ABCD, the Language of Replication Protein A (RPA) Ronald Wilson Reagan College Preparatory High School, Milwaukee Public Schools Caleb Anderson, Rohit Bhatia, Max Ehlers, Kiley Ledger, Hailey Lentz, Sage Marsh, Brenden McLaughlin Haralson, Samantha Pieper, Angelise Puls, Samia Sheikh, Amaya Smith, Max Spellecy, Mayumi Sweeney, Ah Yu Ya, Eliza Borysenko Teachers: Molly Schuld, Jose Perez Research Mentor Dr. Edwin Antony, Marquette University Replication Protein A (RPA) is a eukaryotic single stranded DNA binding protein that is required for DNA replication and repair. In the cell, RPA plays four important functions that are required for all DNA metabolism events including DNA replication, recombination, and repair. Functions i) RPA binds to ssDNA and protects it from being degraded; ii) RPA binds to over 30 different enzymes and recruits it to the ssDNA; iii) RPA informs the cell that ssDNA is present and thus signals the cell cycle checkpoint response; iv) RPA keeps the DNA from getting tangled. RPA binds tightly to ssDNA, but also has to give it away to other proteins for DNA maintenance in the cell. How this occurs is not understood. The current hypothesis is that each DNA binding domain can be individually remodeled by the interacting proteins. Since the overall function of RPA is to ensure that ssDNA is protected, defects in RPA function lead to genome instability and an accumulation of high frequency mutations. Thus, mutations in RPA lead to an increased likelihood of genetic mutations resulting in hereditary disorders and cancers. RPA binds to ssDNA and protects it from degradation. This is achieved through very tight interactions between RPA and ssDNA. RPA is made up of three subunits RPA70, RPA32 and RPA14. The protein can be further subdivided into unique DNA binding domains labeled: A (red), B (yellow), C (green), and D (blue) (Figure 1). The DNA binding domains are referred to as DBDs. DBD-C and DBD-D form the trimerization core with the RPA14 subunit (pink) and this holds all three subunits of RPA together. RPA also includes domain E (pink) and domain F (N-terminus) that do not bind to ssDNA (purple), but instead interact with other proteins. If one were to think of the domains as ‘fingers in a hand’, then the strength of RPA comes from the fingers working together to tightly hold on to the DNA. If RPA needs to be removed from ssDNA, it might be easier to remove one ‘finger’ at a time. In conclusion, RPA is a crucial protein that binds and protects the ssDNA and also communicates with other DNA repair processes to ensure that DNA doesn’t become mutated or damaged. RPA consists of multiple domains. The data shows that DBD-A binds the fastest, but also falls off rapidly. Hence it is in a highly dynamic state. DBD-D is more stable on the DNA. The binding/dissociation of other DBDs are currently being investigated. In the future, the investigators will explore how the dynamics of these DBDs are altered by enzymes that displace RPA from ssDNA. RPA is relevant to current medical studies because the information regarding the speed at which each DBD binds to the DNA provides possible insight to treatment of genetic mutations, such as cancer. References Fan, J., Pavletich, N.P., 2012. Structure and conformational change of a replication protein A heterotrimer bound to ssDNA. Genes. Dev. 26, 2337e2347. Sugitani N, Chazin WJ (2015) Characteristics and concepts of dynamic hub proteins in DNA processing machinery from studies of RPA. Prog Biophys Mol Biol 117:206–211 Pokhrel, et. al. (2017) Monitoring Replication Protein A (RPA) dynamics in homologous recombination through site-specific incorporation of non-canonical amino acids. Nucleic Acids Research 45(16):9413-9426 Introduction Introduction Molecular Story Molecular Story Figure 1: A diagram of RPA showing the three subunits: RPA70, RPA 32 and RPA14, and the individual DNA binding domains Figure 1: A diagram of RPA showing the three subunits: RPA70, RPA 32 and RPA14, and the individual DNA binding domains Figure 2: A Jmol model of RPA (4GNX) Figure 2: A Jmol model of RPA (4GNX) Summary Summary Acknowledgements The MSOE Center for BioMolecular Modeling would like to acknowledge and thank the National Institutes of Health Science Education Partnership Award (NIH-SEPA 1R25OD010505-01) and the National Institutes of Health Clinical and Translational Science Award (NIH-CTSA UL1RR031973) for their support in funding the 2017-2018 SMART Team program. Data presented here are unpublished work from a manuscript that is currently