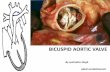
doi:10.1510/mmcts.2008.003806 Repair of aortic valve cusp prolapse Munir Boodhwani a, *, Laurent de Kerchove b , David Glineur b , Phillipe Noirhomme b , Gebrine El Khoury b a Division of Cardiac Surgery, University of Ottawa Heart Institute, Ottawa, Ontario, Canada b Department of Cardiovascular and Thoracic Surgery Cliniques Universitaires Saint-Luc, Avenue Hippocrate 10, 1200 Brussels, Belgium Aortic valve preservation and repair is emerging as a feasible and attractive alternative to aortic valve replacement in young patients with aortic valve insufficiency. Cusp pathology requiring repair is present in up to 50% of patients undergoing aortic valve repair or valve preserving surgery and may occur in isolation or in conjunction with ascending aortic disease. Diagnosis of cusp prolapse can usually be made on preoperative echocardiography and is confirmed on surgical inspection. Techniques available for the correction of cusp prolapse in a trileaflet aortic valve include free margin plication, and free-margin resuspension. These techniques can be used alone or in combination and both provide stable midterm results. Choice of technique may, therefore, be tailored to the cusp pathology encountered. Keywords: Aortic valve; Cusp repair; Surgical technique; Valve repair; Valve sparing Introduction Although valve preserving root replacement surgery, using the reimplantation w1x or remodeling w2x techniques, is increasingly being used to treat aortic root disease, techniques for aortic valve repair for isolated aortic insufficiency (AI) are applied heterogeneously and infrequently. A major limitation to the more generalized application of aortic 1

Aortic Valve Repiar
Dec 26, 2015
Aortic Valve Repiar
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

doi:10.1510/mmcts.2008.003806
Repair of aortic valve cusp prolapse
Munir Boodhwania,*, Laurent de Kerchoveb, David Glineurb, Phillipe Noirhommeb, Gebrine El Khouryb
a
Division of Cardiac Surgery, University of Ottawa Heart Institute, Ottawa, Ontario, Canada
b
Department of Cardiovascular and Thoracic Surgery Cliniques Universitaires Saint-Luc,Avenue Hippocrate 10, 1200 Brussels, Belgium
Aortic valve preservation and repair is emerging as a feasible and attractive alternative to aortic valve replacement in young patients with aortic valve insufficiency. Cusp pathology requiring repair is present in up to 50% of patients undergoing aortic valve repair or valve preserving surgery and may occur in isolation or in conjunction with ascending aortic disease. Diagnosis of cusp prolapse can usually be made on preoperative echocardiography and is confirmed on surgical inspection. Techniques available for the correction of cusp prolapse in a trileaflet aortic valve include free margin plication, and free-margin resuspension. These techniques can be used alone or in combination and both provide stable midterm results. Choice of technique may, therefore, be tailored to the cusp pathology encountered.
Keywords: Aortic valve; Cusp repair; Surgical technique; Valve repair; Valve sparing
Introduction
Although valve preserving root replacement surgery, using the reimplantation w1x or remodeling w2x techniques, is increasingly being used to treat aortic root disease, techniques for aortic valve repair for isolated aortic insufficiency (AI) are applied heterogeneously and infrequently. A major limitation to the more generalized application of aortic valve repair techniques is the absence of a common framework for valve assessment which can help to guide the approach to valve repair. Over the past decade, inspired by other classifications w3, 4x, we have developed a classification for AI which encompasses all types of AI, provides a common language for communication across
* Corresponding author. Dr. Munir Boodhwani, Service de Chirurgie Cardiovasculaire et Thoracique, Cliniques Universitaires Saint-LucUCL 90, Avenue Hippocrate 10, 1200 Brussels, Belgium.
Tel.: q32-2-764-6106; fax: q32-2-764-8960.E-mail: [email protected]
2009 European Association for Cardio-thoracic Surgery
different disciplines, guides the repair techniques employed, and can help to predict mid-term outcome w5x (Schematic 1).
Type I lesions, as in Carpentier’s classification of mitral valve regurgitation, are associated with normal leaflet motion. This is largely due to lesions of the functional aortic annulus with type Ia AI due to sino-tubular junction enlargement and dilatation of the ascending aorta, type Ib due to dilatation of the sinuses of Valsalva and the sino-tubular junction, type Ic due to dilatation of the ventriculo-aortic junction, and lastly type 1d due to cusp perforation without a primary functional aortic annulus lesion. Type II AI is due to cusp prolapse secondary to excessive cusp tissue or due to commissural disruption. Type III AI is
1

