A novel p.S34N mutation of CAMP gene in patients with periodontal disease Oya Tu ¨ rkog ˘ lu a, *, Afig Berdeli b , Gu ¨ lnur Emingil a , Gu ¨ l Atilla a a Department of Periodontology, School of Dentistry, Ege University, Bornova 35100, Izmir, Turkey b Molecular Medicine Laboratory, Department of Pediatrics, School of Medicine, Ege University, Izmir, Turkey 1. Introduction Periodontitis is a multifactorial inflammatory disease that involves microbial dental plaque, genetic and environmen- tal factors, and affects the supporting tissues of teeth. 1 Tissue destruction in periodontitis depends on the balance between both host protective and destructive mechanisms. 2 The innate immune response has a primary role in the defense against plaque-associated bacteria. 3,4 In addition, inflammatory and immune processes protecting the gingi- val tissues against microbial attack are harmful to the host in that inflammation can contribute to the tissue injury observed in periodontitis. 5 Chronic periodontitis is the common form of periodontitis and has a slow on-going course. 6,7 Aggressive type of periodontitis is the severe form of the periodontal diseases affecting mainly young subjects, and have a rapid attachment loss and bone resorption. 8 In addition, aggressive periodontitis subjects present polymor- phonuclear leucocyte defects that can produce high levels inflammatory mediators. 9 archives of oral biology 56 (2011) 573–579 article info Article history: Accepted 15 November 2010 Keywords: Cathelicidin antimicrobial peptide Mutation Periodontitis abstract Objective: Recent studies have showed that genetic factors involved in the host responses might determine the severity of periodontitis. hCAP-18/LL-37 is a part of the innate immune response in the oral cavity. The aim of the present study was to investigate the mutation of CAMP gene encoding hCAP-18/LL-37 in the patients with different periodontal diseases. Design: Seventy-eight chronic periodontitis, 72 generalized aggressive periodontitis, and 149 controls were analysed for mutation of CAMP gene using direct DNA sequencing method. Frequencies of p.S34N mutation were compared by Pearson chi-square test. Logistic regres- sion analysis was used to analyse the association between periodontitis and p.S34N muta- tion adjusting for bleeding on probing, age and gender. Results: Twenty-five subjects had a novel missense mutation of CAMP gene. Single base substitution (c.101G>A) in exon 1 led to p.S34N mutation. All amino acid substitutions were heterozygous mutation. The patients with generalized aggressive periodontitis had signifi- cantly higher p.S34N mutation prevalence compared to the others, whilst there was no significant difference in prevalence of p.S34N mutation between the patients with chronic periodontitis and the control subjects. Logistic regression analysis adjusted for BOP, age and gender revealed that the patients with generalized aggressive periodontitis were 5.32 times more likely to have p.S34N mutation compared to the controls (OR = 5.32, 95% CI: 1.3–22.1). Conclusion: We report a novel missense mutation of CAMP gene. p.S34N mutation in CAMP gene seems to be contributing factor for generalized aggressive periodontitis, but not for chronic periodontitis. # 2010 Elsevier Ltd. All rights reserved. * Corresponding author. Tel.: +90 232 3881105; fax: +90 232 3880325. E-mail address: [email protected] (O. Tu ¨ rkog ˘ lu). available at www.sciencedirect.com journal homepage: http://www.elsevier.com/locate/aob 0003–9969/$ – see front matter # 2010 Elsevier Ltd. All rights reserved. doi:10.1016/j.archoralbio.2010.11.016

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
A novel p.S34N mutation of CAMP gene in patients withperiodontal disease
Oya Turkoglu a,*, Afig Berdeli b, Gulnur Emingil a, Gul Atilla a
aDepartment of Periodontology, School of Dentistry, Ege University, Bornova 35100, Izmir, TurkeybMolecular Medicine Laboratory, Department of Pediatrics, School of Medicine, Ege University, Izmir, Turkey
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9
a r t i c l e i n f o
Article history:
Accepted 15 November 2010
Keywords:
Cathelicidin antimicrobial peptide
Mutation
Periodontitis
a b s t r a c t
Objective: Recent studies have showed that genetic factors involved in the host responses
might determine the severity of periodontitis. hCAP-18/LL-37 is a part of the innate immune
response in the oral cavity. The aim of the present study was to investigate the mutation of
CAMP gene encoding hCAP-18/LL-37 in the patients with different periodontal diseases.
Design: Seventy-eight chronic periodontitis, 72 generalized aggressive periodontitis, and 149
controls were analysed for mutation of CAMP gene using direct DNA sequencing method.
Frequencies of p.S34N mutation were compared by Pearson chi-square test. Logistic regres-
sion analysis was used to analyse the association between periodontitis and p.S34N muta-
tion adjusting for bleeding on probing, age and gender.