under review: Pokhrel N., Caldwell C., Corless E., Tillison E., Wold M.S., Spies M,, and Antony E. – Please contact Dr. Edwin Antony ([email protected]) for more information about the scientific aspects of the work. Funding for this research in Dr. Antony’s group is supported by a grant from the National Institutes of Health 7R15GM110671 Scientific Process Scientific Process Researchers are trying to find out which domain of RPA stays bound to ssDNA for the longest amount of time by measuring the strength and duration of each DBD using rapid kinetic tools such as the stopped flow analysis of RPA-DNA interactions. • DBD-A and DBD-D were chemically modified using unnatural amino acids to carry a fluorophore, which was excited with a particular wavelength of light, so the emission could be captured in real-time using a stopped flow spectrophotometer (Figure 3). • When a particular DBD is bound to the DNA, the fluorescence enhances. • Monitoring the change in fluorescence as a function of time yields kinetic data, or how fast the DBDs bind to DNA. This information can be used to calculate the ON-rate and OFF-rate for each DBD. • Data shows that DBD-A is very dynamic and falls off the DNA as soon as it binds to the DNA. DBD-D on the other hand is more stable on the DNA. Figure 3: Stopped Flow Instrument Figure 3: Stopped Flow Instrument Figure 4: Stopped Flow Experiments. A) Changes in DBD-A fluorescence upon binding to increasing amounts of ssDNA and its B) kinetic analysis. C) Similar stopped flow analysis of DBD- D binding to DNA and D) its kinetic analysis. E) and F) are models showing the binding properties of the individual DBDs of RPA. Figure 4: Stopped Flow Experiments. A) Changes in DBD-A fluorescence upon binding to increasing amounts of ssDNA and its B) kinetic analysis. C) Similar stopped flow analysis of DBD- D binding to DNA and D) its kinetic analysis. E) and F) are models showing the binding properties of the individual DBDs of RPA. e f DBD-B DBD-A Domain E DBD-D DBD-C ssDNA

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

ABCD, the Language of Replication Protein A (RPA)Ronald Wilson Reagan College Preparatory High School, Milwaukee Public Schools
Caleb Anderson, Rohit Bhatia, Max Ehlers, Kiley Ledger, Hailey Lentz, Sage Marsh, Brenden McLaughlin Haralson,
Samantha Pieper, Angelise Puls, Samia Sheikh, Amaya Smith, Max Spellecy, Mayumi Sweeney, Ah Yu Ya, Eliza Borysenko
Teachers: Molly Schuld, Jose Perez
Research Mentor Dr. Edwin Antony, Marquette University
Replication Protein A (RPA) is a eukaryotic
single stranded DNA binding protein that is
required for DNA replication and repair. In the
cell, RPA plays four important functions that are
required for all DNA metabolism events
including DNA replication, recombination, and
repair.
Functions
i) RPA binds to ssDNA and protects it from
being degraded;
ii) RPA binds to over 30 different enzymes and
recruits it to the ssDNA;
iii) RPA informs the cell that ssDNA is present
and thus signals the cell cycle checkpoint
response;
iv) RPA keeps the DNA from getting tangled.
RPA binds tightly to ssDNA, but also has to
give it away to other proteins for DNA
maintenance in the cell. How this occurs is not
understood. The current hypothesis is that each
DNA binding domain can be individually
remodeled by the interacting proteins.
Since the overall function of RPA is to
ensure that ssDNA is protected, defects in RPA
function lead to genome instability and an
accumulation of high frequency mutations.
Thus, mutations in RPA lead to an increased
likelihood of genetic mutations resulting in
hereditary disorders and cancers.
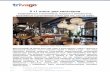
RPA binds to ssDNA and protects it from
degradation. This is achieved through very tight
interactions between RPA and ssDNA. RPA is
made up of three subunits RPA70, RPA32 and
RPA14. The protein can be further subdivided into
unique DNA binding domains labeled: A (red), B
(yellow), C (green), and D (blue) (Figure 1). The
DNA binding domains are referred to as DBDs.
DBD-C and DBD-D form the trimerization core with
the RPA14 subunit (pink) and this holds all three
subunits of RPA together. RPA also includes
domain E (pink) and domain F (N-terminus) that do
not bind to ssDNA (purple), but instead interact with
other proteins. If one were to think of the domains
as ‘fingers in a hand’, then the strength of RPA
comes from the fingers working together to tightly
hold on to the DNA. If RPA needs to be removed
from ssDNA, it might be easier to remove one
‘finger’ at a time.
