Epilepsy Research (2014) 108, 1853—1863 jo ur nal ho me p ag e: www.elsevier.com/locate/epilepsyres Alterations of 5-HT 1A receptor-induced G-protein functional activation and relationship to memory deficits in patients with pharmacoresistant temporal lobe epilepsy Manola Cuellar-Herrera a,∗ , Ana Luisa Velasco a , Francisco Velasco a , David Trejo a , Mario Alonso-Vanegas b , Avril Nuche-Bricaire a , Daruni Vázquez-Barrón a , Rosalinda Guevara-Guzmán c , Luisa Rocha d a Epilepsy Clinic, Hospital General de México Dr. Eduardo Liceaga, Mexico City, Mexico b National Institute of Neurology and Neurosurgery ‘‘Manuel Velasco Suarez’’, Mexico City, Mexico c Departamento de Fisiología, Facultad de Medicina, Universidad Nacional Autónoma de México, Mexico City, Mexico d Department of Pharmacobiology. Center of Research and Advanced Studies, Mexico City, Mexico Received 11 April 2014; received in revised form 29 August 2014; accepted 13 September 2014 Available online 22 September 2014 KEYWORDS [ 35 S]GTPS; Neuropsi; Human tissue; Epilepsy; Serotonin; Memory Summary The 5-hydroxytryptamine-1A (5-HT 1A ) receptors are known to be involved in the inhibition of seizures in epilepsy. Moreover, studies propose a role for the 5-HT 1A receptor in memory function; it is believed that the higher density of this receptor in the hippocampus plays an important role in its regulation. Positron emission tomography (PET) studies in patients with mesial temporal lobe epilepsy (mTLE) have demonstrated that a decrease in 5-HT 1A receptor binding in temporal regions may play a role in memory impairment. The evidences lead us to speculate whether this decrease in receptor binding is associated with a reduced receptor number or if the functionality of the 5-HT 1A receptor-induced G-protein activation and/or the second messenger cascade is modified. The purpose of the present study is to determine 5-HT 1A receptor-induced G-protein functional activation by 8-OH-DPAT-stimulated [ 35 S]GTPS binding assay in hippocampal tissue of surgical patients with mTLE. We correlate functional activity ∗ Corresponding author. Tel.: +52 55 2789 2000x1332. E-mail addresses: [email protected] (M. Cuellar-Herrera), [email protected] (A.L. Velasco), [email protected] (F. Velasco), [email protected] (D. Trejo), [email protected] (M. Alonso-Vanegas), [email protected] (A. Nuche-Bricaire), [email protected] (D. Vázquez-Barrón), [email protected] (R. Guevara-Guzmán), [email protected] (L. Rocha). http://dx.doi.org/10.1016/j.eplepsyres.2014.09.013 0920-1211/© 2014 Elsevier B.V. All rights reserved.

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Epilepsy Research (2014) 108, 1853—1863
jo ur nal ho me p ag e: www.elsev ier .com/ locate /ep i lepsyres
Alterations of 5-HT1A receptor-inducedG-protein functional activation andrelationship to memory deficits in patientswith pharmacoresistant temporal lobeepilepsy
Manola Cuellar-Herreraa,∗, Ana Luisa Velascoa,Francisco Velascoa, David Trejoa, Mario Alonso-Vanegasb,Avril Nuche-Bricairea, Daruni Vázquez-Barróna,Rosalinda Guevara-Guzmánc, Luisa Rochad
a Epilepsy Clinic, Hospital General de México Dr. Eduardo Liceaga, Mexico City, Mexicob National Institute of Neurology and Neurosurgery ‘‘Manuel Velasco Suarez’’, Mexico City, Mexicoc Departamento de Fisiología, Facultad de Medicina, Universidad Nacional Autónoma de México, MexicoCity, Mexicod Department of Pharmacobiology. Center of Research and Advanced Studies, Mexico City, Mexico
Received 11 April 2014; received in revised form 29 August 2014; accepted 13 September 2014Available online 22 September 2014
KEYWORDS[35S]GTP�S;Neuropsi;Human tissue;Epilepsy;
Summary The 5-hydroxytryptamine-1A (5-HT1A) receptors are known to be involved in theinhibition of seizures in epilepsy. Moreover, studies propose a role for the 5-HT1A receptor inmemory function; it is believed that the higher density of this receptor in the hippocampus playsan important role in its regulation. Positron emission tomography (PET) studies in patients withmesial temporal lobe epilepsy (mTLE) have demonstrated that a decrease in 5-HT1A receptor
Serotonin;Memory
binding in temporal regions may play a role in memory impairment. The evidences lead usto speculate whether this decrease in receptor binding is associated with a reduced receptornumber or if the functionality of the 5-HT1A receptor-induced G-protein activation and/or thesecond messenger cascade is modified. The purpose of the present study is to determine 5-HT1A
receptor-induced G-protein functional activation by 8-OH-DPAT-stimulated [35S]GTP�S bindingassay in hippocampal tissue of surgical patients with mTLE. We correlate functional activity
∗ Corresponding author. Tel.: +52 55 2789 2000x1332.E-mail addresses: [email protected] (M. Cuellar-Herrera), [email protected] (A.L. Velasco), [email protected]
(F. Velasco), [email protected] (D. Trejo), [email protected] (M. Alonso-Vanegas), [email protected](A. Nuche-Bricaire), [email protected] (D. Vázquez-Barrón), [email protected] (R. Guevara-Guzmán), [email protected] (L. Rocha).
http://dx.doi.org/10.1016/j.eplepsyres.2014.09.0130920-1211/© 2014 Elsevier B.V. All rights reserved.
1854 M. Cuellar-Herrera et al.
with epilepsy history and neuropsychological assessment of memory. We found that maximumfunctional activation stimulation values (Emax) of [35S]GTP�S binding were significantly increasedin mTLE group when compared to autopsy samples. Furthermore, significant correlations werefound: (1) positive coefficients between the Emax with the age of patient and frequency ofseizures; (2) negative coefficients between the Emax and working memory, immediate recall anddelayed recall memory tasks. Our data suggest that the epileptic hippocampus of patients withmTLE presents an increase in 5-HT1A receptor-induced G-protein functional activation, and thatthis altered activity is related to age and seizure frequency, as well as to memory consolidationdeficit.© 2014 Elsevier B.V. All rights reserved.
I
Ahlmaa1pdir2ersr
titoi
fteailnos1portltMpaodm2
EioiAlilTat5eoinictafuateaao
M
P
Tp
pemlto verify that they were within therapeutic range, magneticresonance imaging to detect the presence of hippocampalsclerosis or atrophy, four serial electroencephalograms to
ntroduction
t present, experimental data show that 5-ydroxytryptamine-1A (5-HT1A) receptors are predominantlyocated in limbic areas, and also suggest that serotoninediates — via these receptors — an anti-epileptic and
nticonvulsant effect (Andrade and Nicoll, 1987; Colinond Halliwell, 1987; Salgado-Commissariat and Alkadhi,997). Positron emission tomography (PET) studies inatients with mesial temporal lobe epilepsy (mTLE) haveemonstrated a decrease in 5-HT1A receptor binding in thensula, anterior cingulate, and medial and lateral temporalegions, ipsilateral to the epileptic focus (Toczek et al.,003; Savic et al., 2004; Giovacchini et al., 2005). Thisvidence was associated with a greater decrease in 5-HT1A
eceptor binding in regions that show interictal activity,eizure propagation and seizure onsets, in comparison toegions with no epileptic activity (Merlet et al., 2004).
