interactions: a case study in aphid wing polymorphism.Oecologia 135, 304e312.
Kunert, G., Weisser, W.W., 2005. The importance of antennae for pea aphid winginduction in the presence of natural enemies. Bull. Entomol. Res. 95, 125e131.
Kunert, G., Otto, S., Röse, U.S., Gershenzon, J., Weisser, W.W., 2005. Alarm phero-mone mediates production of winged dispersal morphs in aphids. Ecol. Lett. 8,596e603.
Kunert, G., Reinhold, C., Gershenzon, J., 2010. Constitutive emission of the aphidalarm pheromone, (E)-beta-farnesene, from plants does not serve as a directdefense against aphids. BMC Ecol. 10, 23.
Le Ralec, A., Anselme, C., Outreman, Y., Poirié, M., van Baaren, J., Le Lann, C., vanAlphen, J.J.M., 2010. Evolutionary ecology of the interactions between aphidsand their parasitoids. C. R. Biol. 333, 554e565.
Lewis, M.J., Prosser, I.M., Mohib, A., Field, L.M., 2008. Cloning and characterisation ofa prenyltransferase from the aphid Myzus persicae with potential involvementin alarm pheromone biosynthesis. Insect Mol. Biol. 17, 437e443.
Ma, G.-Y., Sun, X.-F., Zhang, Y.-L., Li, Z.-X., Shen, Z.-R., 2010. Molecular cloning andcharacterization of a prenyltransferase from the cotton aphid, Aphis gossypii.Insect Biochem. Mol. Biol. 40, 552e561.
Micha, S.G., Wyss, U., 1996. Aphid alarm pheromone (E)-beta-farnesene: a hostfinding kairomone for the aphid primary parasitoid Aphidius uzbekistanicus(Hymenoptera: Aphidiinae). Chemoecology 7, 132e139.
Mölck, G., Pinn, H., Wyss, U., 2000. Manipulation of plant odour preference bylearning in the aphid parasitoid Aphelinus abdominalis (Hymenoptera: Aphe-linidae). Eur. J. Entomol. 97, 533e538.
Mondor, E., Addicott, J., 2007. Do exaptations facilitate mutualistic associationsbetween invasive and native species? Biol. Invasions 9, 623e628.
Mondor, E.B., Roitberg, B.D., 2000. Has the attraction of predatory coccinellids tocornicle droplets constrained aphid alarm signaling behavior? J. Insect Behav.13, 321e329.
Mondor, E.B., Roitberg, B.D., 2003. Age-dependent fitness costs of alarm signaling inaphids. Can. J. Zool. 81, 757e762.
Mondor, E.B., Roitberg, B.D., 2004. Inclusive fitness benefits of scent-markingpredators. Proc. R. Soc. Lond. B. 271, S341eS343.
Mondor, E.B., Baird, D.S., Slessor, K.N., Roitberg, B.D., 2000. Ontogeny of alarmpheromone secretion in pea aphid, Acyrthosiphon pisum. J. Chem. Ecol. 26,2875e2882.
Mondor, E.B., Tremblay, M.N., Lindroth, R.L., 2004. Transgenerational phenotypicplasticity under future atmospheric conditions. Ecol. Lett. 7, 941e946.
Mondor, E.B., Rosenheim, J.A., Addicott, J.F., 2005. Predator-induced transgenera-tional phenotypic plasticity in the cotton aphid. Oecologia 142, 104e108.
Montgomery, M.E., Nault, L.R., 1977. Comparative response of aphids to alarmpheromone, (E)-beta-farnesene. Entomol. Exp. Appl. 22, 236e242.
Montgomery, M.E., Nault, L.R., 1978. Effects of age and wing polymorphism onsensitivity of Myzus persicae to alarm pheromone. Ann. Entomol. Soc. America71, 788e790.
Morgan, E.D., 2010. Biosynthesis in Insects: Advanced Edition. Royal Society ofChemistry, London, p. 362.
Mostafavi, R., Henning, J., Gardea-Torresday, J., Ray, I., 1996. Variation in aphid alarmpheromone content among glandular and eglandular-haired Medicag acces-sions. J. Chem. Ecol. 22, 1629e1638.
Müller, F., 1983. Differential alarm pheromone responses between strains of theaphid Acyrthosiphon pisum. Entomol. Exp. Appl. 34, 347e348.
Nakamuta, K., 1991. Aphid alarm pheromone component, (E)-beta-farnesene, andlocal search by a predatory lady beetle, Coccinella septempunctata Bruckiimulsant (Coleoptera, Coccinellidae). Appl. Entomol. Zool. 26, 1e7.
Nault, L.R., Phelan, P.L., 1984. Alarm pheromones and sociality in pre-social insects.In: Bell, W.J., Cardé, R.T. (Eds.), Chemical Ecology of Insects. Chapman and Hall,London, pp. 237e256.
Nault, L.R., Montgomery, M.E., Bowers, W.S., 1976. Ant-aphid association: role ofaphid alarm pheromone. Science 192, 1349e1351.