Results: Twenty-five subjects had a novel missense mutation of CAMP gene. Single base
substitution (c.101G>A) in exon 1 led to p.S34N mutation. All amino acid substitutions were
heterozygous mutation. The patients with generalized aggressive periodontitis had signifi-
cantly higher p.S34N mutation prevalence compared to the others, whilst there was no
significant difference in prevalence of p.S34N mutation between the patients with chronic
periodontitis and the control subjects. Logistic regression analysis adjusted for BOP, age and
gender revealed that the patients with generalized aggressive periodontitis were 5.32 times
more likely to have p.S34N mutation compared to the controls (OR = 5.32, 95% CI: 1.3–22.1).
Conclusion: We report a novel missense mutation of CAMP gene. p.S34N mutation in CAMP
gene seems to be contributing factor for generalized aggressive periodontitis, but not for
chronic periodontitis.
# 2010 Elsevier Ltd. All rights reserved.
avai lab le at www.sc iencedi rec t .com
journal homepage: http://www.elsevier.com/locate/aob
1. Introduction
Periodontitis is a multifactorial inflammatory disease that
involves microbial dental plaque, genetic and environmen-
tal factors, and affects the supporting tissues of teeth.1
Tissue destruction in periodontitis depends on the balance
between both host protective and destructive mechanisms.2
The innate immune response has a primary role in the
defense against plaque-associated bacteria.3,4 In addition,
inflammatory and immune processes protecting the gingi-
* Corresponding author. Tel.: +90 232 3881105; fax: +90 232 3880325.E-mail address: [email protected] (O. Turkoglu).
0003–9969/$ – see front matter # 2010 Elsevier Ltd. All rights reservedoi:10.1016/j.archoralbio.2010.11.016
val tissues against microbial attack are harmful to the host
in that inflammation can contribute to the tissue injury
observed in periodontitis.5 Chronic periodontitis is the
common form of periodontitis and has a slow on-going
course.6,7 Aggressive type of periodontitis is the severe form
of the periodontal diseases affecting mainly young subjects,
and have a rapid attachment loss and bone resorption.8 In
addition, aggressive periodontitis subjects present polymor-
phonuclear leucocyte defects that can produce high levels
inflammatory mediators.9
d.
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9574
It has been suggested that genetic factors are important
determinants of susceptibility to periodontitis since they
determine and modify host responses to the microbial
challenge.10 Inappropriate or exaggerated immune responses
against bacterial stimulus may cause variations in the
susceptibility for periodontitis.10 Genetic factors involved in
host responses might determine the severity of disease in both
chronic and aggressive periodontitis.2,11–13 Although it is
known that microbial dental plaque is the major factor
causing periodontitis, there is accumulating evidence that
genetic factors play a crucial role in the susceptibility for
especially aggressive periodontitis.14 Aggressive periodontitis
has a tendency towards familial aggregation.8,15 In family
studies, it has been suggested that the pattern of disease
transmission is consistent with Mendelian inheritance of a
gene of major effect.15–17
Human cathelicidin antimicrobial peptide of 18 kDa (hCAP-
18) is the only known cathelicidin for humans.18 These
antimicrobial peptides are products of individual genes encod-
ing the corresponding pre-pro-peptides which are cleaved to
release the mature peptide LL-37.19 It has been suggested that
hCAP18 is expressed in the squamous epithelia,20 epididymis21
and lungs.22 Expression of hCAP18 in epithelial cells is inducible
by infection and inflammation.20,22 hCAP18 binds the microbial
cell membrane and kills the bacteria by disrupting the
metabolism.23 Cathelicidin antimicrobial peptides are impor-
tant defense molecules in the oral cavity because oral
epithelium is constantly exposed to microbial pathogens.24
hCAP-18/LL-37 proteinand mRNAhavebeendetectedinhuman
tongue, buccal mucosa and saliva.20,25 hCAP-18/LL-37 is
expressed from neutrophils in gingival tissues and particularly
in junctional epithelium which serves as a route for neutrophil
migration from the connective tissue into the gingival crevice.24
The production of hCAP-18/LL-37 is upregulated in inflamed
gingival tissues compared to healthy tissues.26 In our previous
study, we demonstrated that gingival tissue samples of chronic
periodontitis had significantly higher immunostainingof hCAP-
18/LL-37 on neutrophils infiltrating in both epithelium and
connective tissue compared to controls.27 We also showed that
hCAP-18/LL-37 mRNA expression levels in gingival tissue
samples of chronic periodontitis seemed to be upregulated
compared to controls.27 On the other hand, generalized
aggressive periodontitis patients showed less immunostaining
neutrophils for hCAP-18/LL-37 in tissue samples and down-
regulated mRNA levels for hCAP-18/LL-37.27 The lack of salivary
hCAP-18/LL-37 production in patients with morbus Kostman
syndrome who have increased susceptibility to periodontal
disease28 might suggest the protective role of hCAP-18/LL-37 in
host defense. The gene encoding human hCAP-18/LL-37,
defined as CAMP, is localized on chromosome 3, and consists
of 4 exons and 3 introns.19 Up to date, the biology of the
promoters of a few cathelicidin has been studied.22,29 However,
there is currently no detailed information about the gene
encoding hCAP-18/LL-37 antimicrobial peptide.