In vitro autoradiography of neocortical tissue surroundinghe epileptic focus of patients with mTLE showed a decreasen 5-HT1A receptor binding in layers I—II (Rocha et al., 2007);his could be associated to high excitability and facilitationf seizure activity, as the neocortex plays an important rolen the seizure generation and/or propagation.
Hippocampus research in epilepsy has been approachedrom several neuroscience disciplines. Physicians have notedhe high predisposition of the hippocampus to generatepileptic activity because of its sensitiveness to hypoxiand temperature (Engel, 1996). Basic research has centeredts interest on hippocampal sclerosis, the most frequentesion in mTLE patients. This alteration is associated witheuronal loss, gliosis, neuronal sprouting, reorganizationf neurotransmitter receptors, alterations in second mes-enger systems, and hyperexcitability (De Lanerolle et al.,989; Babb et al., 1991; Sloviter, 1994). The hippocam-us is not isolated in the brain, the contrary, it is partf many functional circuits in the limbic system that areelated to learning and memory, emotional memory, execu-ive functions and spatial recognition. The relation betweenearning and memory consolidation and the specialized func-ion of the hippocampus is well established (Scoville andilner, 2000; Milner, 1972; Ogren et al., 2008). In mTLEatients, the evidence has shown an association between
left hippocampal epileptogenic zone and verbal mem-
ry deficits. Also, a right hippocampal epileptogenic zoneisrupts the ability to learn and store new visual infor-ation (Drake et al., 2000; Lezak, 2004; Marques et al.,007; Helmstaedter and Elger, 2009; Campo et al., 2013).
danr
xperimental studies propose a role for the 5-HT1A receptorn memory function; it is believed that the higher densityf this receptor in the hippocampus plays an important rolen its regulation (Buhot et al., 2000; Ogren et al., 2008).
PET study in patients with mTLE suggests that reducedeft hippocampal 5-HT1A receptor binding may play a rolen memory impairments of these patients, regardless of theocation of the epileptogenic zone (Theodore et al., 2012).he limitation of this study was that this technique can onlyssess receptor binding, not its functional activity. By itself,his information cannot lead to inferences about how the-HT1A receptor participates in memory impairment. Thevidence that the 5-HT1A receptor may be involved in mem-ry loss in mTLE leads us to speculate whether this decreasen receptor binding is associated with a reduced receptorumber or if the functionality of the 5-HT1A receptor-nduced G-protein activation and/or the second messengerascade are modified. The purpose of the present study waso correlate 5-HT1A receptor-induced G-protein functionalctivation to neuropsychological assessment of memoryunctions in hippocampal tissue from mTLE patients whonderwent epilepsy surgery, in order to obtain evidence of
more specific role of 5-HT1A receptors in memory func-ions in mTLE. In addition, we investigated the influence ofpilepsy history on the 5-HT1A receptor-induced G-proteinctivation, correlating functional activation to the subject’sge, age of seizure onset, duration of epilepsy and frequencyf seizures.
aterials and methods
atients group
he scientific committees of the institutions involved in theresent research previously approved the protocol.
The inclusion criteria for the patients included are:atients with pharmacoresistant mesial temporal lobepilepsy who had a detailed seizure calendar of at least 3onths prior to surgery in order to confirm the presence of at
east two seizures per month, blood levels of anticonvulsants
etermine the presence of temporal epileptiform activitynd neuropsychological assessment to determine basal cog-itive condition and identify behavioral or memory disorderselated to seizures.
ilep
vmtdcdoiief1
ATFsiow
ssaeaab
B
MCp(hutp3t(uotu
[Rp2baE(oe
Functional alteration of 5-HT1A receptor in temporal lobe ep
All patients were surgically-treated at the age they firstcame to our clinic. Since we are a highly specialized clinic,the patient’s first contact is not with us and this delaythe attention. Multiple factors contribute to these delays,including lack of knowledge of general practitioners, anderroneous attitudes and referral practices. This delay isapproximately 20 years (Berg, 2004); in our clinic it isapproximately 14 years (Guerrero-Pérez et al., 2014).
Epileptic hippocampal tissue was obtained from eightpatients with pharmacoresistant mTLE history (Table 1).Each patient signed an informed consent. None of thepatients involved in the present study demonstrated grossstructural lesions other than hippocampal sclerosis in MRI.In 5 (63%) patients, non invasive studies with concordantresults were enough to indicate temporal lobectomy. In 3(37%) patients, a phase II invasive protocol was manda-tory. It included either bilateral hippocampal electrodes orplacement of a grid on the basotemporal cortex, as well ascontinuous video-EEG monitoring (Velasco et al., 2000).
A standard anterior temporal lobectomy, ipsilateral tothe epileptogenic zone, was performed in all patients after48 h from the last seizure occurrence. During the surgicalprocedure, hippocampal biopsies were collected immedi-ately after resection from the anterior hippocampus andneocortical areas; then quickly frozen in pulverized dry iceand stored at -70 ◦C. The epileptogenic zone was found in theleft hippocampus of three (37%) patients, and in the rightone of five (63%) patients. Clinically, the area of brain tis-sue with or without an obvious structural abnormality thancan be demonstrated to be the origin of recurrent epilep-tic seizures is called the epileptic zone (Engel, 1987; Gloor,1987).
Neuropsychological assessment
A comprehensive neuropsychological assessment protocolwas applied to seven patients according to the clinicalguide of the Epilepsy Clinic at Mexico’s General Hospital. Inone patient neuropsychological testing was not performedbecause he had psychiatric comorbidity (bipolar disorder).In addition, the patient HUM-123 does not return to its post-operative neuropsychological assessment.
All neuropsychological tests were performed by Dr. DavidTrejo, neuropsychologist. Pre-operative neuropsychologicaltests were performed three months before surgery. Thepostoperative neuropsychological tests were performed 12months after surgery. Pre and post operative neuropsychol-ogical tests of each patient were reported in Table 2.
The neuropsychological testing typically includes a com-prehensive examination of the major cognitive domains,including hemispheric language lateralization, intelligencescales and ‘‘NEUROPSI Attention and Memory’’ neuropsy-chological test battery (Ostrosky-Solis et al., 1999). Thisbattery was normalized by age and educational level forthe Mexican population and included tasks that allow theassessment of four cognitive domains: Orientation, atten-tion, memory and executive functions.
Given that the purpose of this study was to correlate[35S]GTP�S binding assay coefficients of the 5-HT1A recep-tor to memory functioning, we selected specific memorytasks in order to differentiate immediate recall (visual and
iNuc
sy: relationship to memory deficit 1855
erbal learning) from delayed recall (visual and verbalemory consolidation and retrieval). We also monitored
he integrity of the attention and working memory (WM)omains, since we know they are required for the successfulodification of new information (see Table 2 for a completeescription of task selection). The patients’ performancen each task was scored (number of hits and errors) accord-ng to the test battery manual and the score transformednto normalized data based on patient’s age and years ofducational level. The normalized scores are categorized asollows: 1—3 severe; 4—6 mild to moderate; 7—13 normal;4—19 high normal.
utopsy sampleshe Institute of Forensic Sciences, in agreement with theaculty of Medicine of the National Autonomous Univer-ity of Mexico, provided the control autopsy samples. Thenformed donation consent was obtained from the familyf healthy subjects who died because of various causes,ithout evidence of neurological disease.