M. Boodhwani et al. / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2008.003806
due to leaflet restriction which may be found in bicuspid, degenerative, or rheumatic valvular disease due to calcification, thickening, and fibrosis of the aortic valve leaflets. Repair of cusp prolapse (type II dysfunction), thus, is one component of a larger frame-
Schematic 1. Repair-oriented functional classification of aortic insufficiency with a description of disease mechanisms and repair techniques employed. (STJ, sino-tubular junction; SCA, subcommissural annuloplasty). Multiple mechanisms of insufficiency may be present in one valve. In particular, patients with type Ia (ascending aortic dilatation) and type Ib (aortic root dilatation) should be examined for the presence of cusp prolapse (type II). Adapted from ref. w5x.
work of aortic valve repair techniques that need to be applied in a systematic manner in order to achieve a durable outcome. It is important to note that patients may present with multiple lesions contributing to their AI. In particular, patients with dilatation of the supracoronary ascending aorta (type Ia) and aortic root dilatation (type Ib) may present with concomitant cusp prolapse (type II) which should be carefully assessed.
Definition and echocardiographic diagnosis of cusp prolapse
Normal aortic valve cusp coaptation occurs approximately at a level corresponding to the middle of the sinuses of Valsalva, i.e. halfway between the ventriculo-aortic junction and the sinotubular junction. Cusp prolapse, therefore, is strictly defined as the motion of the cusp free margin below this level. In the setting of a trileaflet aortic valve and single cusp prolapse, the prolapse can be appreciated relative to the other normal cusps, or may be defined, in an absolute manner, as the free margin of the cusp is observed below the physiologic coaptation level. Occasionally, symmetric prolapse of multiple cusps may be present, typically after a valve sparing root
replacement procedure. In this scenario, all three cusps may coapt below the physiologic coaptation level without the presence of significant AI. Despite the absence of AI in this setting, cusp repair with the objective of restoring normal coaptation is critical for the long-term function of the valve.Cusp prolapse can usually be detected echocardiographically but requires confirmation and quantification during surgical inspection. Echocardiographic features of cusp prolapse include the presence of an eccentric AI jet in the opposite direction of the prolapsing cusp, visualization of the valve cusp below the level of the aortic annulus during diastole, and a diminished length of aortic leaflet coaptation. These features can be appreciated during transesophageal echocardiography on the mid-esophageal long axis view of the aortic valve. Furthermore, a transverse fibrous band may be observed on the prolapsing cusp, both on long axis and short axis views, which helps to confirm the diagnosis and localize the prolapsing cusp (Video 1). Surgical assessment of the aortic valve is critical for the diagnosis and quantification of cusp prolapse and is discussed below.
Quantification of AI on echocardiography using jet area can be challenging in the setting of an eccentric AI jet, because these wall-hugging jets can flatten out against the wall of the left ventricle. Thus, jet area may underestimate the severity of AI. Use of vena contracta and flow convergence methods e.g. proximal isovelocity surface area (PISA) can help to quantify the insufficiency but also have some limitations in the setting of eccentric AI. In patients with chronic AI, the presence of symptoms and/or evidence of left ventricular dilatation (LV end diastolic diameter G70 mm or LV end systolic diameter G50 mm) or reduction in LV function are indications for surgical intervention.
Surgical techniquesA median sternotomy is performed with cannulation of the distal ascending aorta and right atrium in patients with isolated lesions of the aortic valve and/ or root. The aorta is cross-clamped, and a left ventricular vent is inserted via the right superior pulmonary vein. Antegrade, normothermic blood cardioplegia is administered either through the aortic root or directly through the coronary ostia in the case of moderate-severe AI.
2