Ng, J.C.K., Perry, K.L., 2004. Transmission of plant viruses by aphid vectors. Mol.Plant Pathol. 5, 505e511.
Nishino, C., Bowers, W.S., Montgomery, M.E., Nault, L.R., Nielson, M.W., 1977. Alarmpheromone of the spotted alfalfa aphid, Therioaphis maculata Buckton(Homoptera:Aphididae). J. Chem. Ecol. 3, 349e357.
Nordlund, D.A., Lewis, W.J., 1976. Terminology of chemical releasing stimuli inintraspecific and interspecific interactions. J. Chem. Ecol. 2, 211e220.
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
Norin, T., 2007. Semiochemicals for insect pest management. Pure Appl. Chem. 79,2129e2136.
Payne, T.L., 1974. Pheromone perception. In: Birch, M.C. (Ed.), Pheromones. North-Holland publishing Company, Amsterdam, pp. 35e61.
Pickett, J.A., Griffith, D.C., 1980. Composition of aphid alarm pheromones. J. Chem.Ecol. 6, 349e360.
Pickett, J.A., Wadhams, L.J., Woodcock, C.M., 1992. The chemical ecology of aphids.Ann. Rev. Entomol. 37, 67e90.
Podjasek, J.O., Bosnjak, L.M., Brooker, D.J., Mondor, E.B., 2005. Alarm pheromoneinduces a transgenerational wing polyphenism in the pea aphid, Acyrthosiphonpisum. Can. J. Zool. 83, 1138e1141.
Poulter, C.D., Rilling, H.C., 1981. Prenyl transferases and isomerase. In: Porter, J.W.,Spurgeon, S.L. (Eds.), Biosynthesis of Isoprenoid Compounds. John Wiley andSons, New York, p. 161.
Rehman, A., Powell, W., 2010. Host selection behaviour of aphid parasitoids(Aphidiidae: Hymenoptera). J. Plant Breeding Crop Sci. 2, 299e311.
Robert, Y., Woodford, J.A.T., Ducray-Bourdin, D.G., 2000. Some epidemiologicalapproaches to the control of aphid-borne virus diseases in seed potato crops innorthern Europe. Virus Res. 71, 33e47.
Robertson, I., Roitberg, B., Williamson, I., Senger, S., 1995. Contextual chemicalecology: an evolutionary approach to the chemical ecology of insects. Am.Entomol. 41, 237e239.
Roitberg, B.D., Myers, J.H., 1978. Adaptation of alarm pheromone reponses of peaaphid Acyrthosiphon pisum (Harris). Can. J. Zool. 56, 103e108.
Schwab,W.,Williams, D.C., Davis, E.M., Croteau, R., 2001.Mechanismofmonoterpenecyclization: stereochemical aspects of the transformation of noncyclizablesubstrate analogs by recombinant (-)-limonene synthase, (þ)-bornyl diphos-phate synthase, and (-)-pinene synthase. Arch. Biochem. Biophys. 392, 123e136.
Schwartzberg, E.G., Kunert, G., Röse, U.S.R., Gershenzon, J., Weisser, W.W., 2008a.Alarm pheromone emission by pea aphid, Acyrthosiphon pisum, clones underpredation by lacewing larvae. Entomol. Exp. Appl. 128, 403e409.
Schwartzberg, E.G., Kunert, G., Stephan, C., David, A., Röse, U.S.R., Gershenzon, J.,Boland, W., Weisser, W.W., 2008b. Real-time analysis of alarm pheromoneemission by the pea aphid (Acyrthosiphon pisum) under predation. J. Chem. Ecol.34, 76e81.
Sen, S.E., Sperry, A.E., 2002. Partial purification of farnesyl diphosphate synthasefrom whole-body Manduca sexta. Insect Biochem. Mol. Biol. 32, 889e899.
Sen, S.E., Trobaugh, C., Beliveau, C., Richard, T., Cusson, M., 2007. Cloning, expressionand characterization of a dipteran farnesyl diphosphate synthase. Insect Bio-chem. Mol. Biol. 37, 1198e1206.
Sloggett, J.J., Weisser, W.W., 2002. Parasitoids induce production of the dispersalmorph of the pea aphid, Acyrthosiphon pisum. Oikos 98, 323e333.
Strong, F.E., 1967. Observations on aphid cornicle secretions. Ann. Entomol. Soc. Am.60, 668e673.
Thomson, D.R., Gut, L.J., Jenkins, J.W., 1999. Pheromones for insect control: strate-gies and successes. In: Hall, F.R., Menn, J.J. (Eds.), Biopesticides: Use andDelivery. Humana Press Inc., Totowa, New Jersey, pp. 385e412.
Tillman, J.A., Seybold, S.J., Jurenka, R.A., Blomquist, G.J., 1999. Insect pheromones ean overview of biosynthesis and endocrine regulation. Insect Biochem. Mol.Biol. 29, 481e514.
van Oosten, A.M., Gut, J., Harrewijn, P., Piron, P.G.M., 1990. Role of farnesene isomersand other terpenoids in the development of different morphs and forms of theaphids Aphis fabae and Myzus persicae. Acta Phytopathol. Entomol. Hung. 25,331e342.