Hippocampus tissue was obtained from the autopsy ofubjects (n = 6). Previous reports indicate that agonist-timulated [35S]GTP�S binding is preserved for several hoursfter death (González-Maeso et al., 2002; Rodríguez-Puertast al., 2000). Our tissue was dissected at the time ofutopsy, with a postmortem interval of 4 to 10 h, immedi-tely stored at −70 ◦C and then manipulated as describedelow (Table 1).
inding assays
embrane preparationrude membrane fractions from human hippocampus wererepared according to the method previously describedBenyhe et al., 1997). Briefly, samples (200—500 mg) wereomogenized on ice in 50 mM Tris—HCl buffer (pH 7.4)sing a Teflon-glass homogenizer. The homogenate was cen-rifuged at 15,000 rpm for 25 min at 4 ◦C and the resultingellet was re-suspended in fresh buffer and incubated for0 min at 35 ◦C. The centrifugation step was repeated, andhe final pellet was re-suspended in 50 mM Tris—HCl bufferpH 7.4) containing 0.32 M sucrose, and stored at −70 ◦Cntil use. Protein levels were determined by the methodf Lowry et al. (1951). Before use, the membranes werehawed, washed by centrifugation to remove sucrose, andsed immediately in the binding assays.
35S]GTP�S functional assayeceptor-mediated G-protein activation was measured asreviously described (Spetea et al., 1998; Páldyová et al.,008) with slight modifications. Human hippocampal mem-rane fractions (≈10 �g of protein/sample) were incubatedt 30 ◦C for 60 min in Tris—EGTA buffer (1 M Tris—HCl, 0.2 MGTA, 1 M MgCl2, 1 M NaCl, pH 7.4), containing [35S]GTP�S0.05 nM) and increasing concentrations (Log 10−11 to 10−5 M)f 8-OH-DPAT (5-HT1A receptor agonist) in presence ofxcess GDP (10 mM) in a final volume of 1 ml. Total bind-
ng was measured in absence of the tested compound.on-specific binding was determined in presence of 10 mMnlabeled GTP�S and subtracted from total binding to cal-ulate the specific binding. The reaction was initiated by1856
M.
Cuellar-Herrera
et al.
Table 1 Clinical data of patients with pharmacoresistant temporal lobe epilepsy and autopsies.
Patients (n = 8) Gender Age(years)
Age of seizureonset (years)
Duration ofepilepsy(years)
Frequency ofseizures (permonth)
AED Diagnosis or causeof death (PMI inhours)
Surgical outcome(Engel’sclassification)
Side offocus
HUM-114 M 29 2 27 2 PHT/CBZ/VPA MTLE NE LeftHUM-117 F 28 27 1 170 VPA/PHT/LTG MTLE Class IC RightHUM-119 M 24 5 19 6 TPM/VPA/CBZ/PHT/LTG MTLE Class IVB LeftHUM-120 F 30 8 22 10 CBZ/PHT MTLE Class IIB RightHUM-121 F 36 2 34 270 CBZ/VPA MTLE Class IA RightHUM-122 F 52 13 39 3 CBZ/OXC/PHT MTLE NE RightHUM-123 F 24 9 15 10 PHT/CBZ/PB/CZP/OXC MTLE Class IID RightHUM-124 F 33 3 30 5 TPM/OXC/CZP/RISP MTLE Class IIA LeftC1 M 66 - Gastrointestinal
bleeding (4)-
C2 M 50 Hepatitis (6) -C3 F 20 Chronic renal
failure (4)-
C4 M 29 Hypovolemicshock (5)
-
C5 M 33 Hypovolemicshock (10)
-
C6 F 40 Hypovolemicshock (3)
-
M—–male; F—–female; NE—–Not evaluated; AED—–antiepileptic drugs; PHT—–phenytoin; CBZ—–carbamacepine; VPA—–valproic acid; LTG—–lamotrigine; TPM—–topiramate; OXC—–oxcarbazepine;PB—–phenobarbital; CZP—–clonazepam; RISP—–risperidone.
Functional alteration of 5-HT1A receptor in temporal lobe epilepsy: relationship to memory deficit 1857
Table 2 Patient’s performance for each task preoperative and postoperative were scored according to number of hits anderrors. The results transform to performance normalized data depending on patient’s age and years of scholarity.
Patients
HUM-114 HUM-117 HUM-119 HUM-120 HUM-121 HUM-122 HUM-123 HUM-124
NEUROPSYCHOLOGICALSUBTESTS
WORKING MEMORY ANDATTENTION
Pre Post Pre Post Pre Post Pre Post Pre Post Pre Post Pre Post
Digit detection 12 12 9 9 2 2 14 11 7 7 8 10 14 NE NEMental control 13 13 13 13 6 6 13 14 7 12 6 14 13 NE NEDigit backward span 10 7 7 7 7 7 7 16 7 10 8 12 10 NE NESpatial backward span 15 12 7 7 6 6 7 6 10 13 9 9 14 NE NEIMMEDIATE RECALL MEMORYWord list 13 3 6 7 5 8 8 10 12 10 10 12 10 NE NEVerbal paired associates 4 4 4 9 6 1 7 14 8 10 8 11 8 NE NELogical memory (stories) 3 2 1 10 4 1 3 6 5 4 7 8 8 NE NERey—Osterreith complex figure 9 11 6 8 7 1 5 2 5 11 6 7 12 NE NEFaces 11 3 11 11 11 11 12 12 11 11 12 4 12 NE NEDELAYED RECALL MEMORYWord list (free recall) 8 1 7 8 5 1 6 11 11 8 9 10 8 NE NEWord list (cued recall) 10 3 1 7 8 1 5 11 4 10 8 7 1 NE NEWord list (recognition) 12 10 2 7 12 1 8 8 2 13 2 7 6 NE NEVerbal paired associates 11 4 1 7 2 1 5 13 3 11 12 10 8 NE NELogic memory 5 3 2 7 1 1 3 8 7 7 7 7 7 NE NERey-Osterreith complex figure 8 4 3 7 7 1 1 1 6 11 3 7 5 NE NEFaces (recognition) 8 8 8 12 8 4 9 10 12 12 7 7 13 NE NE
Values represent: 1—3, severe; 4—6, mild to moderate; 7—13, normal; 14—19, high normal. NE: not evaluated.
ds
R
A
Am(owlE(v
P
E(f
adding [35S]GTP�S and completed with filtration of thesamples through Whatman GF/B glass fiber filters; it waswashed three times with ice-cold 50 mM Tris—HCl buffer (pH7.4) using Brandel M-48 Cell Harvester. The dried filters wereimmersed in a Sigma-FluorTM scintillation cocktail (Sigma)and then used to determine bound radioactivity as describedabove. [35S]GTP�S binding experiments were performed intriplicates.
Data analysis
The data were expressed as mean ± S.E.M. [35S]GTP�Sbinding data were subjected to non-linear regression anal-ysis of concentration-effect curves performed by Prism(GraphPad Software, Inc.). We determined agonist potencyexpressed as pEC50, which is the negative logarithm base10 of the agonist molar concentration that produces 50%of the maximum possible effect. The maximum stimu-lation (Emax) of [35S]GTP�S over the basal was given infmol/mg of protein that an agonist can elicit in a specifiedtissue.
