A Novel Self-regenerating Water Filtration System for the Removal of Lead Ions Using N,S-modified Graphene Oxide Nanoparticles Julian Olivarez, Maria Medina, Alexis Castañon, Cesar Hernandez, Brenden Salazar, Juan Noveron Acknowledgement This project is sponsored by the National Science Foundation No. DRL- 1322600. We would like to thank Gabriel Salazar, Carmen Abril Chavez, Cesar Hernandez, Tariqul Islam, Andrew Pardo and Noemi Dominguez for their assistance throughout the whole project. Method • The design of the conductive porous material is based on functionalizing graphene oxide modified with sulfur-nitrogen lead-binding sites. • This will be accomplished by poking holes into graphene and attaching sulfur atoms on the edges of the pores. • Lead ions will bind to the sulfur groups • After saturation of the lead-binding sites, we will employ an electrical current attached to a copper wire to desorb the lead ions with an electro dialysis action, thus regenerating the lead filter. • The lead flushed out of the system will be immobilized into a suitable solid waste matrix Results References [1] Liu, Y., Lou, J., Ni, M., Song, C., Wu, J., Dasgupta, N. P., Deng, T., Tao P., Shang W. (2016). Bioinspired Bifunctional Membrane for Efficient Clean Water Generation. ACS Appl. Mater. Interfaces ACS Applied Materials & Interfaces, 8(1), 772-779. doi:10.1021/acsami.5b09996 [2] Geneva, R. (2013, October 18). Stop Lead Poisoning in Children. Retrieved June 15, 2016, from http://www.who.int/mediacentre/news/notes/2013/lead-20131018/en/en/ [3] National Science Foundation. (2015). Lead in Drinking Water. Retrieved June 15, 2016, from http://www.nsf.org/consumer-resources/health-and-safety-tips/water-quality-treatment-tips/lead-in-drinking-water [4] Wang, N., Xu, X., Li, H., Zhai, J., Yuan, L., Zhang, K., & Yu, H. (2016). Preparation and Application of a Xanthate-Modified Thiourea Chitosan Sponge for the Removal of Pb(II) from Aqueous Solutions. Industrial & Engineering Chemistry Research Ind. Eng. Chem. Res., 55(17), 4960-4968. doi:10.1021/acs.iecr.6b00694 [5] Pieper, K. J., Krometis, L., Gallagher, D., Benham, B., & Edwards, M. (2015). Profiling Private Water Systems to Identify Patterns of Waterborne Lead Exposure. Environmental Science & Technology Environ. Sci. Technol., 49(21), 12697-12704. doi:10.1021/acs.est.5b03174 [6] Ai, W., Luo, Z., Jiang, J., Zhu, J., Du, Z., Fan, Z., Xie, L., Zhang, H., Huang, W., Yu, T. (2014). Nitrogen and Sulfur Codoped Graphene: Multifunctional Electrode Materials for High-Performance Li-Ion Batteries and Oxygen Reduction Reaction. Adv. Mater. Advanced Materials, 26(35), 6186-6192. doi:10.1002/adma.201401427 [7] Marcano, D. C., Kosynkin, D. V., Berlin, J. M., Sinitskii, A., Sun, Z., Slesarev, A., Alemany, B. L., Lu, W., Tour, J. M. (2010). Improved Synthesis of Graphene Oxide. American Chemical Society-Marcano ET AL., 4(8), 4806-4814. doi:10.1021/nn1006368 [8] Huang, Z., Zheng, X., Lv, W., Wang, M., Yang, Q., & Kang, F. (2011). Adsorption of Lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir, 27(12), 7558-7562. doi:10.1021/la200606r [9] Zhao, G., Li, J., Ren, X., Chen, C., & Wang, X. (2011). Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management.Environmental Science & Technology Environ. Sci. Technol., 45(24), 10454-10462. doi:10.1021/es203439v Abstract An urgent problem that is especially important in the future development of modern society, is the practical effects of water pollution due to lead contamination [1] . According to the Environmental Protection Agency, more than 10 million homes and buildings in America receive and utilize water from service lines made of lead [2] . Over 600,000 cases of lead poisoning in children are reported annually and 143,000 deaths are reported due to lead poisoning [3] . Homes that were built before 1986 have solder joints that can leach lead into the pipe lines, contaminating clean water [5] . There is no amount of lead in water considered to be healthy, but the level to begin action to remove contamination from water is when the amount exceeds 15 parts per billion [4] . Lead in drinking water cannot be seen, tasted or smelled. The only way to determine the level of lead in your water is to have it tested. Graphene nanoparticles have shown many potential applications in removing targeted toxins in water. In our research, we modified graphene oxide with sulfur and nitrogen