Since hCAP-18/LL-37 antimicrobial peptide regulates in-
nate immune response in periodontitis, we hypothesized that
a mutation in CAMP gene might modify the individual
susceptibility to periodontitis. To the best of our knowledge,
there is no study investigating the CAMP gene mutation in the
patients with different types of periodontitis, and also no
reported CAMP gene mutation to date. Therefore, the present
study aimed to investigate the mutation of CAMP gene in the
patients with different types of periodontitis.
2. Materials and methods
2.1. Study population
A total of 299 unrelated Caucasians residing in the Aegean
region of Turkey and belonging to a low to moderate
socioeconomic and education level were included in the
present study. All subjects were recruited from Ege University,
School of Dentistry, and Department of Periodontology
between 2005 and 2008. Exclusion criteria were systemic
diseases, immunological disorders, infectious diseases, smok-
ing, current pregnancy or lactation, oral contraceptive usage,
antibiotic regimen within the last 3 months or periodontal
treatment within the last 6 months. Study protocol was
approved by Ethics Committee of the Ege University School of
Medicine. Subjects gave written informed consent in accor-
dance with Helsinki declaration.
2.2. Determination of periodontal status
Subjects were evaluated clinically and radiographically at the
first visit, and all clinical measurements were performed by a
calibrated examiner (O.T.). The whole mouth periodontal
parameters including probing depth (PD), clinical attachment
level (CAL), and presence of bleeding on probing (BOP)30 were
determined at six sites per tooth excluding third molars.
Plaque index (PI)31 and papilla bleeding index (PBI)32 were also
assessed. Measurements of PD (mm) and CAL (mm) were
performed with William’s periodontal probe (Hu-Friedy,
Chicago, IL, USA). The intra-examiner reliability was high as
was revealed by intraclass correlation coefficient 0.87 and 0.85
for PD and CAL measurements, respectively.
2.3. Study groups
The diagnosis of chronic and aggressive periodontitis was
established on consensus reports of International World
Workshop.6,8 The classification of the study groups was based
on the classification system proposed by the 1999 Interna-
tional World Workshop for a Classification of Periodontal
Disease and Conditions.33 In this case control study, the
subjects were categorized into three groups as follows:
Generalized, severe chronic periodontitis group: Seventy-eight
patients were included in chronic periodontitis group. The
patients who had at least 4 non-adjacent teeth with sites
CAL � 5 mm and PD � 6 mm were defined as chronic
periodontitis patients. All chronic periodontitis patients
included in this group had generalized, severe chronic
periodontitis, i.e. they had affected sites (with CAL � 5 mm)
greater than 30% of sites involved.33 Diagnosis of chronic
periodontitis was made if the CAL was commensurate with
the amount of plaque accumulation of the patients. All
patients were older than 35 years of age. All patients had at
least 16 teeth in their mouth, and BOP was higher than 50%
for whole mouth.
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9 575
Generalized aggressive periodontitis group: Seventy-two
patients were included in this group. These patients demon-
strated a generalized pattern of severe destruction and
CAL � 5 mm and PD � 6 mm on eight or more teeth, at least
three of these were other than central incisors of first molars.
Additionally, CAL was not consistent with the amount of
plaque accumulation or local contributing factors. Generalized
aggressive periodontitis was diagnosed in patients younger
than 35 years of age. All subjects had at least 16 teeth.
Control group: A hundred and forty-nine systemically
healthy subjects were included into the study. The periodon-
tally healthy subjects had PD � 3 mm, no gingival recession
due to periodontitis, CAL � 2 mm at more than or equal to 90%
of the measured tooth sites. Some control subjects who had
CAL > 2 mm at <10% of sites were included into the control
group, as judged by the examining clinician this CAL was not
due to periodontitis but to traumatic tooth brushing, over-
hanging fillings, orthodontic therapy, etc. None of the patients
had clinical signs of periodontitis. No radiographic evidence of
alveolar bone loss was observed in these patients (i.e., distance
between the cemento-enamel junction and bone crest �3 mm
at >95% of the proximal tooth sites). All subjects in this group
were older than 35 years so that they could not be too young to
have periodontitis.