The results from the specific [35S]GTP�S binding wereexamined statistically by Student’s t-test to find differences
of Emax and pEC50 between autopsy samples and mTLE tissue.Pearson’s correlation coefficients were calculated to estab-lish a significant correlation (p < 0.05) of Emax and pEC50 withthe obtained values of subject’s age, age of seizure onset,thpW
uration of epilepsy, frequency of seizures and normalizedcores of WM and memory tasks.
esults
utopsy samples
utopsy samples were acquired from six subjects (fourale and two female) ranging in age from 20 to 66 years
39.6 ± 6.6 years), who died by different causes and with-ut history of neurological disease. The postmortem intervalas 5.3 ± 1.0 h (Table 1). We found no significant corre-
ation in autopsy samples between the coefficients of themax of [35S]GTP�S binding and pEC50 values, with the ager = 0.3293, r = 0.2717, respectively) and postmortem inter-al (r = −0.4988, r = −0.4490, respectively).
atients
pileptic hippocampi were obtained from eight patientstwo male and six female) of age 32.0 ± 3.2 years old (rangedrom 24 to 52 years) with 23.3 ± 4.2 years of epilepsy dura-
ion; the age of seizure onset was 8.6 ± 2.9 years and theyad 59.7 ± 36.2 seizures per month (Table 1). One patientresented bipolar disorder as a psychiatric comorbidity.e found no significant differences in age between mTLE1858 M. Cuellar-Herrera et al.
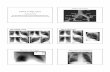
Fig. 1 (A) Representation of absolute [35S]GTP�S basal bind-ing obtained from autopsies and epileptic patients. Valuesare represented as mean ± S.E.M. of fmol/mg of protein. (B)Specific [35S]GTP�S binding to membranes of samples fromautopsies (–�–) and from patients with mTLE (-�-) as a functionof increasing concentration of the 5-HT1A receptor agonist 8-OH-DPAT. Each point represents the mean ± S.E.M of the fmol/mgof protein stimulation over basal values. Note that in patientsws
py
cThsvmwpm−
c[ftc
N
Wds(
aa
Table 3 Pearson’s r between [35S]GTP�S binding parame-ters from hippocampal neuron membranes and clinical data.
Clinical data [35S]GTP�S binding assay
Emax pEC50
Age of patient 0.6375* −0.2374Seizure onset age 0.2261 0.3034Duration of epilepsy 0.3238 −0.3911Frequency of seizures 0.6625* −0.3444
* p < 0.05 Emax values (expressed as maximal stimulation of
rspo[prrvppnificant correlation between the pEC50 values and attentionor memory functions (Table 5).
Fig. 2 Representation of correlation of Emax [35S]GTP�S bind-ing with (A) immediate recall memory scores (Rey—Osterriethcomplex figure) and (B) delayed recall memory scores (wordlist recognition and Rey—Osterrieth complex figure). Note that
ith mTLE, [35S]-GTP�S binding stimulation by 8-OH-DPAT wasignificantly different from that of autopsy samples.
atients and the autopsy samples (32.0 ± 3.2 and 39.6 ± 6.6ears, respectively, p = 0.2836).
8-OH-DPAT-stimulated [35S]GTP�S binding wasoncentration-dependent and had a saturation level.he basal activity from the mTLE group was significantlyigher (459%, p < 0.05) when compared with autopsyamples (Fig. 1A). On the other hand, we found that Emax
alues of [35S]GTP�S binding significantly increased in theTLE group (153.2 ± 23.8 fmol/mg of protein, * p < 0.05)hen compared to autopsy samples (57.5 ± 10.6 fmol/mg ofrotein) (Fig. 1B). However, the pEC50 values in the microolar range were similar in both groups (−7.2 ± 0.60 and7.3 ± 0.19, autopsy samples and mTLE, respectively).
Regarding the correlation analysis, we found signifi-antly positive correlation coefficients between the Emax of35S]GTP�S binding with age of patient (0.6375, p < 0.05) andrequency of seizures (0.6625, p < 0.05) (Table 3). However,he pEC50 values revealed no significant correlations withlinical data (Table 3).
europsychological assessment
e found no significant correlation between the normalizedata of the memory subtests and the subject’s age, age ofeizure onset, duration of epilepsy and frequency of seizures
Table 4).In neuropsychological domains, we found significant neg-tive correlations between Emax [35S]GTP�S binding andttention and WM subtests (Table 5): digit detection
wi(r
[35S]GTP�S binding) and pEC50 values (expressed as the negativelogarithm of EC50).
= −0.7547, serial subtraction r = −0.7033, digit backwardpan r = −0.8966 and spatial backward span r = −0.7607,
< 0.05. In the immediate recall memory domain, wenly found a significant negative correlation between Emax35S]GTP�S binding and visual learning (Rey—Osterrieth com-lex figure, r = −0.7799, p < 0.05, Fig. 2A). On the delayedecall memory domain, we found significant negative cor-elations between Emax [35S]GTP�S binding with verbal andisual memory retrieval (word list recognition, r = −0.9343,
< 0.05 and Rey—Osterrieth complex figure, r = −0.8744, < 0.05, respectively, Fig. 2B). However, we found no sig-
e found a significant negative correlation of 5-HT1A receptor-nduced G-protein functional activation with visual learningimmediate recall memory) and verbal and visual memoryetrieval (delayed recall memory).
Functional alteration of 5-HT1A receptor in temporal lobe epilepsy: relationship to memory deficit 1859
Table 4 Pearson’s r between clinical data and neuropsychological assessment results of temporal lobe epilepsy patients.
Neuropsychological subtests Subject’s age Age of seizure onset Duration of epilepsy Frequency of seizures
Working memory and attentionDigit detection −0.1208 0.0475 −0.1264 −0.2429Mental control −0.5283 0.2708 −0.5956 −0.1450Digit backward span −0.1447 −0.2710 0.0747 −0.4702Spatial backward span −0.0831 −0.3954 0.2080 −0.1660
Immediate recall memoryWord list 0.3341 −0.5323 0.6254 0.1139Verbal paired associates 0.4181 −0.3202 0.5443 0.0255Logical memory 0.3456 −0.3322 0.4964 −0.2554Rey—Osterrieth complex figure −0.4414 −0.1539 −0.2357 −0.4514Faces 0.3328 0.0614 0.2153 −0.5150
Delayed recall memoryWord list (free recall) 0.5500 −0.1954 0.5604 0.5760Word list (cued recall) 0.3141 −0.5452 0.6188 −0.4721Word list (recognition) −0.5630 −0.5445 −0.0607 −0.6239Verbal paired associates 0.5082 −0.2746 0.5827 −0.5762Logic memory 0.5275 −0.3033 0.6174 0.1279Rey—Osterrieth complex figure −0.3093 −0.5766 0.1579 0.0225Faces (recognition) −0.3332 −0.3087 −0.0453 0.3574
Table 5 Pearson’s r between [35S]GTP�S binding param-eters from hippocampal neuron membranes and neuropsy-chological subtest of patients with temporal lobe epilepsy.