M. Boodhwani et al. / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2008.003806
Video 1. Echocardiographic features of aortic insufficiency due to cusp prolapse on transesophageal (A) long axis, and (B) short axis views. (This video is looping 5 times).
Valve exposure
A transverse aortotomy is performed 1 cm above the sinotubular junction starting above the non-coronary sinus and the posterior 2–3 cm of aortic wall is left intact (Video 2). The distal aorta is retracted cephalad. Full thickness 4-0 polypropylene traction sutures are placed at the three commisures. An additional retraction suture may be placed to facilitate exposure (Video3).
Valve assessment
Axial traction is applied (perpendicular to the level of the annular plane) on the commissural traction sutures. This maneuver demonstrates physiological aortic valve closure position and the area and height of coaptation can be observed. A prolapsing cusp will exhibit a transverse fibrous band at this time. The center of the cusp free margin can then be held with a forceps and gently pushed down into the left ventricle. A non-prolapsing cusp will remain at its physiologic coaptation level (halfway between the base of the cusp and the commissure), whereas a prolapsing cusp will be able to be pushed lower due to the presence of excessive cusp tissue (Video 4).
Reference cusps
A 7-0 polypropylene suture is passed through the center (nodule of Arantis) of the two non-prolapsing cusps which serve as a reference. This maneuver
Video 2. Transverse aortotomy and retraction of the distal aorta.
Video 3. Aortic valve exposure using commissural retraction sutures.
Video 4. Surgical inspection and analysis of the valve reveals prolapse of the right coronary cusp, whereas the left coronary and non-coronary cusps appear to be normal.
Video 5. Non-prolapsing cusps are retracted and used as a reference to determine optimal free margin length and height of coaptation.
helps to define the desired height and free margin length that needs to be achieved on the prolapsing cusp (Video 5).
Free margin plication
The quantification of excess free margin for the free margin plication procedure has been previously described w6x. Gentle traction is applied on the reference cusp suture keeping the free margin of the prolapsing cusp parallel with that of the reference cusp. The prolapsing cusp is gently pulled in the direction of the reference cusp and a 6-0 polypropylene suture is passed through the prolapsing cusp at the point at which it meets the center of the reference cusp going from the aortic to the ventricular side. Next, the direction of the traction is reversed and the same suture is passed from the ventricular to the aortic side of the cusp at the point where it meets the middle of the reference cusp. The
3

M. Boodhwani et al. / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2008.003806
length of the cusp free margin between the two ends of this 6-0 suture represents the excess cusp tissue which is then plicated by tying the suture ensuring that the excess tissue remains on the aortic side (Video 6).
Lastly, the plication is extended by about 5–10 mm onto the body of the aortic cusp by adding interrupted or running locked 6-0 polypropylene sutures (Video 7). If a large amount of tissue is being excluded as part of the plication, it may be shaved off using a scalpel or scissors in order to prevent any impingement of cusp motion.
Free margin resuspension
Excess length of the cusp free margin may also be corrected using resuspension with polytetrafluoroethylene (PTFE) suture. This technique may be used in isolation or in combination with other cusp repair techniques and is particularly useful in the setting of a fragile free margin with multiple fenestrations or to homogenize the free margin when a pericardial patch is used for cusp augmentation. This technique is depicted in Video 8.
As shown above in Video 5, a 7-0 polypropylene suture is first passed through the center (nodule of Arantis) of the two non-prolapsing cusps which serve as a reference. A 7-0 PTFE suture is passed twice at the top of the commissure. Next, one arm of the suture is passed over and over the length of the free margin in a running fashion (Video 8). The suture is locked at the other commissure. A second 7-0 PTFE is then passed in the same manner along the cusp free margin (Video 9). The length of the free margin is reduced by applying gentle traction on each branch of the PTFE sutures and applying opposite resistance with a forceps at the middle of the free margin. This maneuver is used to plicate and shorten the free margin until it reaches the same length as the adjacent reference cusp free margin. The same maneuver is applied for the second half of the free margin. This
Video 6. Determination of excess cusp tissue and exclusion using free margin plication.
Video 7. Extension of the cusp plication onto the cusp body.
Video 8. Free margin resuspension is performed using a 7-0 PTFE suture passed over and over along the cusp free margin.
Video 9. A second PTFE suture is placed in the same manner as the first and the free margin is shortened to the desired length.
two-step technique for free margin resuspension allows symmetric and homogeneous shortening. When the appropriate amount of free margin shortening is achieved, the two suture ends at each commissure are tied.
Functional aortic annulus
In addition to repair of cusp prolapse, it is important to stabilize the functional aortic annulus, which consists of the ventriculo-aortic junction and the sinotubular junction. In patients with associated dilatation of the aortic root, this is performed by root replacement using a re-implantation technique. In patients with isolated cusp prolapse or with associated dilatation of the supra-coronary ascending aorta, subcommissural annuloplasty sutures are added to stabilize the proximal portion of the functional aortic annulus.
Pledgeted 2-0 braided sutures are used (Videos 10 and 11). The first arm of the suture is passed from the aortic to the ventricular side, in the interleaflet triangle, and comes back out to the aortic side at the same
4