2.4. Blood samples and DNA isolation
Peripheral venous blood collected in EDTA coated vacutainer
sterile tubes from each subject by standard venipuncture.
Genomic DNA was isolated using a commercially available
genomic DNA isolation kit (QIAamp Mini Blood DNA, Qiagen,
Ontario, Canada) according to the manufacturers’ instruc-
tions. DNA concentration was determined by spectrophotom-
etry at 280 nm and diluted as 50 ng/ml in 200 ml volume. All
genotyping processes were performed using quantified geno-
mic DNA.
2.5. Sequence analysis of the CAMP gene
All 4 exons of CAMP gene were amplified by polymerase chain
reaction (PCR) using flanking intronic primers with designed
using ExonPrimer-Primer Design programme (http://
igh.gsf.de/igh) obtained from Helmholz Center Institute of
Human Genetics (Munich, Germany). All synthetic oligonucle-
otide primers synthesized and purchased by Invitrogen Ltd.
(Paisley, UK) as the HPLC purification grade.
Primer sequences were 50-CAT AAA GGA GGC TCC TGT
GGG-30 sense and 50R-AGGC AGT GCA GCA GGA GCA GA-30
nonsense for exon 1, 50-GAGGTCATGG ACACAAATCA-30 sense
and 50-GAG TAG TTA ATT GAC CCA TT-30 nonsense for exon 2,
50-AACCTGTT TCTTCCTGTA CACAACCCC-30 sense and 50-
CAG AGC CCA GAA GCC TGA GCC-30 nonsense for exons 3 and
4. PCR amplification was carried out on a BioNeerMyGenieTM 96
Gradient Thermal Block the peltier-based systems (BioNeer
Corp, Daejeon, Korea) in a 25 ml reaction mixture in 0.2 ml
thin-wall PCR strip tubes (Axygen Scientific, Inc., CA, USA)
containing 1 ml genomic DNA solution, Platinium TAQ with
Enhancer Buffer (Invitrogen Ltd., Paisley, UK) 50 mmol/l each of
the dGTP, dATp, dTTP and dCTP (Promega, Madison, WI, USA),
5 pmol each forward and reverse primers and 1.0 U Platinium
TAQ Polymerase (Invitrogen Ltd., Paisley, UK). The cycling
conditions comprised a hot start at 95 8C for 10 min, followed
by 35 amplification cycles at gradient programme. Yielded PCR
products were purified using Exo-SAP PCR purification kit
(Amersham Life Science, Buckinghamshire HP7 9NA, UK).
Yielded purified PCR products were done cycle sequencing PCR
using the BigDye Terminator Cycle Sequencing Ready Reac-
tion Kit v.3.1 (Applied Biosystems, Forster City, CA, USA)
according to manufacturers’ instructions. Before sequencing
the PCR products were purified using BigDye XT Purification
Kit (Applied Biosystems, Forster City, CA, USA). DNA direct
sequence analysis in both forward and reverse direction was
performed by ABI3130xl Genetic Analyzer (Applied Biosys-
tems, Foster City, CA, USA). For sequence evaluation, the
SeqScape 2.0 sequencing analysis software (Applied Biosys-
tems, Foster City, CA, USA) was used. The obtained nucleotide
sequences were compared to publish Genbank (NM_004345)
and protein reference sequence (NP_004336) obtained from
NCBI (http://www.ncbi.nlm.nih.gov).
2.6. Statistical analysis
Minimal sample size was determined to detect 7% difference
in intergroup comparisons for allele frequency with 80%
power and p = 0.05 significance level. Statistical analysis was
performed a statistician who is blinded to the clinical
diagnosis of the study subjects. One-way ANOVA test was
used for comparisons of demographic and periodontal
parameters amongst the study groups. When there were
significant differences (p < 0.05), post hoc two group compar-
isons were performed with Dunnett T3 or Bonferroni tests.
Chi-square tests were used to evaluate the concordance of
genotype frequencies with Hardy–Weinberg equilibrium in
each group. The N34 allele and carrier frequencies for p.S34N
mutation were compared by Pearson chi-square test amongst
study groups. The risk of genotypes with periodontitis was
estimated by computing the odds ratio (OR) and 95% CI from
logistic regression analysis, adjusting for BOP, age and gender.
All data analysis was performed using a statistical package
(SPSS Inc., ver. 17.0, Chicago, IL, USA), and p-values < 0.05
were considered to be statistically significant.
3. Results
The genotype frequencies of the whole study population as
well as the frequencies of the three study groups were in
Hardy–Weinberg equilibrium (p > 0.05, x2 < 5.99). Minimum
required sample size was calculated as 70 and 145 subjects for
patient and control groups, respectively.