Neuropsychological subtests [35S]GTP�S Binding Assay
Working memory and attention Emax pEC50
Digit detection −0.7547* 0.6488Mental control −0.7033* 0.6016Digit backward span −0.8966* 0.1487Spatial backward span −0.7607* −0.1316
Immediate recall memoryWord list −0.0283 −0.6159Verbal paired associates 0.1983 −0.2332Logical memory (stories) −0.0659 0.0609Rey—Osterrieth complexfigure
−0.7799* 0.3718
Faces 0.0484 0.3266Delayed recall memory
Word list (free recall) −0.2397 −0.3652Word list (cued recall) −0.5166 −0.4347Word list (recognition) −0.9343* 0.2754Verbal paired associates −0.0480 −0.6742Logic memory −0.0551 −0.3347Rey—Osterrieth complexfigure
−0.8744* −0.1572
Faces (recognition) −0.4557 0.5726
* p < 0.05 Emax values (expressed as maximal stimulation of[35S]GTP�S binding) and pEC50 values (expressed as the negativelogarithm of EC50).
Discussion
Our results revealed that the hippocampus of patients withmTLE shows an increase of the 5-HT1A receptor-induced G-protein functional activation compared to autopsy samples.These results demonstrate that high values of functionalactivation were associated with increased age of patient,frequency of seizures and neuropsychological deficit in WMand memory.
Regarding the tissue from autopsy samples, inherent vari-ables such as postmortem delay and age of death mayproduce changes that could alter the results. In this context,these results showed that postmortem delay (up to 10 h) didnot correlate with the 5-HT1A receptor-induced G-proteinfunctional activation. It is important to consider that theseresults were concordant with those reported previously(González-Maeso et al., 2002). The obtained informationand results lead us to suggest that tissue from autopsy sam-ples can be used as control.
Studies have indicated that 5-HT1A receptor densitydecreases with age in brain regions such as cortex, hip-pocampal regions, locus coeruleus and dorsal raphe nuclei(Dillon et al., 1991; Burnet et al., 1994). This evidence sug-gests that a decrease in receptor density could indirectlyproduce changes in functional 5-HT1A receptor activation.Therefore, we used tissue from mTLE surgical patients andautopsy samples from subjects of similar age, in order toreduce the possible influence of this variable. An impor-tant point to mention is age ranges from 24 to 52 yearsold, which may easily lead to the misinterpretation of the
results. Regarding the wide age range, we did not find acorrelation with the result of memory tests (see Table 4),which confirms that the memory deficits are associatedwith the disease and not with age. Impaired memory and1
cpnasae
iobeescndiGpavoe55Gfa
fa(iTHrivsH(iwat
sswaemi
l1pu(ts
atparmmsdbii(fttdo2wtai
pb
pmrittGcbirdpcti
taOtcIucmid
tT
860
ognitive functions have been reported in mTLE inde-endently of age. Moreover, it is noteworthy that theeuropsychological test used in this study, NEUROPSI, has
rating system that converts natural scores to normalizedcores in order to standardize for age and schooling. Thisllows comparing heterogeneous groups of different age andducation avoiding erroneous results.
Our results showed an increase in 5-HT1A receptor-nduced G-protein functional activation in the hippocampusf mTLE patients, in spite of the decreased 5-HT1A receptorinding found in other studies (Toczek et al., 2003; Savict al., 2004; Giovacchini et al., 2005). This finding can bexplained as a consequence of epilepsy, causing increasedignaling efficiency of 5-HT1A receptors due to adaptivehanges. At this moment, we do not have a plausible expla-ation for these changes in epilepsy. However, in psychiatricisorders such as mania and bipolar disorder, an increasen the functional activity of the receptors that activate-proteins has been reported as a consequence of the patho-hysiology of the disease (Schreiber et al., 1991; Friedmannd Wang, 1996). It is known that anti-epileptic drugs ele-ate and/or stimulate basal serotonin (5-HT) levels as partf their anticonvulsant action (Okada et al., 1992; Daileyt al., 1997; Ahmad et al., 2005). Increased hippocampal-HT release might also result in the down-regulation of-HT1A receptors (Toczek et al., 2003; Savic et al., 2004;iovacchini et al., 2005), providing a potential mechanism
or increased 5-HT1A receptor-induced G-protein functionalctivation.
On the other hand, no correlation between age andunctional activation of the 5-HT1A receptor was found inutopsy samples, which is consistent with previous reportsGonzález-Maeso et al., 2002); however, we did find a pos-tive correlation between these variables in epilepsy cases.his indicates that increased functional activation of the 5-T1A receptor is present only in mTLE. The increased 5-HT1A
eceptor-induced G-protein functional activation observedn this study could represent a compensatory anticon-ulsant mechanism due to changes in the serotoninergicystem in epilepsy. It is known that the activation of 5-T1A receptors plays an inhibitory role on seizure activity
Wada et al., 1992; López-Meraz et al., 2007) by induc-ng hyperpolarization via opening of potassium channelsith consequent inhibitory effects (Salgado-Commissariatnd Alkadhi, 1997). Further studies are necessary to supporthis idea.
An issue to consider is that all our surgically excised tis-ue samples have mesial temporal sclerosis. Previous reportsuggest that the sclerosis-induced changes are associatedith a reorganization of neurotransmitter receptors and/orlterations in second messenger systems (De Lanerollet al., 1989; Babb et al., 1991; Sloviter, 1994), and probablyight be associated to an increase in 5-HT1A receptor-
nduced G-protein functional activation.In mTLE, alterations of executive functions, attention,
earning and memory have been reported (Aldenkamp,997; Jokeit and Ebner, 1999). Each of these functions iserformed by the prefrontal cortex (attention and exec-
tive functions) and hippocampus (learning and memory)Robbins, 2000; Miller and Cohen, 2001; Cohen, 2011). Onhe other hand, in secondarily generalized complex partialeizures are characteristic of mTLE, the structures involvedssae
M. Cuellar-Herrera et al.
re the hippocampus (epileptogenic zone) and temporal cor-ex (propagation area) and the rest of the brain. Continuousropagation of these seizures affects cognitive functionsbove mentioned. The involvement of 5-HT1A receptor asesponsible for the alterations in cognitive function andemory has been suggested by experimental studies in ani-als (Carli et al., 1993; Meneses and Hong, 1999) and clinical
tudies (Sumiyoshi et al., 2001, 2007). PET studies haveemonstrated the presence of the 5-HT1A receptor in therain of normal subjects showing variability of distributionn different brain areas of frontal cortex, temporal cortex,nsula, anterior cingulate, hippocampus and raphe nucleusBorg, 2008). The wide distribution of 5-HT1A receptor in dif-erent brain areas has allowed to propose it as a therapeuticarget for increasing cognitive functions (executive, atten-ion, learning and memory) in the treatment of cognitiveysfunction in schizophrenia, Alzheimer’s disease, declinef age-related cerebral trauma and memory (Kline et al.,002; Schechter et al., 2002; Roth et al., 2004). However,hat happens in patients with mTLE regarding the role of
he 5-HT1A function in memory and WM? Our results revealed significant negative correlation between 5-HT1A receptor-nduced G-protein functional activity and attention and WM.
The WM and attention are strongly related to dorsolateralrefrontal structures. In patients with mTLE deficits haveeen observed in these functions (Jokeit and Ebner, 1999).