Vandermoten, S., Charloteaux, B., Santini, S., Sen, S.E., Béliveau, C., Vandenbol, M.,Francis, F., Brasseur, R., Cusson, M., Haubruge, E., 2008. Characterization ofa novel aphid prenyltransferase displaying dual geranyl/farnesyl diphosphatesynthase activity. FEBS Lett. 582, 1928e1934.
Vandermoten, S., Haubruge, E., Cusson, M., 2009a. New insights into short-chainprenyltransferases: structural features, evolutionary history and potential forselective inhibition. Cell. Mol. Life Sci. 66, 3685e3695.
Vandermoten, S., Santini, S., Haubruge, E., Heuze, F., Francis, F., Brasseur, R.,Charloteaux, B., 2009b. Structural features conferring dual geranyl/farnesyldiphosphate synthase activity to an aphid prenyltransferase. Insect Biochem.Mol. Biol. 39, 707e716.
Vanhaelen, N., Haubruge, E., Lognay, G., Francis, F., 2001. Hoverfly glutathione S-transferases and effect of Brassicaceae secondary metabolites. Pest Biochem.Physiol. 71, 170e177.
Verheggen, F.J., Fagel, Q., Heuskin, S., Lognay, G., Francis, F., Haubruge, E., 2007.Electrophysiological and behavioral responses of the multicolored asian ladybeetle, Harmonia axyridis (Pallas), to sesquiterpene semiochemicals. J. Chem.Ecol. 33, 2148e2155.
Verheggen, F.J., Arnaud, L., Bartram, S., Gohy, M., Haubruge, E., 2008a. Aphid andplant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol.34, 301e307.
Verheggen, F.J., Mescher, M.C., Haubruge, E., De Moraes, C.M., Schwartzberg, E.G.,2008b. Emission of alarm pheromone in aphids: A non-contagious phenom-enon. J. Chem. Ecol. 34, 1146e1148.
Verheggen, F.J., Haubruge, E., De Moraes, C.M., Mescher, M.C., 2009. Social enviro-ment influences aphid production of alarm pheromone. Behav. Ecol. 20,283e288.
Verheggen, F., Haubruge, E., Mescher, M.C., 2010. Alarm pheromones. In: Litwack, G.(Ed.), Vitamins and Hormones. Elsevier, New York, pp. 215e239.
Vet, L.E.M., Dicke, M., 1992. Ecology of infochemical use by natural enemies ina tritrophic context. Annu. Rev. Entomol. 37, 141e172.
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
S. Vandermoten et al. / Insect Biochemistry and Molecular Biology xxx (2012) 1e9 9
Weisser, W.W., Braendle, C., 2001. Body colour and genetic variation in wingedmorph production in the pea aphid. Entomol. Exp. Appl. 99, 217e223.
Weisser, W.W., Braendle, C., Minoretti, N., 1999. Predator-induced morphologicalshift in the pea aphid. Proc. R. Soc. Lond. B. 266, 1175e1181.
Wientjens, W.H., Lakwijk, A.C., Vanderma, T., 1973. Alarm pheromone of grainaphids. Experientia 29, 658e660.
Witzgall, P., Kirsch, P., Cork, A., 2010. Sex pheromones and their impact on pestmanagement. J. Chem. Ecol. 36, 80e100.
Wohlers, P., 1981. Effects of (E)-beta-farnesene on dispersal behavior of the peaaphid Acyrthosiphon pisum. Entomol. Exp. Appl. 29, 117e124.
Please cite this article in press as: Vandermoten, S., et al., Aphid alarm pfunctions, Insect Biochemistry and Molecular Biology (2012), doi:10.1016
Woodford, J.A.T., Jolly, C.A., Aveyard, C.S., 1995. Biological factors influencing the trans-mission of potato leafroll virus by different aphid species. Potato Res. 38, 133e141.
Wu, G.-M., Boivin, G., Brodeur, J., Giraldeau, L.-A., Outreman, Y., 2010. Altruisticdefence behaviours in aphids. BMC Evol. Biol. 10, 19.
Yu, F., Utsumi, R., 2009. Diversity, regulation, and genetic manipulation of plantmono- and sesquiterpenoid biosynthesis. Cell. Mol. Life Sci. 66, 3043e3052.
Zhu, J.W., Cossé, A.A., Obrycki, J.J., Boo, K.S., Baker, T.C., 1999. Olfactory reactions ofthe twelve-spotted lady beetle, Coleomegilla maculata and the green lacewing,Chrysoperla carnea to semiochemicals released from their prey and host plant:electroantennogram and behavioral responses. J. Chem. Ecol. 25, 1163e1177.
heromone: An overview of current knowledge on biosynthesis and/j.ibmb.2011.11.008
Related Documents











![[ pheromone ] 01](https://static.cupdf.com/doc/110x72/568caab71a28ab186da2ad9b/-pheromone-01.jpg)