3.1. Demographic and clinical periodontal findings
The demographics and periodontal parameters of the study
groups are presented in Table 1. Generalized aggressive
periodontitis patients were younger than the other subjects
(p < 0.05). There was no significant difference in gender
amongst the study groups (p > 0.05).
The whole mouth PD score of the control subjects was
statistically lower than those of the others (p < 0.05). CAL
Table 1 – Demographic characteristics and clinical periodontal data of the study groups (distribution or mean W SE).
Generalized aggressive periodontitisN = 72
Chronic periodontitisN = 78
ControlN = 149
Age 28.9 � 0.7* 47.6 � 0.8* 40.5 � 0.6
Gender (N)
Male 40 42 88
Female 32 36 61
PD 3.8 � 0.09 3.8 � 0.09 1.9 � 0.03*
CAL 4.5 � 0.13 4.7 � 0.13 0.07 � 0.008*
BOP % 71.4 � 2.3 82.8 � 2.1* 37 � 2.9*
PBI 1.9 � 0.12 2.2 � 0.07 1 � 0.08*
PI 2.6 � 0.13 3 � 0.09* 2.3 � 0.06*
PD = probing depth, CAL = clinical attachment level, BOP = bleeding on probing, PBI = papilla bleeding index, PI = plaque index, SE = standard
error.* Significant difference from the other groups ( p < 0.05).
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9576
scores were similar in generalized aggressive periodontitis
and chronic periodontitis patients (p > 0.05). Periodontitis
patients (both aggressive and chronic) had significantly higher
CAL scores than those of control subjects (p < 0.05). Control
subjects had significantly less percentages of sites with BOP
when compared with the others (p < 0.05). Control subjects
had lower PBI and PI scores compared to the other groups
(p < 0.05) (Table 1).
3.2. Molecular genetic findings
Seventy-eight chronic periodontitis subjects, 72 generalized
aggressive periodontitis subjects, 149 unrelated controls were
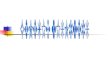
[()TD$FIG]Fig. 1 – Exon 1’s nucleotide sequences and amino acid codon ele
panel: mutant type.
analysed by the direct DNA sequencing method for the
mutation of CAMP gene. All four coding exons and exon–
intron bounder region of CAMP gene from all subjects were
sequenced. Twenty-five subjects had missense mutation in
exon 1 of the CAMP gene. Single nucleotide substitution in
exon 1 (c.101G>A) leads to missense amino acid mutation
(p.S34N) (AGC>AAC) (Fig. 1). Homozygous mutation p.N34N
was not observed in the study subjects. All amino acid
substitutions were heterozygous mutation (Table 2). The
carrier rate for the N34 allele was 8.4% (25 subjects) in whole
study population. The prevalence of the p.S34N mutation for
generalized aggressive periodontitis, chronic periodontitis,
and control groups were 16.7%, 6.4%, and 5.4%, respectively
ctropherogram of CAMP gene. Upper panel: wild type, lower
Table 2 – Distribution of genotypes and allele frequenciesof the study groups (N, %).
Generalizedaggressive
periodontitisN = 72
Chronicperiodontitis
N = 78
ControlN = 149
Genotype
Wild type 60 (83.3) 73 (93.6) 141 (94.6)
S34N 12 (16.7)* 5 (6.4) 8 (5.4)
N34N 0 (0.0) 0 (0.0) 0 (0.0)
Allele
S34 132 (91.7) 151 (96.8) 290 (97.3)
N34 12 (8.3)* 5 (3.2) 8 (2.7)
* Significant difference from the other groups ( p < 0.05).
Table 3 – Logistic regression analysis for an associationbetween the prevalence of p.S34N mutation and period-ontitis (adjusted for BOP, age, and gender).
OR 95% CI p
BOP 0.98 0.97–1 0.09
Age 0.97 0.91–1.04 0.47
Gender (reference male) 2.87 1.08–7.6 0.03
Generalized aggressive
periodontitis (reference control)
5.32 1.3–22.1 0.02
Chronic periodontitis
(reference control)
3.32 0.64–17.21 0.15
OR = odds ratio, CI = confidence interval.
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9 577
(Table 2). There was no significant difference in prevalence of
p.S34N mutation between chronic periodontitis and control
group (p > 0.05) (Table 2). The prevalence of p.S34N mutation
and N34 allele frequency in generalized aggressive periodon-
titis group was significantly higher compared to those of the
other groups ( p < 0.05) (Table 2).