Our results support the hypothesis that the hippocam-us and the prefrontal cortex interact on WM and long-termemory processes (Cohen, 2011). Campo et al. (2013)
ecently demonstrated that unilateral hippocampal sclerosisnduced local and remote changes in the dynamic organiza-ion of a distributed network that supports verbal WM. Athis moment, we ignore whether 5-HT1A receptor-induced-protein functional activation is altered in the prefrontalortex (PFC) as it is in the hippocampus of mTLE patients,ut there is evidence of the 5-HT1A receptor having annhibitory function in PFC by hyperpolarizing pyramidal neu-ons (Celada et al., 2013). As mentioned previously, in otheriseases where associated areas are not only the hippocam-us, 5-HT1A receptor participation in the improvement ofognitive function has been clearly demonstrated. However,here is no evidence of its involvement in cognitive functionsn patients with mTLE.
Our analysis also showed a significant negative correla-ion between 5-HT1A receptor-induced G-protein functionalctivation and immediate visual memory scores (Rey-sterreith complex figure task) and delayed recall memoryasks in both modalities, visual and verbal ((Rey—Osterreithomplex figure task and word list recognition, respectively).mmediate recall memory tasks requires numerous prereq-isites before the information arriving to the hippocampusan be consolidated, such as perception, attention, workingemory or modality-dependent codification. An impairment
n any of these processes could be responsible for the imme-iate memory recall deficit.
These results suggest that the greater the receptor func-ional activity, the greater the deterioration in memory.he results agree with those reported previously in healthy
ubjects by PET, that high doses of 5-HT1A agonist showedignificant negative correlation between receptor bindingnd the acquisition of memory in the hippocampus (Yasunot al., 2003). This evidence suggests that the memory deficitilep
aol
dut
tf
C
N
A
WEV
R
A
A
A
B
B
B
B
B
B
B
C
C
Functional alteration of 5-HT1A receptor in temporal lobe ep
observed in healthy subjects is related to the high dose ofthe agonist of the 5HT1A receptor. Collectively, it has beenreported that stimulation of 5-HT1A receptor can induceamnesia in rats (Carli et al., 1993). This clearly confirmsthe involvement of 5-HT1A receptor in memory in healthysubjects. Theodore et al. (2012) found that 5-HT1A recep-tor binding was reduced in the left hippocampus of mTLEpatients, and suggested that this receptor played a role inmemory deficits, regardless of the location of the epilepto-genic zone; this information does not allow us to establisha direction for this role of the 5-HT1A receptor for G-proteins. Our findings trace a significant negative correlationbetween 5-HT1A receptor-induced G-protein functional acti-vation with the score of delayed recall memory tasks in bothmodalities, visual and verbal, regardless of the location ofthe epileptogenic zone. This could mean that consolidation,a function related to the integrity of the hippocampus, wasimpaired in both modalities, and suggests that even whenlearning (immediate recall) could be performed, a consoli-dation deficit impairs the whole retrieval memory process,regardless of the stimuli modality.
It is important to mention that we found no signifi-cant correlation between neuropsychological assessmentsand epilepsy history of mTLE patients. Other authors havereported similar results (Dodrill, 2002; Rausch et al., 2003;Campo et al., 2013); however, other reports suggest thatmemory deficits are related to duration of epilepsy andseizure frequency (Jokeit and Ebner, 1999; Elger et al., 2004;Thompson and Duncan, 2005). The differences betweenreports might depend on the tests used to assess memoryfunction, type of epilepsy or the sample size used for eachstudy.
It has been known, until now, that treatment withpartial 5HT1A agonists improved memory in patients withschizophrenia, anxiety and depression (Carli and Samanin,1988; Sumiyoshi et al., 2000; Riedel et al., 2002). Onthe other side, it has been reported that 5-HT1A recep-tor antagonists improved memory in Alzheimer’s disease byenhancing the activation of neural circuits involved in cog-nitive processes (Schechter et al., 2002). Taken together,these evidences point our attention to the way that the 5-HT1A receptor function could affect other neurotransmissionsystems, causing secondary effects on cognitive functions.It has also been reported that 5-HT1A receptor stimulationdecreases glutamate release (Dijk et al., 1995; Matsuyamaet al., 1996). Glutamate plays a critical role on neuro-plasticity changes that have been associated with memoryconsolidation, such as long-term potentiation in hippocam-pal neurons (Bliss and Collingridge, 1993). An importantfactor to be considered is the glutamate increase in pharma-coresistant mTLE patients (During and Spencer, 1993). Thus,it is plausible that the increase in 5-HT1A receptor-inducedG-protein functional activation observed in this study causesa glutamate decline, indirectly exerting a detrimental effecton memory consolidation in our epilepsy patients.
Conclusion
Our results demonstrated an increase in 5-HT1A receptor-induced G-protein functional activation in the hippocampusof mTLE patients; this altered activity is strongly related to
C
sy: relationship to memory deficit 1861
ge and seizure frequency as well as to a deficit in mem-ry consolidation, both visual and verbal, regardless of theocation of the epileptogenic zone.
The relationship of different factors such as antiepilepticrugs, mesial temporal sclerosis, cortical atrophy or othernderlying processes in epilepsy and 5-HT1A receptor func-ion is still to be determined.
Further studies should be conducted to establish a rela-ionship between alterations in the 5-HT1A receptor androntal executive function deficits.
onflict of interest statement
one of the authors has any conflict of interest to disclose.
cknowledgements
e thank Ms. Leticia Neri Bazan and Mr. Hector Vazquezspinosa for their excellent technical assistance and Mr.ladímir Barberena for English improvement.
eferences
hmad, S., Fowler, L.J., Whitton, P.S., 2005. Lamotrigine, carba-mazepine and phenytoin differentially alter extracellular levelsof 5-hydroxytryptamine, dopamine and amino acids. EpilepsyRes. 63, 141—149.
ldenkamp, A.P., 1997. Effect of seizures and epileptiform dis-charges on cognitive function. Epilepsia 38, S52—S55.
ndrade, R., Nicoll, R.A., 1987. Pharmacologically distinct actionsof serotonin on single pyramidal neurones of the rat hippocam-pus recorded in vitro. J. Physiol. 394, 99—124.
abb, T.L., Kupfer, W.R., Pretorius, J.K., Crandall, P.H., Levesque,M.F., 1991. Synaptic reorganization by mossy fibers in humanepileptic fascia dentata. NeuroScience 42, 351—363.
enyhe, S., Farkas, J., Tóth, G., Wollemann, M., 1997. Met5-enkephalin-Arg6-Phe7, an endogenous neuropeptide, binds tomultiple opioid and nonopioid sites in rat brain. J. Neurosci.Res. 48, 249—258.
erg, A.T., 2004. Understanding the delay before epilepsy surgery:who develops intractable focal epilepsy and when? CNS Spectr.9, 136—144.
liss, T.V., Collingridge, G.L., 1993. A synaptic model of memory:long-term potentiation in the hippocampus. Nature 361, 31—39.
org, J., 2008. Molecular imaging of the 5-HT(1A) receptor in rela-tion to human cognition. Behav. Brain Res. 16, 103—111.
uhot, M.C., Martin, S., Segu, L., 2000. Role of serotonin in memoryimpairment. Ann. Med. 32, 210—221.
urnet, P.W., Eastwood, S.L., Harrison, P.J., 1994. Detection andquantitation of 5-HT1A and 5-HT2A receptor mRNAs in humanhippocampus using a reverse transcriptase-polymerase chainreaction (RT-PCR) technique and their correlation with bindingsite densities and age. Neurosci. Lett. 178, 85—89.
ampo, P., Garrido, M.I., Moran, R.J., García-Morales, I., Poch, C.,Toledano, R., Gil-Nagel, A., Dolan, R.J., Friston, K.J., 2013. Net-work reconfiguration and working memory impairment in mesialtemporal lobe epilepsy. Neuroimage 72, 48—54.
arli, M., Samanin, R., 1988. Potential anxiolytic properties of 8-hydroxy-2-(di-n-propylamino)tetralin, a selective serotonin 1Areceptor agonist. Psychopharmacology (Berl.) 94, 84—91.
arli, M., Tatarczynska, E., Cervo, L., Samanin, R., 1993. Stim-ulation of hippocampal 5-HT1A receptors causes amnesia andanxiolytic-like but not antidepressant-like effects in the rat. Eur.J. Pharmacol. 234, 215—221.