functional groups while increasing the surface area and maintain the electrical conductivity of the material. The covalent functionalization of graphene was carried out with 2-aminothiophenol as a source of the heteroatoms N and S. Lead(II) adsorption experiments was carried out and analyzed with ICP-OES. Adsorption isotherms for lead (II) ions in water at varying conditions will be presented. Re-generation experiments of the nanoparticles using electro dialysis will also be presented. Conclusion After performing numerous tests with the ICP-OES, TEM, SEM and UV-Vis, we came to the conclusion that the smaller the concentration of the lead (II) ions in DI water solution, the higher the percentage that graphene oi is able to absorb. We discovered that the copper wire connected to the 12 V battery was the most successful in desorbing lead from graphene oxide. Future Work For future experiments, another way to adsorb the lead (II) ions would be with electroplating. Electroplating is a procedure used to dissolve a heavy metal with the use of an electric current to cause the cations to form a layer on an electrode. We hope that copper nanoparticles will electroplate with the lead as well as devise a system that automatically disposes the lead into a solid waste matrix. The product will be tested with but not limited to; X-ray powder diffraction, EDS elemental analysis, and Infrared Spectroscopy. Image 1: TEM image of N,S GO coated with lead ions 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 200 220 240 260 280 300 ABS WAVELENGTH Desorption of lead 20 PPM after copper_Pb stock 1 Abs 20 PPM after copper_Pb exp. silver paint Abs 20 PPM after copper_Pb exp. galvanic cell Abs 20 PPM after copper_Pb exp. copper wire Abs Image 2: SEM ,Before lead Image 3:SEM Before lead Image 4:SEM, After Lead -0.5 0 0.5 1 1.5 2 2.5 200 220 240 260 280 300 320 Before Pb(II) After Pb(II) Pb(II) release Pb Pb Pb Pb Pb Pb N,S-graphene N,S-graphene removing lead ions from water N,S-graphene electro- desorption regeneration The University of Texas at El Paso, Department of Chemistry, El Paso, TX, 500 University Ave. El Paso, TX 79968 E-mail: [email protected]

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

A Novel Self-regenerating Water Filtration System for the Removal of Lead Ions Using N,S-modified
Graphene Oxide NanoparticlesJulian Olivarez, Maria Medina, Alexis Castañon, Cesar Hernandez, Brenden Salazar, Juan Noveron
Acknowledgement
This project is sponsored by the National Science Foundation No. DRL- 1322600. We would like
to thank Gabriel Salazar, Carmen Abril Chavez, Cesar Hernandez, Tariqul Islam, Andrew Pardo
and Noemi Dominguez for their assistance throughout the whole project.
Method
• The design of the conductive porous material is based on functionalizing graphene
oxide modified with sulfur-nitrogen lead-binding sites.
• This will be accomplished by poking holes into graphene and attaching sulfur atoms
on the edges of the pores.
• Lead ions will bind to the sulfur groups
• After saturation of the lead-binding sites, we will employ an electrical current
attached to a copper wire to desorb the lead ions with an electro dialysis action, thus
regenerating the lead filter.
• The lead flushed out of the system will be immobilized into a suitable solid waste
matrix
Results
References[1] Liu, Y., Lou, J., Ni, M., Song, C., Wu, J., Dasgupta, N. P., Deng, T., Tao P., Shang W. (2016). Bioinspired Bifunctional Membrane for Efficient Clean Water Generation. ACS Appl. Mater. Interfaces ACS Applied Materials & Interfaces, 8(1), 772-779. doi:10.1021/acsami.5b09996 [2] Geneva, R. (2013, October 18). Stop Lead Poisoning in Children. Retrieved June 15, 2016, from http://www.who.int/mediacentre/news/notes/2013/lead-20131018/en/en/[3] National Science Foundation. (2015). Lead in Drinking Water. Retrieved June 15, 2016, from http://www.nsf.org/consumer-resources/health-and-safety-tips/water-quality-treatment-tips/lead-in-drinking-water[4] Wang, N., Xu, X., Li, H., Zhai, J., Yuan, L., Zhang, K., & Yu, H. (2016). Preparation and Application of a Xanthate-Modified Thiourea Chitosan Sponge for the Removal of Pb(II) from Aqueous Solutions. Industrial & Engineering Chemistry Research Ind. Eng. Chem. Res., 55(17), 4960-4968. doi:10.1021/acs.iecr.6b00694 [5] Pieper, K. J., Krometis, L., Gallagher, D., Benham, B., & Edwards, M. (2015). Profiling Private Water Systems to