The logistic regression analysis adjusted for BOP, age and
gender showed that generalized aggressive periodontitis was
significantly associated with p.S34N mutation ( p < 0.05) (Table
3). In addition, gender was also found to be associated with
p.S34N mutation (p < 0.05). The logistic regression analysis
demonstrated that generalized aggressive periodontitis
patients were 5.32 (95% CI = 1.3–22.1) times more likely to
have p.S34N mutation than control subjects (p < 0.02). Whilst
age and chronic periodontitis did not appear to be associated
with p.S34N mutation, females were 2.87 (95% CI = 1.3–22.1)
times more likely to have p.S34N mutation than male subjects
(p < 0.03).
4. Discussion
We report a novel missense mutation in CAMP gene in the
present study. Our results showed significant difference in
prevalence of p.S34N mutation between generalized aggres-
sive periodontitis and the other groups. p.S34N mutation
prevalence was higher in generalized aggressive periodontitis
group compared to chronic periodontitis and control groups.
However, no significant difference in prevalence of p.S34N
mutation between chronic periodontitis patients and controls
was presented. To the best of our knowledge, this is the first
study reporting a novel missense p.S34N mutation in CAMP
gene.
cDNA encoding region for four exons and exon–intron
bounder region of CAMP gene from 299 subjects were
sequenced in the present study. Twenty-five missense
p.S34N mutations in CAMP gene were found in the whole
study population. p.S34N mutation in CAMP gene includes a
single base substitution in exon 1. This single base substitu-
tion in exon1 (c.101G>A) alters the DNA codon from the amino
acid serine substitution (AGC) to asparagine substitution
(AAC). This nucleotide change (AGC/AAC) caused p.S34N
missense mutation.
In the present study, eight of 149 control subjects had
p.S34N mutation although they had no sign of periodontitis.
Periodontal diseases are multifactorial inflammatory diseases
caused by microbial dental plaque, genetic and environmental
factors,10,13,34,35 and there is no factor responsible for
periodontal disease alone. Periodontal tissue destruction
depends on the balance between host protective and
destructive mechanism.2 Our results demonstrated that
generalized aggressive periodontitis patients have significant-
ly higher prevalence of p.S34N mutation compared to chronic
periodontitis patients and control subjects. Similarly, N34
allele frequency was also significantly higher in generalized
aggressive periodontitis patients than the others. However,
the prevalence of p.S34N mutation of chronic periodontitis
group was similar to that of the control group. Logistic
regression analysis adjusted for BOP, age and gender showed
that there was a significant association between p.S34N
mutation and generalized aggressive periodontitis. General-
ized aggressive periodontitis patients were 5.3 times more
likely to have p.S34N mutation than control subjects.
Nevertheless, chronic periodontitis was not associated with
p.S34N mutation after logistic regression analysis. Host
immune reactions are in part genetically determined, and
genetic predisposition is an important factor for especially
aggressive periodontitis.2,34,35 Furthermore, familial aggrega-
tion is possible in aggressive periodontitis.8 In our previous
study, we demonstrated that generalized aggressive peri-
odontitis patients showed less immunostaining neutrophils in
gingival tissue samples, besides downregulated mRNA levels
for hCAP-18/LL-37 compared to healthy controls.27 We
suggested that the lack of hCAP-18/LL-37 might be an
important factor in the pathogenesis of the generalized form
of the aggressive periodontitis.27 Based on the present data, we
suggest that p.S34N mutation might be one of the contributing
factors for the pathogenesis of generalized aggressive peri-
odontitis.
hCAP-18/LL-37 is mainly released from neutrophils emi-
grating into inflammatory sites and plays an important role in
the balance between health and disease of the oral cavity.19,24
Dale et al.24 showed that antimicrobial peptides in the gingiva
are localized in specific sites, and have different roles in
various regions of the periodontium. Researchers stated that
hCAP-18/LL-37 was expressed from neutrophils in gingival
tissues and particularly in junctional epithelium which serves
as a route for neutrophil migration from the connective tissue
into the gingival crevice.24 Hosokawa et al.26 demonstrated
that neutrophils expressed hCAP-18/LL-37, and that expres-
sion was more prominent in the inflammatory lesions when
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9578
compared to healthy gingiva. This higher expression of hCAP-
18/LL-37 in inflammatory lesions might be explained by the
fact that microbial dental plaque stimulates both inflamma-
tory and immune responses during the progression of
periodontal disease. Moreover, Puklo et al.36 stated that local
deficiency in hCAP-18/LL-37 could be considered as a
supporting factor in the pathogenesis of severe periodontitis.