1
C
C
C
D
D
D
D
D
D
D
E
E
E
F
G
G
G
G
H
J
K
L
L
L
M
M
M
M
M
M
O
O
O
P
R
R
R
R
R
862
elada, P., Puig, M.V., Artigas, F., 2013. Serotonin modulation ofcortical neurons and networks. Front. Integr. Neurosci. 7, 1—20.
ohen, M.X., 2011. Hippocampal-prefrontal connectivity predictsmidfrontal oscillations and long-term memory performance.Curr. Biol. 21, 1900—1905.
olino, A., Halliwell, A.J., 1987. Differential modulation of threeseparate K-conductances in hippocampal CA1 neurons by sero-tonin. Nature 2, 73—77.
ailey, J.W., Reith, M.E., Yan, Q.S., Li, M.Y., Jobe, P.C., 1997.Anticonvulsant doses of carbamazepine increase hippocampalextracellular serotonin in genetically epilepsy-prone rats: doseresponse relationships. Neurosci. Lett. 227, 13—16.
e Lanerolle, N.C., Kim, J.H., Robbins, R.J., Spencer, D.D., 1989.Hippocampal interneuron loss and plasticity in human temporallobe epilepsy. Brain Res. 495, 387—395.
ijk, S.N., Francis, P.T., Stratmann, G.C., Bowen, D.M., 1995.NMDA-induced glutamate and aspartate release from rat cor-tical pyramidal neurones: evidence for modulation by a 5-HT1Aantagonist. Br. J. Pharmacol. 115, 1169—1174.
illon, K.A., Gross-Isseroff, R., Israeli, M., Biegon, A., 1991. Autora-diographic analysis of serotonin 5-HT1A receptor binding in thehuman brain postmortem: effects of age and alcohol. Brain Res.554, 56—64.
odrill, C.B., 2002. Progressive cognitive decline in adolescents andadults with epilepsy. Prog. Brain Res. 135, 399—407.
rake, M., Allegri, F., Thomson, A., 2000. Alteración CognitivaEjecutiva de Tipo Frontal en Pacientes con Epilepsia de LóbuloTemporal Mesial. Medicina 60, 453—456.
uring, M.J., Spencer, D.D., 1993. Extracellular hippocampal glu-tamate and spontaneous seizure in the conscious human brain.Lancet 341, 1607—1610.
lger, C.E., Helmstaedter, C., Kurthen, M., 2004. Chronic epilepsyand cognition. Lancet Neurol. 3, 663—672.
ngel Jr., J., 1987. Approaches to localization of the epileptogeniclesion. In: Engel Jr., J. (Ed.), Surgical Treatment of the Epilep-sies. Raven Press, New York, NY, pp. 75—95.
ngel, J., 1996. Introduction to temporal lobe epilepsy. EpilepsyRes. 26, 141—150.
riedman, E., Wang, H.Y., 1996. Receptor-mediated activation of Gproteins is increased in postmortem brains of bipolar affectivedisorder subjects. J. Neurochem. 67, 1145—1152.
iovacchini, G., Toczek, M.T., Bonwetsch, R., Bagic, A., Lang, L.,Fraser, C., Reeves-Tyer, P., Herscovitch, P., Eckelman, W.C., Car-son, R.E., Theodore, W.H., 2005. 5-HT 1A receptors are reducedin temporal lobe epilepsy after partial-volume correction. J.Nucl. Med. 46, 1128—1135.
loor, P., 1987. Comment: approaches to localization of the epilep-togenic lesion. In: Engel Jr., J. (Ed.), Surgical Treatment of theEpilepsies. Raven Press, New York, NY, pp. 97—100.
onzález-Maeso, J., Torre, I., Rodríguez-Puertas, R., García-Sevilla,J.A., Guimón, J., Meana, J.J., 2002. Effects of age, post-mortem delay and storage time on receptor-mediated activationof G-proteins in human brain. Neuropsychopharmacology 26,468—478.
uerrero-Pérez, R., Aguado-Carrillo, G., Vázquez-Barrón, D.,Velasco, F., Velasco, A.L., 2014. Tiempo estimado entre el ini-cio de las crisis y el tratamiento quirúrgico en pacientes conepilepsia refractaria. Archivos de Neurociencias 19, 151—154.
elmstaedter, C., Elger, C.E., 2009. Chronic temporal lobe epilepsy:a neurodevelopmental or progressively dementing disease? Brain132, 2822—2830.
okeit, H., Ebner, A., 1999. Long term effects of refractory temporallobe epilepsy on cognitive abilities: a cross sectional study. J.Neurol. Neurosurg. Psychiatry 67, 44—50.
line, A.E., Yu, J., Massucci, J.L., Zafonte, R.D., Dixon, C.E.,2002. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin against traumatic brain
R
M. Cuellar-Herrera et al.
injury-induced cognitive deficits and neuropathology in adultmale rats. Neurosci. Lett. 333, 179—182.
ezak, M.D., 2004. Neuropsychological Assessment, fourth ed.Oxford University Press, Oxford, pp. 986.
ópez-Meraz, M.L., Martínez, A., Rocha, L., 2007. Effect of 8-OH-DPAT on electrographic activity during the kainic acid-inducedstatus epilepticus in rats. Seizure 16, 365—370.
owry, O.H., Rosenbrough, N.J., Farr, R.J., Randall, R.J., 1951. Pro-tein measurement with the Folin phenol reagent. J. Biol. Chem.193, 265—273.
arques, C.M., Caboclo, L.O., da Silva, T.I., Noffs, M.H., CarreteJr., H., Lin, K., Lin, J., Sakamoto, A.C., Yacubian, E.M., 2007.Cognitive decline in temporal lobe epilepsy due to unilateralhippocampal sclerosis. Epilepsy Behav. 10, 477—485.
atsuyama, S., Nei, K., Tanaka, C., 1996. Regulation of glutamaterelease via NMDA and 5-HT1A receptors in guinea pig dentategyrus. Brain Res. 728, 175—180.
erlet, I., Ostrowsky, K., Costes, N., Ryvlin, P., Isnard, J., Fail-lenot, I., Lavenne, F., Dufournel, D., Le Bars, D., Mauguière,F., 2004. 5-HT1A receptor binding and intracerebral activity intemporal lobe epilepsy: an [18F]-MPPF—PET study. Brain 127,900—913.
eneses, A., Hong, E., 1999. 5-HT1A receptors modulate the con-solidation of learning in normal and cognitively impaired rats.Neurobiol. Learn. Mem. 71, 207—218.