In conclusion, RPA is a crucial protein that binds and protects the ssDNA and also communicates
with other DNA repair processes to ensure that DNA doesn’t become mutated or damaged. RPA consists
of multiple domains. The data shows that DBD-A binds the fastest, but also falls off rapidly. Hence it is in a
highly dynamic state. DBD-D is more stable on the DNA. The binding/dissociation of other DBDs are
currently being investigated. In the future, the investigators will explore how the dynamics of these DBDs
are altered by enzymes that displace RPA from ssDNA. RPA is relevant to current medical studies
because the information regarding the speed at which each DBD binds to the DNA provides possible
insight to treatment of genetic mutations, such as cancer.
References
Fan, J., Pavletich, N.P., 2012. Structure and conformational change of a replication protein A
heterotrimer bound to ssDNA. Genes. Dev. 26, 2337e2347.
Sugitani N, Chazin WJ (2015) Characteristics and concepts of dynamic hub proteins in DNA
processing machinery from studies of RPA. Prog Biophys Mol Biol 117:206–211
Pokhrel, et. al. (2017) Monitoring Replication Protein A (RPA) dynamics in homologous
recombination through site-specific incorporation of non-canonical amino acids. Nucleic
Acids Research 45(16):9413-9426
IntroductionIntroduction Molecular StoryMolecular Story
Figure 1: A diagram of RPA showing the three subunits:
RPA70, RPA 32 and RPA14, and the individual DNA
binding domains
Figure 1: A diagram of RPA showing the three subunits:
RPA70, RPA 32 and RPA14, and the individual DNA
binding domains
Figure 2: A Jmol model of RPA (4GNX)Figure 2: A Jmol model of RPA (4GNX)
SummarySummary
Acknowledgements
The MSOE Center for BioMolecular Modeling would like to acknowledge and thank the National
Institutes of Health Science Education Partnership Award (NIH-SEPA 1R25OD010505-01) and
the National Institutes of Health Clinical and Translational Science Award (NIH-CTSA
UL1RR031973) for their support in funding the 2017-2018 SMART Team program.
Data presented here are unpublished work from a manuscript that is currently under review:
Pokhrel N., Caldwell C., Corless E., Tillison E., Wold M.S., Spies M,, and Antony E. – Please
contact Dr. Edwin Antony ([email protected]) for more information about the scientific
aspects of the work.
Funding for this research in Dr. Antony’s group is supported by a grant from the National Institutes
of Health 7R15GM110671
Scientific ProcessScientific Process
Researchers are trying to find out which domain of RPA stays bound to
ssDNA for the longest amount of time by measuring the strength and
duration of each DBD using rapid kinetic tools such as the stopped flow
analysis of RPA-DNA interactions.
• DBD-A and DBD-D were chemically modified using unnatural amino
acids to carry a fluorophore, which was excited with a particular
wavelength of light, so the emission could be captured in real-time
using a stopped flow spectrophotometer (Figure 3).
• When a particular DBD is bound to the DNA, the fluorescence
enhances.
• Monitoring the change in fluorescence as a function of time yields
kinetic data, or how fast the DBDs bind to DNA. This information can
be used to calculate the ON-rate and OFF-rate for each DBD.
• Data shows that DBD-A is very dynamic and falls off the DNA as
soon as it binds to the DNA. DBD-D on the other hand is more stable
on the DNA.
Figure 3: Stopped Flow InstrumentFigure 3: Stopped Flow Instrument
Figure 4: Stopped
Flow Experiments.
A) Changes in DBD-A
fluorescence upon
binding to
increasing
amounts of ssDNA
and its
B) kinetic analysis.
C) Similar stopped
flow analysis of DBD-
D binding to DNA and
D) its kinetic analysis.
E) and F) are models
showing the binding
properties of the
individual DBDs of
RPA.
Figure 4: Stopped
Flow Experiments.
A) Changes in DBD-A
fluorescence upon
binding to
increasing
amounts of ssDNA
and its
B) kinetic analysis.
C) Similar stopped
flow analysis of DBD-
D binding to DNA and
D) its kinetic analysis.
E) and F) are models
showing the binding
properties of the
individual DBDs of
RPA.
e
f
DBD-B
DBD-ADomain E
DBD-D
DBD-C
ssDNA
Related Documents