After stimulation, neutrophils migrate into gingival crevice
through the junctional epithelium from connective tissue, and
this antimicrobial peptide plays a protective role in defense of
oral cavity against to microbial invasion. Putsep et al.28 have
suggested that a mutation in CAMP gene could prevent or shut
off production of the cathelicidin peptides and have been
suggested as the underlying mechanism in Morbus Kostman
which is characterized with severe neutrophil deficiency. A
mutation in CAMP gene encoding hCAP-18/LL-37 might
increase the severity of the disease by modifying host
responses. Our result shown that generalized aggressive
periodontitis was significantly associated with p.S34N muta-
tion whilst chronic periodontitis did not appear to be
associated with p.S34N mutation. Our results is supported
by the results of our previous study27 and the results of Puklo
et al.36
As a limitation of the present study, sixteen periodontally
healthy subjects who had CAL > 2 mm at <10% of measured
sites were included into the study. As it is well known,
clinically detectable attachment loss may occur as a result of
events other than periodontitis, such as traumatic tooth
brushing, tooth position or subgingival margins of restora-
tions, etc. In clinical view, it is very difficult to find
periodontally healthy subjects who have no clinical attach-
ment loss in her/his dentition, who are 35 years old and more.
The presence of CAL > 2 mm at <10% of sites in some control
subjects in the present study was not due to periodontitis but
to traumatic tooth brushing, overhanging fillings, orthodontic
therapy, etc., as judged by the examining clinician.
As a conclusion, we report a novel missense mutation in
CAMP gene, mutation p.S34N, which changes DNA codon for
the amino acid serine substitution (AGC) to asparagine
substitution (AAC). To best our knowledge, this is the first
study investigating the CAMP gene mutations in periodontal
diseases. Our results demonstrated that generalized aggres-
sive periodontitis was significantly associated with p.S34N
mutation, but not chronic periodontitis. Therefore, it might be
suggested that p.S34N mutation is a contributing factor for
developing generalized aggressive periodontitis, within the
limitations of the present study. The studies investigating the
improvement of the periodontal parameters after periodontal
treatment in periodontitis patients who have p.S34N mutation
would be interesting in view of evaluating of host response to
treatment in these patients. Further studies are necessary to
investigate if p.S34N mutations are associated with suscepti-
bility to periodontitis in combination with polymorphisms in
other genes.
Acknowledgements
We thank to the Scientific and Technological Research Council
of Turkey (TUBITAK).
Funding: This study was supported by grants from The
Scientific and Technological Research Council of Turkey
(TUBITAK, SBAG-105S463).
Competing interests: We do not have any conflict of interests.
Ethical approval: The study protocol was approved by
Research Ethics Committee of Medical Faculty, Ege University
(# 05-9/6).
r e f e r e n c e s
1. Listgarten MA. Pathogenesis of periodontitis. J ClinPeriodontol 1986;13(5):418–30.
2. Kinane DF, Hart TC. Genes and gene polymorphismsassociated with periodontal disease. Crit Rev Oral Biol Med2003;14(6):430–49.
3. Boman HG. Innate immunity and the normal microfora.Immunol Rev 2000;173:5–16.
4. Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. Susceptibilityof various oral bacteria to antimicrobial peptides and tophagocytosis by neutrophils. J Periodont Res 2007;42(5):410–9.
5. Kornman KS. Mapping the pathogenesis of periodontitis: anew look. J Periodontol 2008;79(8):1560–8.
6. Lindhe J, Lamster RI, Charles A, Chung CP, Flemmig T,Kinane D, et al. Consensus report: chronic periodontitis. AnnPeriodontol 1999;1(4):38.
7. Flemmig TF. Periodontitis. Ann Periodontol 1999;4(1):32–8.8. Lang N, Bartold PM, Cullinan M, Jeffcoat M, Mombelli A,
Murakami S, et al. Consensus report: aggressiveperiodontitis. Ann Periodontol 1999;1(4):53.
9. Shapira L, Warbington M, Van Dyke TE. TNF alpha and IL-1 beta in serum of LJP patients with normal and defectiveneutrophil chemotaxis. J Periodontal Res 1994;29:371–3.
10. Page RC, Offenbacher S, Schroeder HE, Seymour GJ,Kornman KS. Advances in the pathogenesis of periodontitis:summary of developments, clinical implications and futuredirections. Periodontology 2000 1997;14:216–48.
11. Michalowicz BS, Wolff LF, Klump DG, Hinrichs JE, Aeppli D,Bouchard TJ, et al. Periodontal findings in adult twins. JPeriodontol 1991;62(5):293–9.
12. Gore EA, Sanders JJ, Pandey JP, Palesch Y, Galbraith GM.Interleukin-1beta13953 allele 2: association with diseasestatus in adult periodontitis. J Clin Periodontol1998;25(10):781–5.
13. Laine ML, Farre MA, Gonzalez G, van Dijk LJ, Ham AJ, WinkelEG, et al. Polymorphisms of the interleukin-1 gene family,oral microbial pathogens, and smoking in adultperiodontitis. J Dent Res 2001;80(8):1695–9.