ilner, B., 1972. Disorders of learning and memory after temporallobe lesions in man. Clin. Neurosurg. 19, 421—446.
iller, E.K., Cohen, J.D., 2001. An integrative theory of prefrontalcortex function. Annu. Rev. Neurosci. 24, 167—202.
gren, S.O., Eriksson, T.M., Elvander-Tottie, E., D’Addario, C.,Ekström, J.C., Svenningsson, P., Meister, B., Kehr, J., Stiedl, O.,2008. The role of 5-HT(1A) receptors in learning and memory.Behav. Brain Res. 195, 54—77.
kada, M., Kaneko, S., Hirano, T., Ishida, M., Kondo, T., Otani, K.,Fukushima, Y., 1992. Effects of zonisamide on extracellular lev-els of monoamine and its metabolite, and on Ca2+ dependentdopamine release. Epilepsy Res. 13, 113—119.
strosky-Solis, F., Ardila, A., Roselli, M., 1999. Neuropsi: a briefneuropsychological test battery in Spanish with norms by ageand educational level. J. Int. Neuropsychol. Soc. 5, 413—433.
áldyová, E., Bereczki, E., Sántha, M., Wenger, T., Borsodi, A.,Benyhe, S., 2008. Noladin ether, a putative endocannabinoidreceptors. Neurochem. Int. 52, 321—328.
ausch, R., Kraemer, S., Pietras, C.J., Le, M., Vickrey, B.G., Passaro,E.A., 2003. Early and late cognitive changes following temporallobe surgery for epilepsy. Neurology 60, 951—959.
iedel, W.J., Klaassen, T., Griez, E., Honig, A., Menheere, P.P.,van Praag, H.M., 2002. Dissociable hormonal, cognitive andmood responses to neuroendocrine challenge: evidence forreceptor-specific serotonergic dysregulation in depressed mood.Neuropsychopharmacology 26, 358—367.
obbins, T.W., 2000. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res.133, 130—138.
ocha, L., Lorigados-Pedre, L., Orozco-Suárez, S., Morales-Chacón,L., Alonso-Vanegas, M., García-Maeso, I., Villeda-Hernández, J.,Osorio-Rico, L., Estupinán, B., Quintana, C., 2007. Autoradiogra-phy reveals selective changes in serotonin binding in neocortexof patients with temporal lobe epilepsy. Prog. Neuropsychophar-macol. Biol. Psychiatry 31, 1208—1218.
oth, B.L., Hanizavareh, S.M., Blum, A.E., 2004. Serotonin recep-tors represent highly favorable molecular targets for cognitiveenhancement in schizophrenia and other disorders. Psychophar-macology (Berl.) 174, 17—24.
odríguez-Puertas, R., González-Maeso, J., Meana, J.J., Pazos, A.,2000. Autoradiography of receptor-activated G-proteins in postmortem human brain. Neuroscience 96, 169—180.
ilep
S
S
T
T
T
V
W
Functional alteration of 5-HT1A receptor in temporal lobe ep
Salgado-Commissariat, D., Alkadhi, K.A., 1997. Serotonin inhibitsepileptiform discharge by activation of 5-HT1A recep-tors in CA1 pyramidal neurons. Neuropharmacology 36,1705—1712.
Savic, I., Lindström, P., Gulyás, B., Halldin, C., Andrée, B., Farde,L., 2004. Limbic reductions of 5-HT1A receptor binding in humantemporal lobe epilepsy. Neurology 62, 1343—1351.
Schechter, L.E., Dawson, L.A., Harder, J.A., 2002. The potentialutility of 5-HT1A receptor antagonists in the treatment of cog-nitive dysfunction associated with Alzheimer’s disease. Curr.Pharm. Des. 8, 139—145.
Schreiber, G., Avissar, S., Danon, A., Belmaker, R.H., 1991. Hyper-functional G proteins in mononuclear leukocytes of patients withmania. Biol. Psychiatry 29, 273—280.
Scoville, W.B., Milner, B., 2000. Loss of recent memory after bilat-eral hippocampal Lesions. J. Neuropsychiatry Clin. Neurosci. 1,103—113.
Sloviter, R.S., 1994. The functional organization of the hippocampaldentate gyrus and its relevance to the pathogenesis of temporallobe epilepsy. Ann. Neurol. 35, 640—654.
Spetea, M., Monory, K., Tömböly, C., Tóth, G., Tzavara, E., Benyhe,S., Hanoune, J., Borsodi, A., 1998. In vitro binding and signalingprofile of the novel mu opioid receptor agonist endomorphin 2in rat brain membranes. Biochem. Biophys. Res. Commun. 250,720—725.
Sumiyoshi, T., Matsui, M., Yamashita, I., Nohara, S., Uehara,
T., Kurachi, M., Meltzer, H.Y., 2000. Effect of adjunctivetreatment with serotonin-1A agonist tandospirone on mem-ory functions in schizophrenia. J. Clin. Psychopharmacol. 20,386—388.Y
sy: relationship to memory deficit 1863
umiyoshi, T., Matsui, M., Yamashita, I., Nohara, S., Kurachi, M.,Uehara, T., Sumiyoshi, S., Sumiyoshi, C., Meltzer, H.Y., 2001.The effect of tandospirone, a serotonin(1A) agonist, on memoryfunction in schizophrenia. Biol. Psychiatry 49, 861—868.
umiyoshi, T., Park, S., Jayathilake, K., Roy, A., Ertugrul, A.,Meltzer, H.Y., 2007. Effect of buspirone, a serotonin1A partialagonist, on cognitive function in schizophrenia: a random-ized, double-blind, placebo-controlled study. Schizophr. Res. 95,158—168.
heodore, W.H., Wiggs, E.A., Martinez, A.R., Dustin, I.H., Khan,O.I., Appel, S., Reeves-Tyer, P., Sato, S., 2012. Serotonin 1Areceptors, depression, and memory in temporal lobe epilepsy.Epilepsia 53, 129—133.
hompson, P.J., Duncan, J.S., 2005. Cognitive decline in severeintractable epilepsy. Epilepsia 46, 1780—1787.
oczek, M.T., Carson, R.E., Lang, L., Ma, Y., Spanaki, M.V., Der,M.G., Fazilat, S., Kopylev, L., Herscovitch, P., Eckelman, W.C.,Theodore, W.H., 2003. PET imaging of 5-HT1A receptor bindingin patients with temporal lobe epilepsy. Neurology 60, 749—756.
elasco, A.L., Boleaga, B., Brito, F., Jiménez, F., Gordillo, J.L.,Velasco, F., Velasco, M., 2000. Absolute and relative predictorvalues of some non-invasive and invasive studies for the outcomeof anterior temporal lobectomy. Arch. Med. Res. 31, 62—74.
ada, Y., Nakamura, M., Hasegawa, H., Yamaguchi, N., 1992. Roleof serotonin receptor subtype in seizures kindled from the felinehippocampus. Neurosci. Lett. 141, 21—24.
asuno, F., Suhara, T., Nakayama, T., Ichimiya, T., Okubo, Y.,Takano, A., Ando, T., Inoue, M., Maeda, J., Suzuki, K., 2003.Inhibitory effect of hippocampal 5-HT1A receptors on humanexplicit memory. Am. J. Psychiatry 160, 334—340.
Related Documents