Identify Patterns of Waterborne Lead Exposure. Environmental Science & Technology Environ. Sci. Technol., 49(21), 12697-12704. doi:10.1021/acs.est.5b03174[6] Ai, W., Luo, Z., Jiang, J., Zhu, J., Du, Z., Fan, Z., Xie, L., Zhang, H., Huang, W., Yu, T. (2014). Nitrogen and Sulfur Codoped Graphene: Multifunctional Electrode Materials for High-Performance Li-Ion Batteries and Oxygen Reduction Reaction. Adv. Mater. Advanced Materials, 26(35), 6186-6192. doi:10.1002/adma.201401427 [7] Marcano, D. C., Kosynkin, D. V., Berlin, J. M., Sinitskii, A., Sun, Z., Slesarev, A., Alemany, B. L., Lu, W., Tour, J. M. (2010). Improved Synthesis of Graphene Oxide. American Chemical Society-Marcano ET AL., 4(8), 4806-4814. doi:10.1021/nn1006368[8] Huang, Z., Zheng, X., Lv, W., Wang, M., Yang, Q., & Kang, F. (2011). Adsorption of Lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir, 27(12), 7558-7562. doi:10.1021/la200606r[9] Zhao, G., Li, J., Ren, X., Chen, C., & Wang, X. (2011). Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management.Environmental Science & Technology Environ. Sci. Technol.,45(24), 10454-10462. doi:10.1021/es203439v
Abstract
An urgent problem that is especially important in the future development of modern society, is the practical effects of water pollution due to lead contamination [1]. According to the Environmental Protection Agency, more than 10 million homes and buildings in America receive and utilize water from service lines made
of lead[2]. Over 600,000 cases of lead poisoning in children are reported annually and 143,000 deaths are reported due to lead poisoning[3]. Homes that were built
before 1986 have solder joints that can leach lead into the pipe lines, contaminating clean water [5]. There is no amount of lead in water considered to be healthy,
but the level to begin action to remove contamination from water is when the amount exceeds 15 parts per billion [4]. Lead in drinking water cannot be seen,
tasted or smelled. The only way to determine the level of lead in your water is to have it tested. Graphene nanoparticles have shown many potential applications
in removing targeted toxins in water. In our research, we modified graphene oxide with sulfur and nitrogen functional groups while increasing the surface area
and maintain the electrical conductivity of the material. The covalent functionalization of graphene was carried out with 2-aminothiophenol as a source of the
heteroatoms N and S. Lead(II) adsorption experiments was carried out and analyzed with ICP-OES. Adsorption isotherms for lead (II) ions in water at varying
conditions will be presented. Re-generation experiments of the nanoparticles using electro dialysis will also be presented.
Conclusion
After performing numerous tests
with the ICP-OES, TEM, SEM and
UV-Vis, we came to the conclusion
that the smaller the concentration
of the lead (II) ions in DI water
solution, the higher the percentage
that graphene oi is able to absorb.
We discovered that the copper wire
connected to the 12 V battery was
the most successful in desorbing
lead from graphene oxide.
Future Work
For future experiments, another way to adsorb the
lead (II) ions would be with electroplating.
Electroplating is a procedure used to dissolve a
heavy metal with the use of an electric current to
cause the cations to form a layer on an electrode.
We hope that copper nanoparticles will
electroplate with the lead as well as devise a
system that automatically disposes the lead into a
solid waste matrix. The product will be tested with
but not limited to; X-ray powder diffraction, EDS
elemental analysis, and Infrared Spectroscopy.
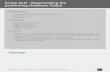
Image 1: TEM image of N,S
GO coated with lead ions
0
0.5
1
1.5
2
2.5
3
3.5
4
4.5
200 220 240 260 280 300
AB
S
WAVELENGTH
Desorption of lead
20 PPM after copper_Pb stock 1 Abs
20 PPM after copper_Pb exp. silver paint Abs
20 PPM after copper_Pb exp. galvanic cell Abs
20 PPM after copper_Pb exp. copper wire Abs
Image 2: SEM ,Before lead Image 3:SEM Before lead Image 4:SEM, After Lead
-0.5
0
0.5
1
1.5
2
2.5
200 220 240 260 280 300 320
Before Pb(II)
After Pb(II)
Pb(II) release
Pb
Pb
Pb
Pb
Pb
Pb
N,S-graphene N,S-grapheneremoving lead
ions from water
N,S-grapheneelectro-
desorption
regeneration
The University of Texas at El Paso, Department of Chemistry, El Paso, TX,
500 University Ave. El Paso, TX 79968 E-mail: [email protected]
Related Documents