14. Loos BG, Van der Velden U, Laine ML. Susceptibility. In:Lindhe J, Karring T, Lang NP, editors. Clinical periodontologyand implant dentistry. 5th ed. Oxford: Blackwell Munksgaard;2008. p. 328–46.
15. Marazita M, Burmeister J, Gunsolley J. Evidence forautosomal dominant inheritance and race-specificheterogeneity in early-onset periodontitis. J Periodontol1994;65(6):623–30.
16. Beaty TH, Boughman JA, Yang P, Astemborski JA, Suzuki JB.Genetic analysis of juvenile periodontitis in familiesascertained through an affected proband. Am J Hum Genet1987;40(5):443–52.
17. Hart TC, Marazita ML, Schenkein HA, Diehl SR. Re-interpretation of the evidence for X-linked dominantinheritance of juvenile periodontitis. J Periodontol1992;63(3):169–73.
a r c h i v e s o f o r a l b i o l o g y 5 6 ( 2 0 1 1 ) 5 7 3 – 5 7 9 579
18. Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the onlyhuman member of the cathelicidin family of antimicrobialpeptides. Biochim Biophys Acta 2006;1758(6):1408–25.
19. Bals R, Wilson JM. Cathelicidins – a family ofmultifunctional antimicrobial peptides. Cell Mol Life Sci2003;60(4):711–20.
20. Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G,Borregaard N, Stahle-Backdahl M. The human cationicantimicrobial protein (hCAP18), a peptide antibiotic, iswidely expressed in human squamous epithelia andcolocalizes with interleukin-6. Infect Immun 1999;67(5):2561–6.
21. Malm J, Sorensen O, Persson T, Frohm-Nilsson M, JohanssonB, Bjartell A, et al. The human cationic antimicrobial protein(hCAP-18) is expressed in the epithelium of humanepididymis, is present in seminal plasma at highconcentrations, and is attached to spermatozoa. InfectImmun 2000;68(7):4297–302.
22. Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M,Liden S, Wigzell H, et al. The expression of the gene codingfor the antibacterial peptide LL-37 is induced in humankeratinocytes during inflammatory disorders. J Biol Chem1997;272(24):15258–63.
23. Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M.Antimicrobial and chemoattractant activity,lipopolysaccharide neutralization, cytotoxicity, andinhibition by serum of analogs of human cathelicidin LL-37.Antimicrob Agents Chemother 2005;49(7):2845–50.
24. Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F,Robinovitch M, O’Neal R, et al. Localized antimicrobialpeptide expression in human gingival. J Periodontal Res2001;36(5):285–94.
25. Murakami M, Ohtake T, Dorschner RA, Gallo RL.Cathelicidin antimicrobial peptides are expressed insalivary glands and saliva. J Dent Res 2002;81(12):845–50.
26. Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB,Karimbux N, Napimoga MH, et al. Innate immune peptide
LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol2006;146(2):218–25.
27. Turkoglu O, Kandiloglu G, Berdeli A, Emingil G, Atilla G.Antimicrobial peptide hCAP-18/LL-37 protein and mRNAexpressions in different periodontal diseases. Oral Dis2011;17(1):60–7. 10.1111/j.1601-0825.2010.01704.x.
28. Putsep K, Carlsson G, Boman HG, Andersson M. Deficiencyof antibacterial peptides in patients with morbusKostmann: an observation study. Lancet 2002;360(9340):144–1149.
29. Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS,Hiemstra PS, et al. Human cathelicidin, hCAP-18, isprocessed to the antimicrobial peptide LL-37 byextracellular cleavage with proteinase 3. Blood2001;97(12):3951–9.
30. Ainamo J, Bay I. Problems and proposals for recordinggingivitis and plaque. Int Dent J 1975;25(4):229–35.
31. Quigley GA, Hein JW. Comparative cleansing efficiency ofmanual and power brushing. J Am Dent Assoc 1962;65:26–9.
32. Saxer UP, Muhlemann HR. Motivation and instruction. SSOSchweiz Monatsschr Zahnheilkd 1975;85(9):905–19.
33. Armitage GC. Development of a classification system forperiodontal diseases and conditions. Ann Periodontol1999;4(1):1–7.
34. Loos BG, John RP, Laine ML. Identification of genetic riskfactors for periodontitis and possible mechanisms of action.J Clin Periodontol 2005;32(6):159–79.
35. Shapira L, Wilensky A, Kinane DF. Effect of geneticvariability on the inflammatory response to periodontalinfection. J Clin Periodontol 2005;32(6):72–86.
36. Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J.Analysis of neutrophil-derived antimicrobial peptides ingingival crevicular fluid suggests importance of cathelicidinLL-37 in the innate immune response againstperiodontogenic bacteria. Oral Microbiol Immunol2008;23(4):328–35.
Related Documents