M. Boodhwani et al. / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2008.003806
level. The second arm of the suture is passed in a similar fashion just below the first. A free pledget is added and the suture is tied. This maneuver helps to stabilize the ventriculo-aortic junction, reduces the width of the interleaflet triangles and increases the coaptation surface of the valve leaflets. Subcommissural annuloplasty is typically performed at midcommissural height, except at the non-coronary/right coronary commissure where it should be performed
Video 10. Subcommissural annuloplasty is performed on the right coronary/non-coronary commissure.
Video 11. Subcommissural annuloplasty of the non-coronary/left coronary and the left coronary/right coronary commissures.
higher in order to avoid the membranous septum and conduction tissue. Care should also be taken in this area during tying of the suture in order to avoid a tear in the septum. At the other two commissures, the subcommissural annuloplasty may be performed at a lower level if greater increase in the coaptation surface is desired.
Post-repair echocardiography
Post-repair echocardiography should focus on the presence of any AI as well as the length and height of coaptation (Photo 1). An eccentric AI jet, length of coaptation -5 mm and a level of coaptation below the mid-height of the sinuses of Valsalva are predictive of late recurrent AI and should be indications for re-exploration of the aortic valve w7x.
ResultsAmong 376 patients having elective aortic valve repair between 1996 and 2008, 89 (24%) had cusp prolapse repair in the setting of a trileaflet aortic valve. Free
margin resuspension using PTFE alone was used in 34 (38%) patients, plication alone in 34 (38%) and PTFEqplication in 21 (24%). Repair of one cusp was performed in 55 (62%) patients, of two cusps in 18 (20%) and three cusps in 16 (18%). Concomitant repair techniques included subcommissural annuloplasty (ns49), supracoronary aortic replacement (ns11) and valve sparing root replacement (ns39).
Photo 1. Post-repair transesophageal echocardiographic view of the aortic valve demonstrating desirable length (green) and height (red) of coaptation.
There was no hospital mortality. Overall survival at 5 years was 95"5%. Echocardiographic follow-up was obtained in 94% of patients and at a median follow-up time of 25 months (range 1–107 months), recurrent AI ()2q) occurred in six patients; three of them had aortic valve replacement. Freedom from reoperation at 5 years was 91"8% for free margin resuspension, 100% for free margin plication and 94"6% for PTFEqplication with no significant differences between groups (Ps0.7). Freedom from recurrent AI ()2q) at 3 years was 87"13% for PTFE, 100% for plication and 89"11% for PTFEqplication (Ps0.6).
These results are consistent with other reported series by Aicher et al. where freedom from reoperation at 5 years after free margin plication was 95% w8x. This series, however, included both tricuspid and bicuspid aortic valves and free margin resuspension was not used. In the context of valve sparing root replacement, David et al. w9x performed free margin plication in 36% and free margin reinforcement with PTFE in 22% of a total of 220 patients. This series also included some patients with bicuspid aortic valves. Overall freedom from reoperation at 10 years was 95% for the entire cohort and separate results for those having cusp intervention were not reported.
5

M. Boodhwani et al. / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2008.003806
Conclusion
Cusp prolapse is a common cause of AI and can be detected on echocardiography and on surgical inspection. Free margin plication and free margin resuspension are both effective techniques for the correction of cusp prolapse with or without aortic root pathology. They can be used alone or in combination with no significant differences in mid-term outcome between techniques.
References
w1x David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617–621; discussion 622.
w2x Yacoub MH, Gehle P, Chandrasekaran V, Birks EJ, Child A, Radley-Smith R. Late results of a valvepreserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg 1998;115:1080–1090.
w3x Carpentier A. Cardiac valve surgery–the ‘French correction’. J Thorac Cardiovasc Surg 1983;86: 323–337.
w4x Haydar HS, He GW, Hovaguimian H, McIrvin DM, King DH, Starr A. Valve repair for aortic insufficiency: surgical classification and techniques. Eur J Cardiothorac Surg 1997;11:258– 265.
w5x Boodhwani M, de Kerchove L, Glineur D, Poncelet A, Rubay J, Astarci P, Verhelst R, Noirhomme P,
El Khoury G. Repair-oriented classificationof aortic insufficiency: impact on surgical
techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286–294.
w6x Boodhwani M, de Kerchove L, Glineur D, El Khoury G. A simple method for the quantification and correction of aortic cusp prolapse by free margin plication. J Thorac Cardiovasc Surg 2009; in press.
w7x de Waroux JB, Pouleur AC, Goffinet C, Vancraeynest D, Van Dyck M, Robert A, Gerber BL, Pasquet A, El Khoury G, Vanoverschelde JL. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007;116(11 Suppl): I264–I269.
w8x Aicher D, Langer F, Adam O, Tscholl D, Lausberg H, Scha¨ fers HJ. Cusp repair in aortic valve reconstruction: does the technique affect
stability? J Thorac Cardiovasc Surg 2007;134: 1533–1538; discussion 1538–1539.
w9x David TE, Feindel CM, Webb GD, Colman JM, Armstrong S, Maganti M. Long-term results of aortic valve-sparing operations for aortic root aneurysm. J Thorac Cardiovasc Surg 2006;132: 347–354.
6
Related Documents